While the benefit of sleep after learning in offline consolidation is established [1], a role for sleep before learning in promoting initial memory formation remains largely uncharacterized. Existing theoretical frameworks speculate that accrued time awake, associated with ongoing experience, decreases learning capacity, while specific NREM oscillations support restoration of learning ability [1, 2]. Despite these model predictions, it remains untested whether episodic learning capacity remains stable across the day, or is progressively compromised by continued time awake. Furthermore, it is similarly unclear whether the presence, rather than the detrimental absence, of sleep restores efficient learning ability, and if so, what aspect(s) of sleep physiology support such reinstatement [3, 4]. Here we test the hypothesis of a learning interaction between wake and sleep, such that episodic encoding capacity deteriorates across a daytime waking interval, but that sleep and associated NREM spindle and slow-wave oscillations restore efficient learning ability.
Forty-four participants (27 females, 20.9±0.3 [mean±s.e.m.] years) performed two learning sessions on an episodic memory-encoding task at 12:00 and 18:00, each followed by immediate testing. After the first learning session, participants were assigned to either a No-Nap group (n=24) that remained awake from 12:00–18:00, or Nap group (n=20) that obtained a polysomnography monitored 100-minute sleep opportunity (14:00–15:40). During the time awake, in both groups, standard daily activities were conducted, allowing for an ecological examination of the impact of waking as assumed by model heuristics [1, 2]. To determine the specificity of effects on episodic learning, participants also performed a procedural motor-skill learning task at both sessions (Supplemental Materials).
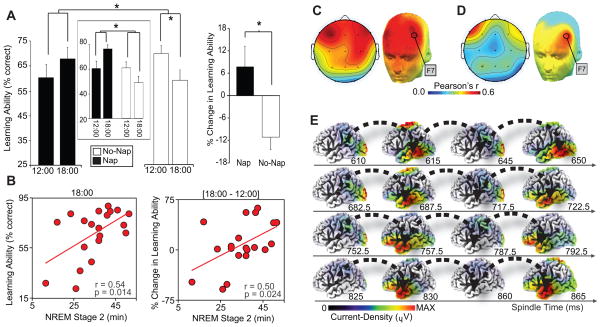
For episodic learning, a significant Group [No-Nap/Nap] by Session [12:00/18:00] interaction was identified (P=0.045; Figure 1A). Specifically, learning ability in the No-Nap group deteriorated significantly from 12:00 to 18:00 (P=0.045), yet demonstrated a non-significant increase in the Nap group (P=0.314). The change in learning ability within each subject was expressly characterized as a difference-score, subtracting performance at 18:00 from that at 12:00, which was also significantly different between groups (P=0.045; Figure 1A). These effects were specific to episodic learning, with no between-session change expressed in procedural motor-skill learning, or in measures of alertness or reaction time (Supplementary Materials).
Figure 1.
(A) Episodic learning ability (% face-names encoded) in the Nap and Non-Nap group at 12:00 and 18:00 (left), and the change in episodic learning ability between sessions [18:00–12:00] (right). Episodic learning ability in a subset of Nap and No-Nap subjects (n=10 per group), matched on initial 12:00 performance, is presented in the center box. Comparisons (line across bars) in both plots reflect significance* at: P<0.05. Error bars represent s.e.m. (B) Within the Nap group, correlations with stage-2 NREM sleep and episodic learning ability at 12:00 (left-panel), 18:00 (middle-panel), and the change in learning ability between session [18:00–12:00] (right-panel). (C) Topographic correlations (color-bar indicates Pearson’s correlation strength) in the Nap-group between fast sleep spindles and episodic learning ability at 18:00 (post-nap), significant in derivations F3, F4, Fz, F7, F8, Fp1 and Fp2 over PFC, maximal at F7 (r=0.536, P=0.018). (D) Of these derivations, the change in episodic learning ability [18:00–12:00], significantly and conjointly correlated with fast-spindles at derivation F7 over left PFC (r=0.535, P=0.018). (E) sLORETA source time-series of fast sleep spindles identified by onset at F7, demonstrating a current-density loop recurring in left temporal lobe, proceeding the peak of the spindle. Spindle-time (ms) is presented below each cortical surface, relative to onset. A movie of this source time-series is provided in Supplemental Movie S1.
Two additional analyses demonstrated that the main interaction effect was not predicated on numerical differences in initial learning level in each group. First, learning ability at 12:00 showed no significant predictive relationship with subsequent learning ability at 18:00, either in the Nap group (r=−0.29, P=0.21) or No-Nap (r=0.20, P=0.361) groups, or both combined (r=−0.12, P=0.446). Second, when matching a subset of participants from the Nap and No-Nap groups, based on initial 12:00 performance (n=10 per group, center box of Figure 1A, P=0.98), the group by session interaction remained significant (P=0.030). That the episodic learning deficit at 18:00 in the No-Nap group was not simply the result of having performed a first learning session (12:00) was demonstrated in an additional Control group that performed two learning sessions back-to-back at 18:00, expressing no change in learning ability (Supplemental Materials).
Within the Nap-group, episodic learning ability after the nap (18:00) correlated positively with prior stage-2 NREM sleep (r=0.54, P=0.014; Figure 1B). Furthermore, the change in episodic learning ability ([18:00–12:00]; difference-score) also correlated with intervening stage-2 NREM sleep (r=0.50, P=0.024; Figure 1B). No significant association was identified between stage-2 NREM and initial episodic learning ability at 12:00 (r=−0.29, P=0.214), indicating that stage-2 NREM was not simply a proxy for general learning ability. No other sleep parameters correlated with learning (Supplemental Materials).
In addition to sleep-stages, and consistent with their proposed role in hippocampal-neocortical memory processing [1], fast sleep spindles (13.5–15Hz) over bilateral prefrontal cortex (PFC) correlated positively with episodic learning ability after the nap at 18:00 (Figure 1C). Moreover, the change in episodic learning ability ([18:00–12:00]) additionally correlated with fast-spindles at derivation F7 over left lateral PFC (Figure 1D). Only associations with F7 fast spindles achieved significance after correcting for multiple comparisons (Supplemental Materials). Neither slow-spindles (12–13.5Hz) nor properties of NREM slow-waves correlated with learning (Supplemental Materials). Finally, and motivated by the role of the lateral prefrontal cortex and medial temporal lobe structures in episodic memory [5], we conducted source current-density analysis across the EEG time-series, time-locked to the fast spindle onset at F7. This analysis revealed an oscillation of activity throughout these fast spindle events looping through the left temporal lobe, as well as superior parietal and occipital cortex (Figure 1E, Supplemental Movie S1).
Though hippocampal activity was not directly measured in the current study, our findings may parsimoniously be accounted for within a hippocampal-neocortical framework of memory processing, predicting decreased episodic learning capacity with continued waking experience [1]; a potential limitation of sparse hippocampal representational coding [6]. Consequently, NREM sleep spindles, which co-occur with hippocampal sharp-wave ripples [1, 7], and have been associated with hippocampal activation in human neuroimaging investigations [8], are proposed to support a proactive shift from hippocampal- to increasing cortical-dependence of previously encoded representations [1], thereby restoring post-sleep episodic encoding ability. Both predictions of wake deterioration and sleep restoration conform to the changes in episodic learning observed in the current study, including the spindle relationships and their associated current-source in temporal lobe. Nevertheless, methods that localize functional activity will be necessary to validate this hypothesized hippocampal account.
Our findings are less congruent with tenants of the slow-wave synaptic homeostasis model [2], although it should be noted that both models are not mutually exclusive. While a deficit in episodic learning was identified with accumulating time awake, which conforms to predictions of the synaptic homeostasis model, the lack of difference in procedural learning is perhaps surprising within this account. However, more time awake may be required before observable detriments emerge, as well as more time spent in slow-wave sleep for restoration. The absence of an association between episodic learning restoration and properties of NREM slow-waves also does not correspond to predictions of this framework [2]. That the hippocampus may not experience canonical slow oscillations [9] could partially explain this latter finding.
These restorative benefits of stage-2 NREM and spindles on associative-memory encoding partially differ to recent findings reporting that slow-wave sleep deprivation in elderly participants impairs item-memory encoding [3]. Three factors may explain these sleep-stage differences, including the sleep paradigm used (nap versus deprivation), age of participants (young versus older adults), and the type of learning (item- versus associative-encoding). Nevertheless, both studies congruently implicate NREM sleep properties in episodic encoding restoration.
More generally, considering their ontogenetic relationship [10], our results offer insights into the potential association between sleep-spindle oscillations and episodic learning ability across the lifespan, both in promoting such learning capacity during formative years, and in compromised episodic encoding ability in aging and disease [10].
Supplementary Material
Acknowledgments
We gratefully acknowledge help from the study research assistants, and Giulio Tononi and colleagues for graciously providing scripts for NREM oscillation analyses. This work was supported by awards R01-AG031164 (MPW) and F32-AG039170 (BAM) from the National Institutes of Health.
References
- 1.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 2.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJ. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 4.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 5.Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Treves A, Skaggs WE, Barnes CA. How much of the hippocampus can be explained by functional constraints? Hippocampus. 1996;6:666–674. doi: 10.1002/(SICI)1098-1063(1996)6:6<666::AID-HIPO9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 8.Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.