Abstract
We report a 51-year old male with herpes simplex encephalitis (HSE) showing unusual progression and magnetic resonance (MR) findings. The initial neurological manifestation of intractable focal seizure with low-grade fever persisted for three days, and rapidly coma, myoclonic status, and respiratory failure with high-grade fever emerged thereafter. The polymerase chain reaction (PCR) result of cerebrospinal fluid (CSF) was positive for HSV-1 DNA. In the early stage, MR images (MRI) were normal. On subsequent MR diffusion-weighted (DW) and fluid-attenuated inversion recovery (FLAIR) images, high-intensity areas first appeared in the left frontal cortex, which was purely extra-temporal involvement, and extended into the basal ganglia, then the white matter, which are relatively spared in HSE. Antiviral therapy and immunosuppressive therapy did not suppress the progression of HSE, and finally severe cerebral edema developed into cerebral herniation, which required emergency decompressive craniectomy. Histological examination of a biopsy specimen of the white matter detected perivascular infiltration and destruction of basic structure, which confirmed non specific inflammatory change without obvious edema or demyelination. The present case shows both MR and pathological findings in the white matter in the acute stage of HSE.
Key words: herpes simplex encephalitis, unusual progression, white matter
Introduction
In herpes simplex encephalitis (HSE), the gray matter of the temporal and frontal lobes is predominantly destroyed by many foci of hemorrhage and necrosis.1 The lesions usually begin either unilaterally or bilaterally in the medial temporal cortex with bilateral spread along limbic pathways to the orbital frontal lobe and insular cortex. Parietal, occipital, brainstem, internal capsule, and cingulate gyrus involvement often occurs with further spread, but the basal ganglia and lobar white matter are relatively spared.2 Here, we report a case of HSE with basal ganglia and white matter involvement evident on MRI during the acute stage, which presented with unusual and severe progression. When there was no response to medical management of HSE, we had to use surgical decompression. Pathological findings of HSE during the acute stage indicated involvement of the white matter. To our knowledge, this is the first report which shows both MR and pathological findings in the white matter during the acute stage of HSE.
Case Report
A previously healthy 51-year old male presented with the sudden onset of generalized tonic clonic convulsion. After two hours he was admitted to our hospital. The convulsion had terminated spontaneously after thirty minutes. On admission, he had no headache, was not vomiting, and had a low-grade fever of 37.4°C. There were no obvious motor or sensory abnormalities, and his reflexes were equal bilaterally in both the upper and lower limbs. Meningeal signs were absent. Physical examination was unremarkable. A hemogram, urinalysis, and other chemical studies exhibited normal results. MRI on admission (three hours after onset) was normal on T1, T2, diffusion-weighted (DW) and fluid-attenuated inversion recovery (FLAIR) images.
One hour after admission, clonic convulsion in his right arm emerged without impaired consciousness. The focal seizure could not be controlled by diazepam, phenytoin and midazolam infusion. This refractory focal seizure persisted until day 3 without other neurological focal signs, and a second MRI on day 2 was normal on T1, T2, DW, FLAIR, and gadolinium-enhanced T1 images. Electroencephalography (EEG) on day 2 showed left parietal focal slow θ activity without epileptiform discharge. Titers for common viruses (HIVs, Epstein-Barr, cytomegalovirus, herpes zoster, and herpes simplex), bacteria, and toxoplasmosis were negative. Tests for autoantibodies (antinuclear, anti-deoxyribonucleic acid, and anti-ribonucleoprotein antibodies), lupus erythematosus factor, syphilis serology, p-and cANCA were all negative. The patient's serum angiotensin-converting enzyme level was normal. Cerebrospinal fluid (CSF) examination on day 3 showed no abnormality, and no herpes simplex virus (HSV) DNA was demonstrated by polymerase chain reaction (PCR). Cytology and culture of the CSF for bacteria, tuberculous bacillus, and fungi were negative.
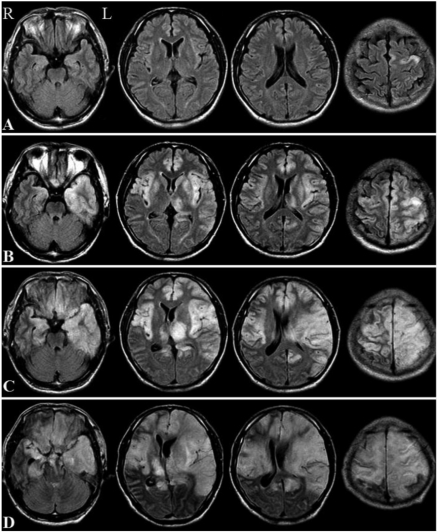
On day 4, he became unconsciousness and rapidly comatose. Axial DW and FLAIR images on day 4 revealed high-intensity areas in the left frontal cortex (Figure 1A). The clonic seizure in the right arm changed to myoclonic status in the mouth, diaphragm, and right arm. Additionally, respiratory failure necessitated artificial ventilation under anesthesia. Continuous propofol infusion did not suppress the myoclonic status migrating around the four limbs, from the right arm to the right leg, the right leg to the left arm, and the left arm to the left leg, every other day. After the myoclonus in the left leg disappeared, myoclonic status persisted in the diaphragm alone. High-dose barbiturate therapy instead of propofol did not resolve this intractable myoclonic status in the diaphragm. EEG on day 7 revealed a burst-suppression pattern observed during barbiturate coma. Axial DW and FLAIR images on day 9 revealed high-intensity areas; bilaterally widespread in frontoparietal cortex, medial temporal lobe, insula, and putamen with left-sided predominance, additionally in the frontal orbital lobes bilaterally, in the left caudate nucleus, and the thalamus (Figure 1B). On lumbar puncture, CSF pressure was 400 mm H2O, cell count was 16/mm3 (56% mononuclear cells), CSF protein concentration and glucose were normal at 27 and 98 mg/dL (blood glucose 115 mg/dL), respectively, and the PCR result was positive for HSV-1 DNA. A diagnosis of HSE was made, and acyclovir was administered on day 9, but did not suppress the emergence of the diaphragmatic myoclonic status. Fever increased to high-grade, and coma persisted, so artificial ventilation did not require sedation. Axial DW and FLAIR images on day 16, compared with the previous MRI, demonstrated more widespread lesions indicating frontoparietal involvement, progression of left thalamic high-intensity areas, and swelling sufficient to cause midline shift to the right and distortion of the lateral ventricle (Figure 1C).
Figure 1.
Progressive white matter change in herpes simplex encephalitis. Axial fluid-attenuated inversion recovery (FLAIR) images: (A) on day 4, showing high signal intensity in the left frontal cortex, and (B) on day 9, showing high signal intensity in the frontoparietal cortex bilaterally, medial temporal lobe, insula, and putamen with left-sided predominance, and in the frontal orbital lobe bilaterally, left caudate nucleus, and thalamus. The high-intensity areas in the gray matter shown on day 9 increased: (C) on day 16, except in the basal ganglia (caudate nucleus, putamen), especially in the left thalamus, which caused midline shift to the right, and (D) on day 23, extending to the white matter, causing an increased midline shift to the right and cerebral edema.
Cerebral edema worsened, and adjunctive immunosuppressive (steroid pulse therapy and intravenous immunoglobulin) and high-dose anti-edematous therapies were added. CSF examination on day 20 revealed a 37/m3 cell count (65% mononuclear cells) and protein concentration of 340 mg/dL. Axial DW and FLAIR images on day 23 showed high-intensity areas in the white matter adjacent to the affected gray matter, and cerebral edema worsened catastrophically (Figure 1D). One hour after the MRI was performed (on day 23), sudden hypotension and mydriasis emerged, which suggested cerebral herniation. Emergency decompressive craniectomy was required. Surgical decompression resolved the cerebral herniation, and rescued our patient from death.
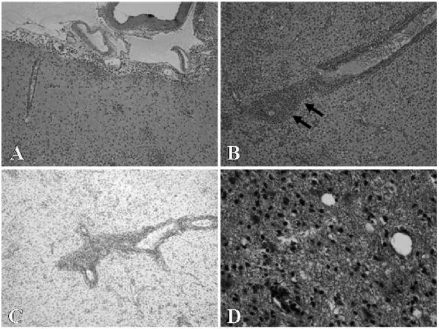
Despite this, coma, diaphragmatic myoclonic status, and high-grade fever persisted without functional recovery. During the surgery, a brain biopsy of the left frontal white matter was carried out. Histological examination detected perivascular infiltration of lymphocytes and destruction of basic structure (Figure 2A, B). Viral inclusion bodies were not detected. Some of the neurons were immunohistochemically positive for HSV-1 antigen. Obvious edema, demyelination and axonal reduction were not seen (Figure 2C, D).
Figure 2.
Microscopic findings of the biopsied tissue in the left frontal lobe (A, B, C, D). (A) and (B, arrows): perivascular infiltration of lymphocytes and destruction of basic structure, which confirmed non specific inflammatory change, hematoxylin-eosin stain, × 10 and ×40, respectively. (C): no obvious demyelination, Kluver-Barrera stain, ×10. (D): no definite axonal reduction or swelling, Bodian stain, ×10.
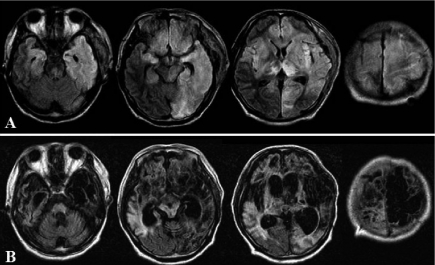
Subsequently, axial DW and FLAIR images on day 30 displayed the extensive white matter lesions extending into the lower midbrain and pons despite surgical decompression (Figure 3A). Resolution of high-grade fever coincided with amelioration of diaphragmatic myoclonic status, while coma and decorticate states persisted. At four months after onset, this patient remained in a comatose and decerebrate state under artificial ventilation. The patient with severe sequelae of HSE was transferred to another hospital. Two years after onset, an axial FLAIR image showed cerebral atrophy, severe ventricular dilatation and multiple cystic changes (3B).
Figure 3.
Axial FLAIR images: (A) after surgical decompression showing high signal intensity in the frontotemporoparietal lobes bilaterally, left occipital lobe, basal ganglia bilaterally, left midbrain, and pons, and (B) two years after onset showing cerebral atrophy, severe ventricular dilatation, and cystic change in the frontal lobes, with left-sided predominancy, and in the left thalamus.
Discussion
Typically, MRI abnormalities in HSE are seen in the temporal and frontal lobes, and the basal ganglia and white matter are relatively spared. Early diagnosis of HSE is difficult because of the finding of a normal MRI or a purely extra-temporal involvement. Clinical diagnosis is suggested by the febrile patient with focal neurologic signs, and the most important factor in the diagnosis of HSE is the consideration of this diagnostic possibility.3 Hence, if HSE is suspected, treatment should be started before a definitive diagnosis is made. Early administration of antiviral therapy is the only parameter that can be modified to improve the prognosis of the patient with HSE.4
In our case, MRI and CSF examinations were normal until day 3, and the initial abnormal DW and FLAIR images on day 4 revealed purely extra-temporal involvement, which delayed antiviral therapy. MR findings in this case have two characteristic points. First, the initial lesion was seen in the left frontal cortex, and extended to have an unusual distribution into the basal ganglia, i.e., from purely extra-temporal involvement, which are usually spared in HSE. Additionally, MRI on days 9 and 16 showed thalamic involvement. In infectious encephalitis, bilateral involvement of the basal ganglia and thalamus is characteristic of flavivirus encephalitis, like Japanese encephalitis.5 Therefore this case suggested that MR findings, which displayed purely extra-temporal involvement in the early stage and subsequently basal ganglia and thalamic involvement, did not rule out HSE conclusively.
Second, MRI revealed the extensive white matter involvement adjacent to the affected gray matter, in parallel with monophasic clinical worsening. This white matter involvement appeared on day 23, during the acute stage. In this report, acute stage means the stage of the illness where the symptoms are the most severe before recovery. This acute white matter involvement revealed high-intensity areas on DW and FLAIR images, and especially markedly high intensities on DW images. Several reports have described white matter lesions that appeared several weeks or months after the onset of illness,2,6–12 not during the acute stage like the present case but during the subacute or chronic stage. The delayed white matter lesions appeared with or without deterioration of conditions after the discontinuation of the acute stage, which developed independently of the affected gray matter lesions.
There have been no reports regarding intensities on DW images in delayed white matter involvement. The mechanism underlying the delayed white matter lesions has not been elucidated, and an immune-mediated complication, such as edema or demyelination, is suspected.2 Besides, another two mechanisms are possible: the occurrence of late-onset symptoms of the initial viral infection, and recurrence of viral replication (as a result of incomplete treatment of the initial HSE or by selection of clones of an acyclovir-resistant virus).13 One autopsy study of HSE with delayed white matter involvement showed diffuse edema, without demyelination or viral inclusion.11 Another biopsy study of HSE with delayed white matter involvement revealed cell-mediated demyelination without reactivation of the HSV infection.14 In the present case, the acute white matter involvement appeared adjacent to the affected gray matter with high intensities on DW images. The speculated mechanism of the acute white matter involvement was direct invasion of HSV different from that with the delayed change.2 On the other hand, it was postulated at the autopsy that focal perivascular myelin destruction in the white matter, without recovery, was dependent on the immune response.15 That is to say, the speculated mechanism underlying either the delayed or acute white matter involvement of HSE has been controversial. Microscopic findings of the biopsied tissue obtained in acute white matter involvement demonstrated perivascular infiltration and destruction of basal structure, which confirmed non specific inflammatory changes.
Viral inclusion bodies were not detected. A brain biopsy was carried out on day 23, after acyclovir administration, which demonstrated some of the neurons to be immunohistochemically positive for HSV-1 antigen. Thus we could not conclude that the acute white matter involvement was not a result of direct invasion of HSV. Obvious edema, demyelination, axonal reduction, and swelling, which could suggest the mechanism underlying the white matter involvement of HSE during the acute stage, were not seen. There was a discrepancy between nonspecific pathological findings and the unusual clinical course of the present HSE, including MR findings. Hence, it is still unclear from the histology whether the acute white matter involvement is because of direct invasion of HSE, like the affected gray matter, or secondary inflammatory change or both, but combination therapy with antiviral therapy and immunosuppressive therapy did not control the condition. Although the white matter involvement had not extended into the medulla oblongata on MRI, prolonged respiratory failure necessitated artificial ventilation. Therefore, it was possible that the respiratory centers located in the medulla oblongata were injured despite there being no corresponding involvement on MRI.
The present case resulted in neurologic deterioration because of cerebral edema and herniation despite antiviral, immunosuppressive, and anti-edematous therapies. Emergency decompressive craniectomy prevented death as a result of cerebral herniation, but lead to severe neurological sequelae. Matthew et al. (reported by Adamo and Deshaies, 2008) discussed cases of infectious encephalitis including HSE, which resulted in excellent recovery after surgical decompression.16 The reason that surgical decompression did not lead to improvement in the present case may be attributed to the devastating white matter involvement, sufficient to extend into the brainstem and cause injury to the respiratory center even after surgical decompression. If extensive progression into the white matter could have been prevented, the sequelae would not have been as severe as in the present case. Therefore, further investigation of the mechanism underlying the white matter involvement of HSE during the acute stage is needed.
References
- 1.Wasay M, Mekan SF, Khelaeni B, et al. Extra temporal involvement in herpes simplex encephalitis. Eur J Neurol. 2005;12:475–9. doi: 10.1111/j.1468-1331.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 2.Mitsufuji N, Ikuta H. Asymptomatic self-limiting white matter lesions in the chronic phase of herpes simplex encephalitis. Brain Dev. 2002;24:300–3. doi: 10.1016/s0387-7604(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 3.Gümü H, Kumanda S, Per H, et al. Unusual presentation of herpes simplex virus encephalitis: bilateral thalamic involvement and normal imaging of early stage of the disease. Am J Emerg Med. 2007;25:87–9. doi: 10.1016/j.ajem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Jabkowski M, Kolasa P, Szubert W, et al. Herpes simplex encephalitis: a case report. Med Sci Monit. 2004;10:CS41–5. [PubMed] [Google Scholar]
- 5.Phowthongkum P, Phantumchinda K, Jutivorakool K, et al. Basal ganglia and brainstem encephalitis, optic neuritis, and radiculomyelitis in Epstein-Barr virus infection. J Infect. 2007;54:e141–4. doi: 10.1016/j.jinf.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Tamura T, Morikawa A, Kikuchi K. Diffuse white matter lesions associated with herpes simplex encephalitis as observed on magnetic resonance imaging. Brain Dev. 1996;18:150–2. doi: 10.1016/0387-7604(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 7.Takanashi J, Sugita K, Ishii M, et al. Longitudinal MR imaging and proton MR spectroscopy in herpes simplex encephalitis. J Neurol Sci. 1997;149:99–102. doi: 10.1016/s0022-510x(97)05401-4. [DOI] [PubMed] [Google Scholar]
- 8.Gaviani P, Leone M, Mula M, et al. Progression of MRI abnormalities in herpes simplex encephalitis despite clinical improvement: natural history or disease progression. Neurol Sci. 2004;25:104–7. doi: 10.1007/s10072-004-0240-5. [DOI] [PubMed] [Google Scholar]
- 9.De Tige X, De Laet C, Mazoin N, et al. Post-infectious immune-mediated ence-phalitis after pediatric herpes simplex encephalitis. Brain Dev. 2005;27:304–7. doi: 10.1016/j.braindev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka H, Tanizawa E, Ueno S. Herpes simplex virus encephalitis with progressive severe white-matter lesions. Eur J Neurol. 2007;14:e18–9. doi: 10.1111/j.1468-1331.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 11.Barthez-Carpentier MA, Rozenberg F, Dussaix E, et al. Relapse of herpes simplex encephalitis. J Child Neurol. 1995;10:363–7. doi: 10.1177/088307389501000504. [DOI] [PubMed] [Google Scholar]
- 12.Ueda N, Miyasaki H, Kuroiwa Y. Diffuse white matter lesions in a case of herpes simplex encephalitis. J Neurol. 2003;250:867–8. doi: 10.1007/s00415-003-1093-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamada S, Kameyama T, Nagaya S, et al. Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J Neurol Neurosurg Psychiatry. 2003;74:262–4. doi: 10.1136/jnnp.74.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig H, Rabinowitz SG, Day E, et al. Post-infectious encephalomyelitis after successful treatment of herpes simplex encephalitis with adenine arabinoside. N England J Med. 1979;19:1089–93. doi: 10.1056/NEJM197905103001906. [DOI] [PubMed] [Google Scholar]
- 15.Hamano K, Robain O, Gray F, et al. Focal perivascular alterations of white matter in herpes simplex encephalitis. A histological and immunocytochemical study. Jpn J Psychiatr Neurol. 1986;40:209–19. doi: 10.1111/j.1440-1819.1986.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 16.Adamo MA, Deshaies EM. Emergency decompressive craniectomy for fulminating infectious encephalitis. J Neurosurg. 2008;108:174–6. doi: 10.3171/JNS/2008/108/01/0174. [DOI] [PubMed] [Google Scholar]