Abstract
Glioblastoma multiforme, a neoplasm with variable histological and biological features, is characterized by diverse imaging features, including highly heterogeneous enhancement. This reflects variable disruption of the blood brain barrier and inherent differences in the vascularity of the tumor. Experience in treating malignant glioma with antiangiogenic drugs is growing, and the most commonly used, in combination with irinotecan or other cytotoxic agents as salvage therapy, is bevacizumab, a monoclonal antibody against vascular endothelial growth factor.
A 42-year-old, right-handed person with recurrent glioblastoma multiforme presented with two synchronous foci of recurrent disease in follow-up: one area with enhancement and another one nonenhancing and infiltrative, which responded differently to treatment with bevacizumab and irinotecan. Our example demonstrates the heterogeneous nature of glioblastoma multiforme and is proof of principle for antiangiogenic treatment in selected enhancing, presumably angiogenic forms of glioblastoma multiforme. Antiangiogenic treatment may be ineffective in more infiltrative, biologically different lesions.
Key words: glioblastomas, antiangiogenic biochemotherapy, imaging, bevacizumab, irinotecan.
Introduction
Glioblastoma multiforme (GBM) is a neoplasm with variable features not only histologically but also biologically. GBM is characterized by diverse imaging features, including highly heterogeneous enhancement, reflecting variable disruption of the blood brain barrier (BBB) and inherent differences in the vascularity of the tumor. There is growing experience with the treatment of malignant glioma with antiangiogenic drugs. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), has been used most commonly in combination with irinotecan or other cytotoxic agents as salvage therapy.
Our case report vividly demonstrates disparate responses to combined biochemotherapy in the same patient with recurrent GBM. Two synchronous foci of recurrent disease, one area with enhancement and another one nonenhancing and infiltrative, responded differently to treatment with bevacizumab and irinotecan. This case illustrates the principle of antiangiogenesis mechanisms in the treatment of enhancing GBM, and provides a dramatic example of the heterogeneity of the tumor biology and its different responses to treatment.
Case Report
A 42-year-old, right-handed, previously healthy man presented with one week of morning headaches, general weakness, vertigo, nausea, and vomiting. His neurological examination revealed a mild pronator drift of the right upper limb, without other abnormalities. Brain magnetic resonance imaging (MRI) showed an enhancing right frontotemporal tumor (Figure 1). The patient underwent right temporal craniotomy for gross total removal of the tumor. The pathology was typical for GBM, with nuclear atypia, multiple mitotic divisions, vascular endothelial proliferation, and focal tumor necrosis.
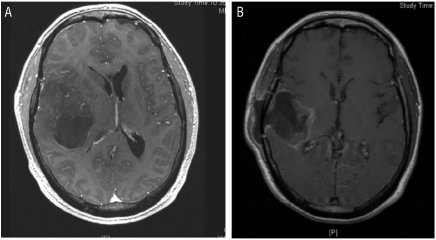
Figure 1.
(A) Initial preoperative magnetic resonance imaging (MRI) scan: axial T1-weighted sequence with gadolinium demonstrates a right temporal, centrally hypointense lesion with mass effect and midline shift. (B) Axial T1-weighted sequence MRI with gadolinium following the second operation, postradiation, demonstrates a cystic cavity with postsurgical changes, resolution of mass effect, and shift.
The patient began standard chemoradiation (conformal external beam radiation, 200 cGy per fraction, with concomitant temozolomide 75 mg/m2 per day). Two weeks into his treatment, he was admitted to the emergency room with headache, vertigo, and a new left homonymous hemianopsia. A brain computerized tomography (CT) scan revealed early recurrence of the tumor with significant vasogenic edema and midline shift. The patient underwent a repeat right temporal craniotomy with gross total removal of the recurrent tumor and implantation of Gliadel wafers. He was treated with intravenous antibiotics for postoperative cerebrospinal fluid leak and meningitis. After resolution of postoperative complications he completed hypofractionated radiation with concurrent temozolomide, and went on to receive 16 adjuvant cycles of monthly temozolomide.
He enjoyed good quality of life throughout this period, with his only symptoms being hemianopsia and mild impulsive behavior. Brain MRI performed after the sixteenth cycle of adjuvant chemotherapy showed recurrent, enhancing, multifocal disease involving the right frontoparietal lobes, and nonenhancing involvement of the contralateral, left medial temporal lobe (Figures 2A and C). These changes were predated by progressive cognitive decline, behavioral changes, and gait instability. The patient was started on a salvage protocol of intravenous irinotecan and bevacizumab, 125 mg/m2 and 5 mg/kg, respectively, every two weeks. After four treatments (over two months) his MRI scan showed dramatic, almost complete resolution of the enhancing component of his disease (Figure 2B), but further progression of the ill-defined, nonenhancing, infiltrative, left medial temporal tumor (Figure 2D). Clinically, he became progressively confused, displayed dressing apraxia, increased emotional lability, impulsiveness, and loss of insight. A fifth treatment of biochemotherapy was given with the addition of carboplatin AUC 5, but the patient continued to decline clinically, and he was transferred to hospice, where he died of progressive disease.
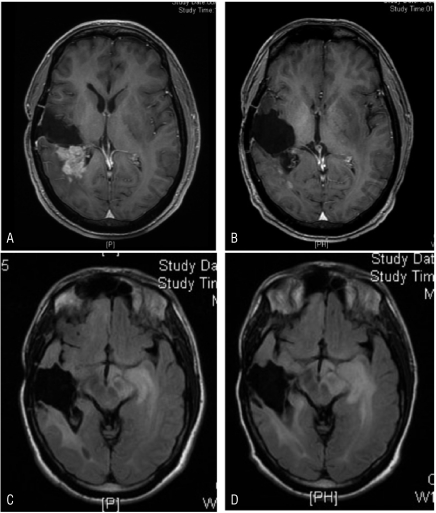
Figure 2.
(A) T1-gadolinium FSPGR magnetic resonance imaging (MRI) scan, revealing a recurrent right frontoparietal focus before treatments with irinotecan and bevacizumab. (B) T1-gadolinium FSPGR MRI, post four treatments with irinotecan and bevacizumab demonstrating almost complete resolution of the enhancing disease. (C) T2 Flair MRI sequence revealing the medial left temporal nonenhancing focus pre irinotecan and bevacizumab treatment. (D) T2 Flair MRI sequence post irinotecan and bevacizumab treatment demonstrating progression of the ill-defined, nonenhancing, infiltrative left medial temporal lobe disease (despite dramatic response of the right-sided disease as depicted in B).
Discussion
The natural history of recurrent GBM is variable, as the tumor is biologically heterogeneous. We are only beginning to understand the molecular genetic components and distinct mechanisms that may determine a given neoplasm's behavior and response to various treatments. Recent studies have shown the importance of a variety of molecular characteristics, including methylation of the methylguanine methyltransferase enzyme (MGMT) promoter,1 epidermal growth factor receptor (EGFR) vIII and phosphatase and tensin homolog (PTEN) status,2 and likely, the status of VEGF and VEGF receptor3 in relation to tumor growth and treatment responses.
Chemotherapy has an important salvage role in the treatment of recurrent GBM.4 Targeting of tumor angiogenesis is a recently developed method for tumor control and stabilization, which shows promise in the treatment of several tumor types including malignant glioma. Biochemotherapy with bevacizumab and cytotoxic agents (including irinotecan) has been shown to improve survival significantly in patients with non-small-cell lung cancer and metastatic colorectal cancer.5,6 Use of antiagiogenic agents with chemotherapy has been shown to be effective in recurrent GBM as well.7 The initial report by Stark-Vance using combined bevacizumab and irinotecan in the treatment of recurrent GBM demonstrated an unprecedented high response rate.8 These findings were supported in phase II trials of bevacizumab and irinotecan in recurrent malignant glioma,9,10 which led to FDA approval of bebacizumab for second-line treatment of recurrent or progressive GBM in May 2009. A phase II randomized, noncomparative clinical trial of bevacizumab alone or in combination with irinotecan in a group of 167 patients revealed six months of progression free survival (PFS) of 42.6% and 50.3% in the bevacizumab arm alone and in combination with irinotecan, respectively, with 9.2 months, with bevacizumab alone and 8.7 months median survival with combined therapy.11 Bokstein, et al.12 reported a series of 20 patients with recurrent malignant glioma who were treated with bevacizumab at 5 mg/kg and irinotecan at 125 mg/m2. These patients treated with a lower dose of bevacizumab showed a six-month PFS and overall survival (OS) of 25% and 55%, respectively, with fewer thromboembolic complications.12
Studies of imaging in combined biochemotherapy have shown impressive radiographic responses. The radiological differences in tumor enhancement pattern may be caused by several factors, among them tumor necrosis, tumor vascularity, and severity of damage to the BBB.13 Pope, et al.14 assessed the early imaging effects of bevacizumab coupled with etoposide, carboplatin, or irinotecan in a cohort of 14 patients with recurrent malignant glioma. An imaging response rate of 50% was seen. Several patients were found to have responses in areas of necrotic-appearing tumor, whereas solid areas of their tumor continued to grow. An explanation for this phenomenon might be found in the differences in requirements of tumor endothelium for VEGF receptor stimulation in necrotic versus solid areas of tumor.14
Our case demonstrates two heterogeneous, synchronous GBM lesions in a patient with recurrence; one focus displayed dense contrast enhancement while the contralateral lesion was ill-defined and nonenhancing. Following biochemotherapy with irinotecan and bevacizumab, the enhancing tumor showed dramatic resolution while the infiltrative, nonenhancing lesion progressed and ultimately lead to the demise of the patient. This example demonstrates the heterogeneous nature of GBM and is proof of principle for antiangiogenic treatment in selected enhancing, presumably angiogenic forms of GBM, although antiangiogenic treatment may be ineffective in more infiltrative, biologically different lesions.
References
- 1.Hegi ME, Diserens AC, Gloria T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 2.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–24. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 3.Hicklin DJ, Ellis LM. Role of vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 4.Hou LC, Anand V, Hsu AR, et al. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20:1–9. doi: 10.3171/foc.2006.20.4.2. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Giantonio BJ. Bevacizumab in the treatment of metastatic colorectal cancer (mRC) in second-and third-line settings. Semin Oncol. 2006;33:S15–8. doi: 10.1053/j.seminoncol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Tuettenberg J, Grobholz R, Korn T, et al. Continiuous low dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme. J Cancer Res Clin Oncol. 2005;131:31–40. doi: 10.1007/s00432-004-0620-5. [DOI] [PubMed] [Google Scholar]
- 8.Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Neurooncology. 2005;7:369–369. [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 10.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 11.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 12.Bokstein F, Shpigel S, Blumenthal DT. Treatment of recurrent high-grade glial tumors with bevacizumab and irinotecan. Cancer. 2008;112:2267–73. doi: 10.1002/cncr.23401. [DOI] [PubMed] [Google Scholar]
- 13.Atlas SW, Lavi E. Intra-axial tumor. In: Atlas SW, editor. Magnetic resonance imaging of the brain and spine. 2nd ed. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 14.Pope WB, Lai A, Nghiemphu P. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–60. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]