Abstract
Fatigue and exercise intolerance are symptoms in children with metabolic myopathy. Frequently this is combined with muscle pain in children with mitochondrial myopathy. Offering therapeutic advice remains challenging in this patient group. Here we describe five children above the age of four years, with normal intelligence, myopathy, exercise intolerance, motor developmental delay, and fatigue, who were diagnosed with a mitochondrial dysfunction. Based on the positive experience of condition training in adults with mitochondrial disease and inactivity, aerobic exercise training was advised for all the children. Because of the lack of clear protocols for individualized mitochondrial myopathies, regular training was initiated. The Movement Assessment Battery of Children, the Jamar dynamometer for grip force, and the Bruce protocol treadmill test were applied for evaluation. No patient showed significant disease progression on a weekly scheme of strength training or on aerobic training during periods varying between 6 and 18 months. Only one out of the five patients has shown an improvement after a period of structured, aerobic training, demonstrating good compliance and motivation over the course of 18 months. Some patients developed severe muscle pain after explosive exercise. Even in a relatively homogenous, intelligent group of patients and motivated parents, we could not reach full compliance. With our case studies, we would like to draw attention to the importance and pitfalls of movement therapy in children with mitochondrial disease.
Key words: aerobic exercise, mitochondria, oxydative phosphorylation, pediatrics.
Introduction
Fatigue, exercise intolerance, muscle pain, or muscle cramps are common symptoms in children diagnosed with a metabolic myopathy.1 One of the most challenging tasks for the pediatrician is offering therapeutic advice and care for children with mitochondrial myopathies. In general, most patients with a mitochondrial disease have a progressive disease. Associated organ involvement, such as cardiomyopathy, severe neurological symptoms, or mental retardation, often limit the choices in therapeutic possibilities. What kind of therapy should be offered? In most cases children with motor developmental delay, hypotonia, or muscle weakness are routinely treated with physical therapy. However, for the physical therapist, the question remains whether patients with a genetic malfunction of oxidative phosphorylation benefit from standard vigorous strengthening exercise, or have a better outcome with a regime of aerobic exercise. Moreover, how can children be motivate to maintain the physical training, and can we improve the quality of life by such an intervention if it proves to be effective?
Strength training or aerobic exercise programs might maximize muscle and cardiorespiratory function and prevent additional disuse atrophy in patients with diverse forms of neuromuscular disease. However, over-exertion might cause more rapid disease progression. In a mouse model of type 2 spinal muscular atrophy, running-based training sustained motor function and increased the life span by 57.3%.2 In humans, in myotonic dystrophy and fascioscapulohumoral dystrophy, patients improved their muscle strength without any observed negative side effects after different training programs.3–6 The work capacity of patients with limb-girdle muscular dystrophy type 2I improved after a three-month aerobic exercise program. Following training, no significant rise in creatine kinase was observed and the muscle morphology remained unaffected.7
Mitochondrial disorders are the most prevalent group of inherited neurometabolic diseases. They affect tissues heavily dependent on oxidative metabolism. Patients usually suffer from exercise intolerance owing to their impaired oxidative capacity and physical deconditioning.8 Fatigue and weakness are physical signs of mitochondrial myopathy that may be treatable with exercise therapy. Several studies evaluated the role and value of regular physical exercise in adult patients.3–7 Following exercise training, the muscle mass as well as mitochondrial function improves. Patients become highly motivated and experience improvement in the quality of life. Although there are different reports regarding the alteration in heteroplasmy in patients carrying mitochondrial DNA mutations, the general conclusion is that aerobic training leads to objective improvement in functional ability.9 It is hypothesized that, through exercise training, the incorporation of satellite cells can be enhanced, increasing the ratio of wild-type to mutant mtDNA-mitochondria (“gene shifting”).3 In addition, exercise can play a significant role in accelerating the rate of mitochondrial biogenesis and may soothe mitochondrial dysfunction that arises in metabolic disease. Exercise induces expression of PPARγ coactivator-1α (PGC-1α), augmenting the activity of several nuclear genes and mitochondrial biogenesis.10 These regeneration processes can lead to a decrease in fatigue and muscle pain and an increase in exercise tolerance, leading to an improved quality of life. The long-term consequences of regular exercise training in mitochondrial dysfunction are not known yet. One should consider possible late effects of training on the muscles that may be exposed to higher levels of oxidative stress, compared to healthy controls.11 Based on theoretical assumptions, we initiated aerobic training in five patients with mitochondrial dysfunction and evaluated their data to be able to select an adequate pediatric patient group that possibly benefits from exercise training. We evaluated the technical requirements, assessed factors in patient motivation, and screened for possible risk factors, like exercise intolerance, pain, muscle cramps, and mitochondrial dysfunction.
Materials and Methods
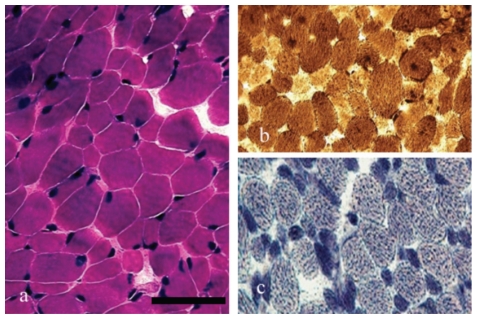
We evaluated a clinically homogenous group of five children, above the age of four years, with a congenital myopathy. These five children had normal intelligence, motor developmental delay, exercise intolerance, and fatigue according to medical history and detailed evaluation by the pediatric physical therapist using standard diagnostic protocols.12 All five patients previously underwent an open muscle biopsy and were diagnosed with a mitochondrial dysfunction and OXPHOS enzyme deficiency in muscle and/or fibroblasts.13 An example of muscle staining for patient #1 can be seen in Figure 1. None of the patients carried a mitochondrial DNA mutation.
Figure 1.
(a) Hematoxylin and eosin, (b) ATPase, and (c) NADH staining in the muscle biopsy of patient 1. A mild fiber type variation (type 1 fibers are smaller than type 2 fibers) can be observed. No complex deficiency was noted histochemically. (Bar equals 100 µm.)
In order to achieve adequate therapeutic compliance we evaluated patients with an IQ above 85. Criteria for exclusion were cardiac dysfunction, renal dysfunction, hepatic dysfunction, severe malnutrition, endocrine abnormalities, loss of vision or hearing, pyramidal involvement, ataxia, epilepsy, or severe extrapyramidal symptoms. No mitochondrial mutation carrier was selected in the pilot group to avoid the effects of possible gene-shifting. All patients included had exercise intolerance as the most significant presentation of their disease. Despite variable complex involvement, no significant clinical differences were noted, as can be seen in Table 1. The Movement Assessment Battery of Children (MABC) was used for evaluation of motor development,14,15 the Jamar dynamometer for assessment of grip force,16 a one-minute circuit test for observation of explosive activities, and a treadmill test was applied following the Bruce protocol.17 In addition, at each evaluation the VO2/kg was calculated.18 The study was performed in agreement with the declaration of Helsinki and was approved by CMO (2007/295).
Table 1. Parameters in mitochondrial myopathy.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| General | |||||
| Gender | F | M | M | M | M |
| Date of birth | 12.10.98 | 12.12.00 | 27.1.01 | 10.11.97 | 5.1.99 |
| Muscular | |||||
| Hypotonia | + | - | + | + | - |
| Muscle weakness | + | + | + | + | + |
| Muscle cramps | - | + | - | + | + |
| Exercise intolerance | + | + | + | + | + |
| Neurological | |||||
| Mental retardation | + | - | - | - | - |
| Epilepsy | - | - | - | - | - |
| Pyramidal involvement | - | - | - | - | - |
| Extrapyramidal symptoms | - | - | - | - | - |
| Visual loss | - | - | - | - | - |
| Hearing loss | - | - | - | - | - |
| Concentration problems | + | + | + | + | + |
| Multisystem | |||||
| Failure to thrive | - | + | - | - | - |
| Cardiac involvement | - | - | - | - | - |
| Renal involvement | - | - | - | - | - |
| Endocrine abnormalities | - | - | - | - | - |
| Chronic diarrhea | - | + | - | - | - |
| Food aversion | + | + | - | - | + |
| Biochemistry | |||||
| Complex deficiency | III + V | I + III | I | III | I |
| Alanin (blood) μmol/L | |||||
| (N<460) | 503 | 301–460 | 275–490 | 430–510 | 392–446 |
| Lactate (blood) mmol/L | |||||
| (N<2.1) | 0.9–2.7 | 1.1–3.8 | 1.1–1.8 | 1.4–1.9 | 1.3–3.2 |
| MDC score | 8 | 6 | 5 | 6 | 6 |
| Exercise tolorence | |||||
| MABC | 15 pc | 50 pc | 5 pc | 5–10 pc | 15 pc |
| Treadmill test 1* (Bruce17) | 8.55 min | 7 min | 11 min | 9.5 min | 9 min |
| Treadmill test 2** (Bruce17) | 8.44 min | 7.3 min | 10 min | 8 min | 9.1 min |
| VO2-max1+ | 45.6 | 51.05 | 48.18 | 49.92 | 51.2 |
| VO2-max2++ | 44.9 | 51.06 | 45.68 | 50.88 | 50.56 |
Exercise endurance according to Bruce17 at the time of first and last measurement;
VO2max at the first and last measurements.
Case Reports
Patient #1
Patient #1, a girl with general hypermobility, had normal development of early motor milestones. At the age of seven years, her MABC score was above the fifteenth percentile. Anamnestically, the patient reported that automatic use of motor skills was difficult for her. The girl's problems consisted of fatigue, pain in her legs, and rigid, clumsy movements especially in the morning after bed rest and at the start of activities. She possibly suffered from anxious and frightened behavior, more often coinciding with general malaise. Observing activities that rely on explosive muscle force, it was notable that after 50 seconds the girl's heart rate had risen to 206 beats per minute (bpm). At the first evaluation of grip force, the girl scored –1 SD for her age and gender. She underwent the Bruce protocol treadmill test for 8.55 minutes (<P10) with a HRmax of 197 (>P90) and a HR6 of 170 (>P90). The VO2/kg, derived from this HR6 was 45.6. The advised training focused on an aerobic training protocol. At the age of eight-and-a-half years, the previously mentioned tests were repeated. The grip force had increased marginally and was between –1 SD and –0 SD. The treadmill test was performed for 8.54 (<P10) minutes, with a HRmax of 200 (>P90) and a HR6 of 174 (>P90). The VO2/kg was 44.96. In conclusion, the intervention did not increase the capacities of this girl but stabilization occurred.
Patient #2
Patient #2 was a boy who developed all motor milestones with a slight delay. All age-related skills were acquired. His main problem was fatigue, causing exercise intolerance and many absences from school. This boy's history included failure to thrive and chronic diarrhea. Owing to feeding problems, he was fed with a stomach-tube, and carried his food around during the day. During activities, which rely on explosive muscle force, a heart rate of 174 bpm was reached within a minute. Several repetitions of an activity clearly provoked the boy's fatigue. At the age of six years and 10 months, the MABC score represented normal motor development. His grip force was –2 SD for both hands. The boy underwent the Bruce protocol treadmill test for 7.00 minutes (<P10) with a HRmax of 159 (<P10) and a HR6 of 136 (P10<X<P50). The VO2/kg was 51.04. After half a year on regular physical therapy (once a week for 30 minutes) and avoiding explosive activities, the subjective complaints of fatigue had increased; the boy's radius of action and school attendance had decreased. At the second evaluation, the grip force was –1 SD. The treadmill test was performed for 7.30 minutes (<P10), with a HRmax of 143 (<P10) and a HR6 of 134 (P10<X<P50). The VO2/kg was 51.36. In conclusion, the patient perceived fatigue in daily life but the objective evaluation showed stabilization in exercise tolerance.
Patient #3
Patient #3, a boy, presented with hypotonia from birth and delayed motor development, with similarly delayed acquirement of motor milestones. In addition, speech development was slow, and he presented behavioral problems in the autistic spectrum. The boy's subjective exercise tolerance was variable. The MABC score at the age of five-and-a-half years was below the P5. Static and dynamic balance was most problematic. The scores in the hand skill domain were between P5 and P15, while ball skills were within the normal rage (>P15). The observation of activities, which rely on explosive muscle force, showed a maximum performance level, however, with a heart rate of 206 bpm within a minute. Repetition of the activities after a recovery period provoked a less coordinated gait pattern and stumbling. Grip force was not measured, considering the boy's age. The treadmill test was performed for 11.12 minutes (P50), with a HRmax of 194 (P10<X<P50) and a HR6 of 154 (P50<X<P90). The VO2/kg was 48.18. An exercise related tremor as well as hypermetrical movements during goal-directed motor tasks could be observed. In this case, regular light movement activity was advised: 920 minutes swimming in warm water, 20 minutes walking, and 20 minutes light gymnastics), with further advice on daily activities (spread more over the day and avoiding explosive activities). At the second evaluation, at the age of six years, the treadmill test was performed for 10.55 minutes (P10<X<P50), with a HRmax of 206 (<P50) and a HR6 of 165 (P90). The VO2/kg was 45.68. Thus, although in this child the VO2/kg decreased, we noted some stabilization as measured with objective tests.
Patient #4
Since he was born, patient #4 had presented with axial and peripheral hypotonia and hypermobility, as well as exercise intolerance. Age-related skills developed more slowly than normal. However, there was a discrepancy between gross and fine motor skills, the latter developing normally, while problems arose in static and dynamic balance. Activities such as soccer, running, jumping, and tag could not be done for longer than a few minutes; the patient could not maintain enough speed in the activities and strength was lacking increasingly. In addition, since he was a toddler the patient had not been able to go to school for an entire day. There was, however, no lack of motivation for exercise and sports. This sometimes results in too much physical strain, causing pain and cramps in the legs, in addition to stomach aches, leading to less school attendance and more feeding problems. At the age of eight-and-a-half years, activities that rely on explosive muscle force were observed. Within 30 seconds the heart rate was raised to 189 bpm. The treadmill test was performed at the same age. The patient could perform the test for 9.57 minutes (<P10), with a HRmax of 189 (<P10) and a HR6 of 143 (P50). The VO2/kg was 49.92. A structured training schedule was started, with treadmill exercise twice a week at 60–70% of maximum exertion (calculated with Karvonen's formula). This schedule was supervised by a children's physical therapist and evaluated every six months. At the second evaluation, after half-a-year of training, the patient performed the test for 9.13 minutes (<P10), with a HRmax of 195 (P10<X<P50) and a HR6 of 165 (P90). The VO2/kg was 46.4. At the third evaluation, after one year of training, the patient performed the test for 8.41 minutes (<P10), with a HRmax of 191 (P10) and a HR6 of 158 (P90). The VO2/kg was 47.52. At the fourth evaluation, after one-and-half years of training, the patient performed the test for 8.00 minutes (<P10), with a HRmax of 172 (<P10) and a HR6 of 137 (P10<X<P50). The VO2/kg was 50.88. There obviously was less motivation owing to puberty. In this child the exercise tolerance was somewhat variable but did stabilize with age.
Patient #5
Patient #5 was a boy with delayed, clumsy motor development, complicated by behavioral features, such as impulsiveness and difficulties in managing concentration and emotions. In addition, the patient had movement characteristics pointing toward dyspraxia. He was short-statured, with possible dyschondroplastic proportions, and had problems with falling asleep. His fatigue had been a central problem since he was a toddler. Owing to this, he could not attend school for the entire day. He could perform all age-related skills, but only for a short amount of time. In explosive activities, he was exhausted after five minutes and complained of muscle aches and cramps shortly thereafter and the following evening. The radius of action was about 500 meters; after walking for this distance, he had pain in his legs and had to rest. At the age of eight-and-a-half years, the MABC score was above the 15th percentile and the grip force was –2 SD for age and gender. Explosive activity was observed during one minute, in which time the heart rate increased to 165 bpm. The treadmill test was performed for 9.00 minutes (<P10), with a HRmax of 175 (<P10) and a HR6 of 136 (P10<X<P50). The VO2/kg was 51.2. During this evaluation, the patient's saturation gradually decreased to 91%. After the first evaluation, an aerobic training scheme was advised, but in the following period this advice was not followed, and the patient received regular physical therapy (once a week for 30 minutes). At the second evaluation, done at the age of nine years, the grip force was still –2 SD. The treadmill test was performed again, this time for 9.10 minutes (<P10), with a HRmax of 180 (<P10) and a HR6 of 144 (P50). The VO2/kg was 49.04. At the age of 10 years, the patient was evaluated again. Anamnestically, the pain in his legs presented mainly a few hours after explosive activity, soccer in this boy's case. The grip force was –1 SD this time and the treadmill test results were 7.53 minutes (<P10), with a HRmax of 185 (<P10) and a HR6 of 139 (P50). The VO2/kg was 50.56. The patient stopped the test rather soon, having learned to regulate his energy use and not going to his maximum exertion. Thus, in this patient a stabilization of the exercise tolerance was also found.
Results
Patient #1 had normal age-related motor function. Exercise intolerance was based on subjective muscle fatigue. In between the evaluations, she received regular physical therapy (strength training once a week for 40 minutes), but no structural aerobic exercise could take place owing to variable compliance. After 18 months follow-up, however, no objective worsening could be detected, despite her increasing complaints of fatigue.
Patient #2 had severe limitations in exercise tolerance owing to muscle fatigue. The clinical situation was complicated by failure to thrive and severe feeding problems treated with tube feeding. In between the evaluations, he received regular physical therapy only (strength training once a week for 30 minutes). Regular (aerobic) exercise training was not successful, however; no measurable worsening occurred in the exercise tolerance after a follow-up period of 20 months, despite increasing complaints of fatigue.
Patient #3 had hypotonia and delayed motor development in addition to behavioral problems in the autistic spectrum. He did short episodes of regular exercise (light physical exercise three times a week for 20 minutes), and was advised to avoid explosive motor activity. Structured aerobic training was unsuccessful. His exercise tolerance remained acceptably stable in the follow-up period of 12 months.
Patient #4 had severe limitations in exercise tolerance. Throughout the years, global strength gradually increased with regular physical therapy (strength training once a week) and his exercise tolerance improved with the aerobic exercise regime (additional treadmill home training exercise twice a week for 20 minutes).
Patient #5 had limited exercise tolerance owing to muscle cramps. Repeated measurements were inconclusive for demonstrating objective exercise intolerance, despite his subjective chronic fatigue. The patient had regular physical therapy, avoided explosive activities, and reported fewer subjective complaints after 12 months. His exercise tolerance remained stable.
Discussion
This pilot study demonstrates a diverse group of young patients with exercise intolerance and mitochondrial dysfunction. One of the five patients showed an improvement after a period of structured aerobic training over the course of 18 months, demonstrating good compliance and motivation. In addition, we have to note that on lower frequency of regular physical exercise and avoiding explosive activities, none of the patients developed objective progression of their muscle disease. However, although aerobic exercise is a safe method of training for some patients with mitochondrial myopathy, others developed severe pain and cramps after physical activity. Compared to the successful training experience in adults, we noted several major complicating factors in applying specific training schedules in these children, including motivation, behavioral problems, and young age. It remains a question whether an improvement in exercise tolerance in children implies an increase in quality of life. One hypothesis is that if exercise tolerance improves in training, patients will not suffer as much from the disease in daily activities and can endure normal daily activities with fewer problems. It is striking that, contrary to comparable research in adults, the importance and success of physical training in children was not at all obvious. Children prefer to undergo explosive activities and more playful exercises, or receive a reward for completing a session, compared to having regular aerobic treadmill or bicycle training. The parents need to be highly motivated as well, to reach adequate compliance.
The Jamar dynamometer and the Bruce-protocol proved to be reliable instruments to evaluate grip force and cardiovascular components. However, it was hard to compare other exercise and fitness parameters because the patients sometimes had to stop because of pain or lack of motivation. Measuring lactate production and blood gas, and a glucose analysis might provide valuable additional markers in this patient group. Inadequate training can give insufficient results or be harmful to the patient because of excessive anaerobic activity. Therefore, it is important to ensure training takes place appropriately; the prevention of an anaerobic load definitely should be taken into account. The next step in studying the value of aerobic exercise in patients is to set up a prospective study considering these points of discussion. Important aspects for such research would be an individualized but still standardized training schedule, and a reliable method for monitoring compliance. Such a method could be regular training supervised by the same therapist, group training, or a video registration. The second most important question is to reach adequate motivation. Children appear to be less motivated regarding their general condition compared to adults. Fitness training in a possible game setting could be the solution for this problem. Our patients had no significant clinical progression of the disease during the period of physical therapy. This is an important finding in patients with mitochondrial disease, where disease progression is characteristic and inactivity increases significantly in childhood in most patients. Finally, learning more about the value of training and choosing the adequate form of exercise may provide physicians with a way to apply proper therapy in mitochondrial disease, which is one of the most complex, frequently progressive, and only partially treatable diseases.
References
- 1.Smeitink JA. Mitochondrial disorders: clinical presentation and diagnostic dilemmas. J Inherit Metab Dis. 2003;26:199–207. doi: 10.1023/a:1024489218004. [DOI] [PubMed] [Google Scholar]
- 2.Grondard C, Biondi O, Armand AS, et al. Regular exercise prolongs survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 2005;25:7615–22. doi: 10.1523/JNEUROSCI.1245-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orngreen MC, Olsen DB, Vissing J. Aerobic training in patients with myotonic dystrophy type 1. Ann Neurol. 2005;57:754–7. doi: 10.1002/ana.20460. [DOI] [PubMed] [Google Scholar]
- 4.Tollbäck A, Eriksson S, Wredenberg A, et al. Effects of high resistance training in patients with myotonic dystrophy. Scand J Rehabil Med. 1999;31:9–16. [PubMed] [Google Scholar]
- 5.Olsen DB, Ørngreen MC, Vissing J. Aerobic training improves exercise performance in facioscapulohumeral muscular dystrophy. Neurology. 2005;6464:1064–6.:E22. doi: 10.1212/01.WNL.0000150584.45055.27. [DOI] [PubMed] [Google Scholar]
- 6.van der Kooi EL, Lindeman E, Riphagen I. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2005;1 doi: 10.1002/14651858.CD003907.pub2.CD003907 [DOI] [PubMed] [Google Scholar]
- 7.Sveen ML, Jeppesen TD, Hauerslev S, et al. Endurance training: an effective and safe treatment for patients with LGMD2I. Neurology. 2007;68:59–61. doi: 10.1212/01.wnl.0000250358.32199.24. [DOI] [PubMed] [Google Scholar]
- 8.Cejudo P, Bautista J, Montemayor T, et al. Exercise training in mitochondrial myopathy: a randomized controlled trial. Muscle Nerve. 2005;32:342–50. doi: 10.1002/mus.20368. [DOI] [PubMed] [Google Scholar]
- 9.Trenell MI, Sue CM, Kemp GJ, et al. Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle Nerve. 2006;33:524–31. doi: 10.1002/mus.20484. [DOI] [PubMed] [Google Scholar]
- 10.Hood DA, Saleem A. Exercise-induced mitochondrial biogenesis in skeletal muscle. Nutr Metab Cardiovasc Dis. 2007;17:332–7. doi: 10.1016/j.numecd.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Adhihetty PJ, Taivassalo T, Haller RG, et al. The effect of training on the expression of mitochondrial biogenesis and apoptosisrelated proteins in skeletal muscle of patients with mtDNA defects. Am J Physiol Endocrinol Metab. 2007;293:E672–80. doi: 10.1152/ajpendo.00043.2007. [DOI] [PubMed] [Google Scholar]
- 12.Morava E, van den Heuvel L, Hol F, et al. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67:1823–6. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- 13.Janssen AJ, Trijbels FJ, Sengers RC, et al. Measurement of the energy-generating capacity of human muscle mitochondria: diagnostic procedure and application to human pathology. Clin Chem. 2006;52:860–71. doi: 10.1373/clinchem.2005.062414. [DOI] [PubMed] [Google Scholar]
- 14.Schoemaker MM, Smits-Engelsman BC, Jongmans MJ. Psychometric properties of the movement assessment battery for children - checklist as a screening instrument for children with a developmental co-ordination disorder. Br J Educ Psychol. 2003;73:425–41. doi: 10.1348/000709903322275911. [DOI] [PubMed] [Google Scholar]
- 15.Smits-Engelsman BC, Fiers MJ, Henderson SE, et al. Interrater reliability of the Movement Assessment Battery for Children. Phys Ther. 2008;88:286–94. doi: 10.2522/ptj.20070068. [DOI] [PubMed] [Google Scholar]
- 16.Trutschnigg B, Kilgour RD, Reinglas J, et al. Precision and reliability of strength (Jamar vs. Biodex handgrip) and body composition (dual-energy X-ray absorptiometry vs. bioimpedance analysis) measurements in advanced cancer patients. Appl Physiol Nutr Metab. 2008;33:1232–9. doi: 10.1139/H08-122. [DOI] [PubMed] [Google Scholar]
- 17.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–62. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 18.Koene S, Rodenburg R, Peters G, et al. Muscle pain, fatigue and hypothermia at night in association with mitochondrial dysfunction. J Ped Neurol. 2009;7:345–50. [Google Scholar]