Abstract
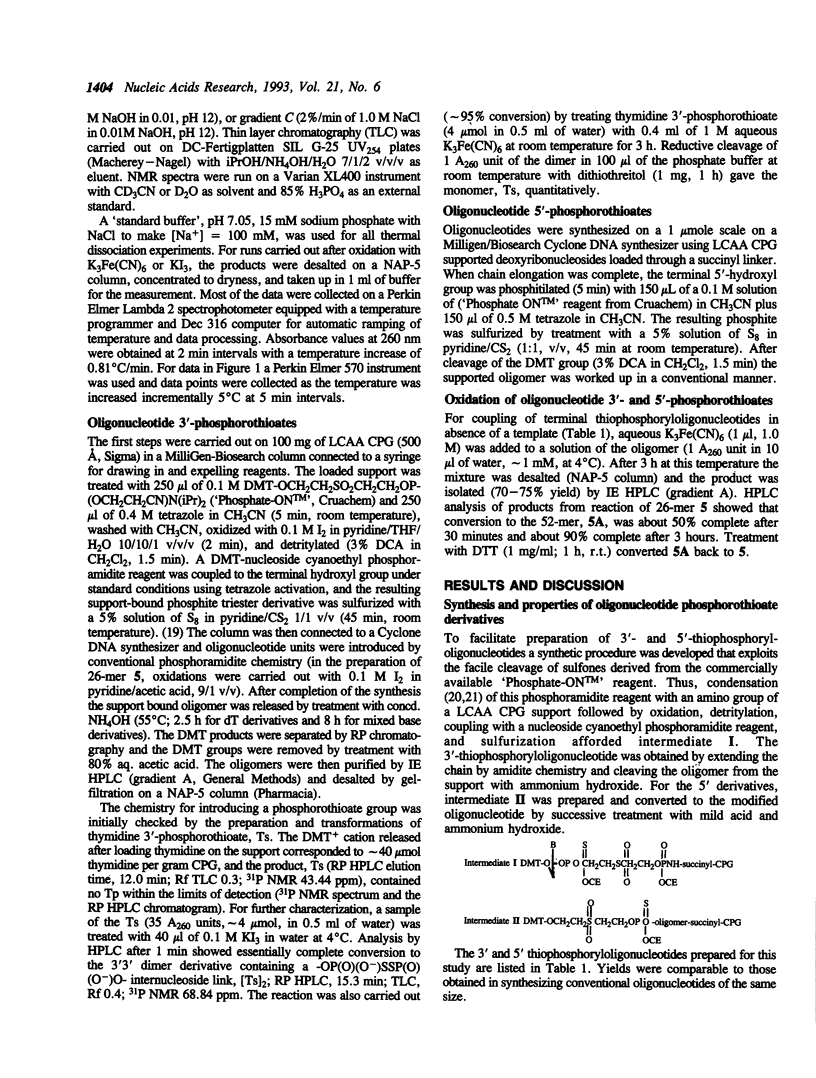
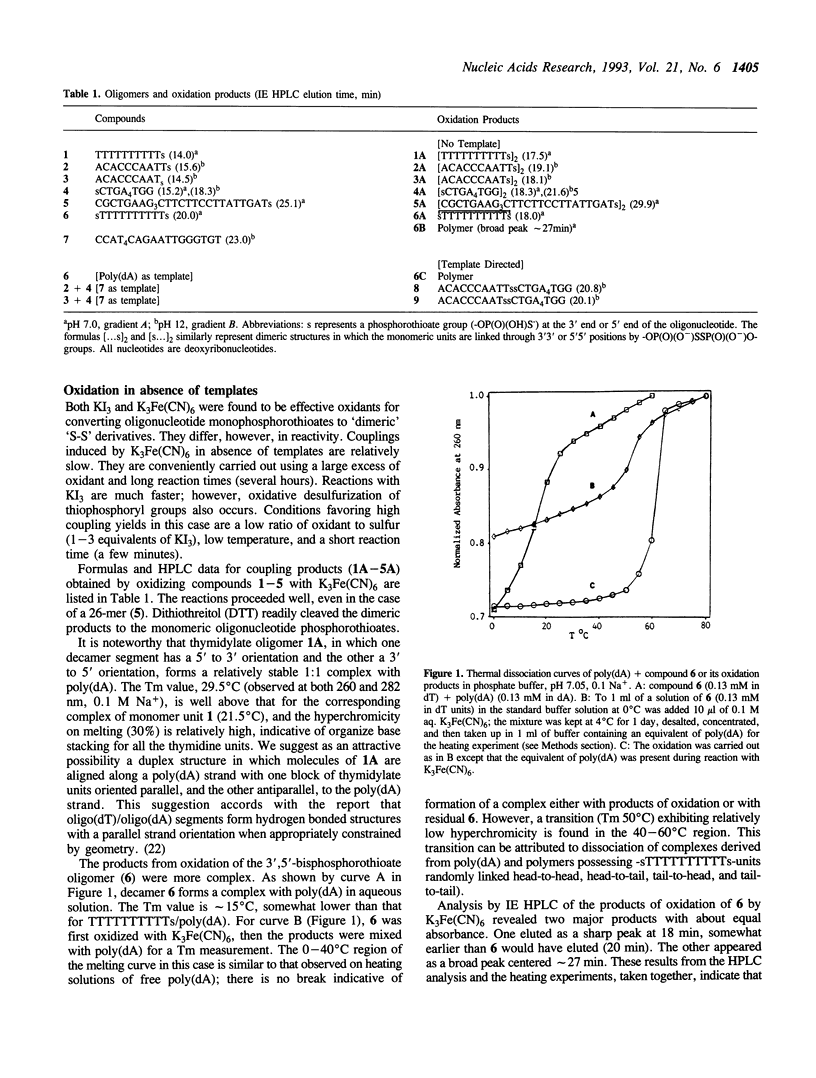
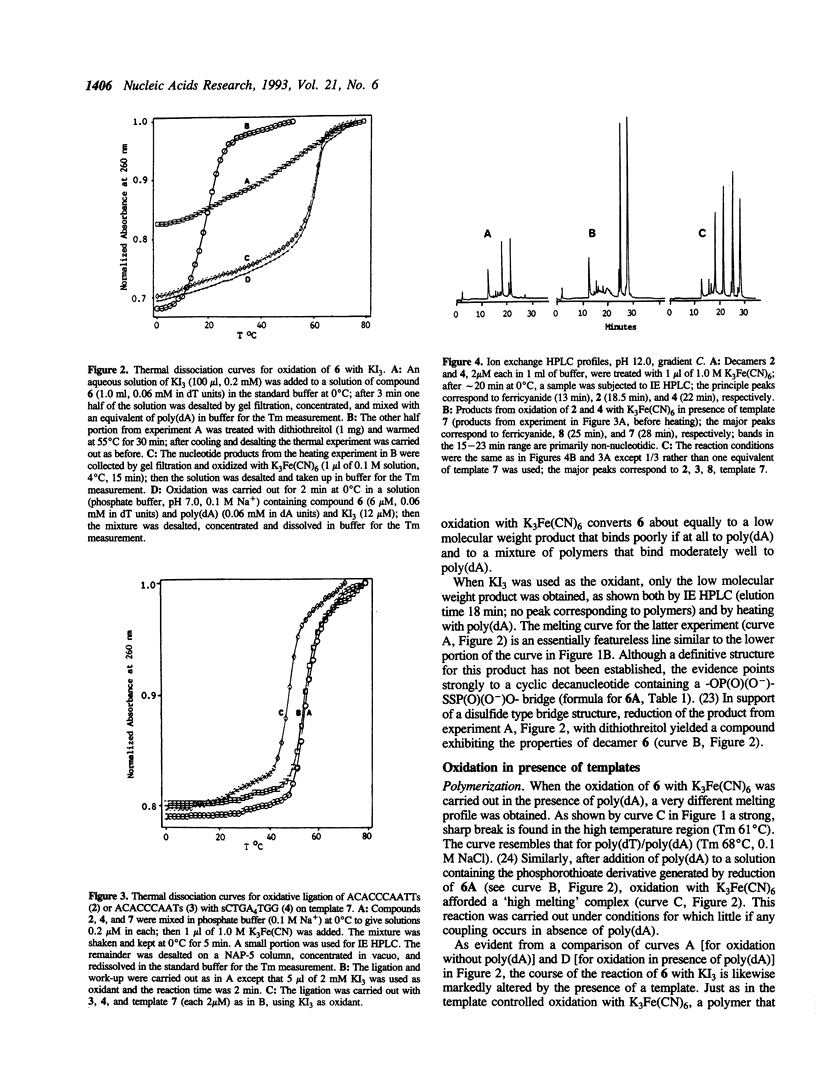
Oxidation of a pair of 3'- and 5'-thiophosphoryloligonucleotides in the presence of a complementary oligonucleotide template is shown to provide an effective means for selectively linking oligonucleotide blocks. Coupling proceeds rapidly and efficiently under mild conditions in dilute aqueous solutions (microM range for oligomers, 2-15 min at 0-4 degrees C with K3Fe(CN)6 or KI3 as oxidant). This chemistry was demonstrated by polymerization of a thymidylate decamer derivative (sTTTTTTTTTTs) in the presence of poly(dA) and by coupling oligomers possessing terminal thiophosphoryl groups (ACACCCAATTs + sCTGAAAATGG and ACACCCAATs + sCTGAAAATGG) in the presence of a template (CCATTTTCAGAATTGGGTGT). Efficient linking of 5' to 3' phosphoryl groups can be achieved under conditions where virtually no coupling takes place in absence of a template. A novel feature of the chemistry is that catalyzed recombinations of oligomers containing internal -OP(O)(O-)SSP(O)(O-)O- linkages can be directed by hydrogen bonding to a complementary oligonucleotide. Convenient procedures are reported for solid phase synthesis of the requisite oligonucleotide 3'- and 5'-phosphorothioates.
Full text
PDF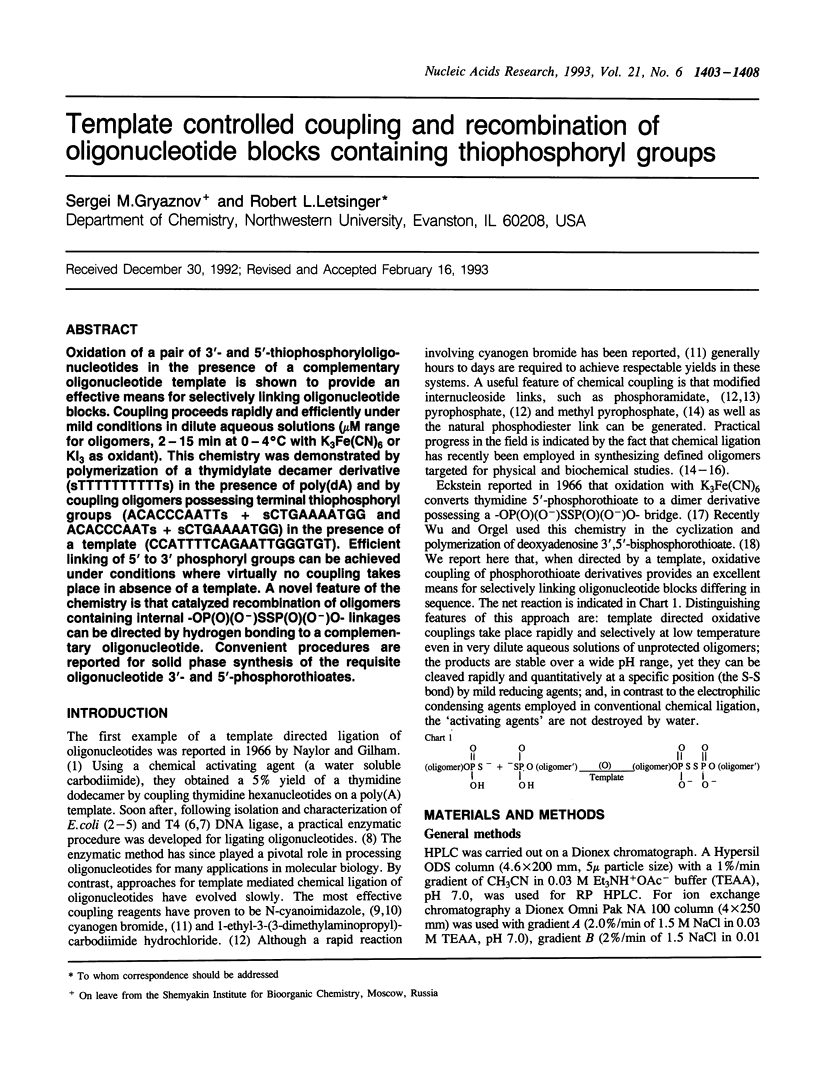

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley G. W., Kushlan D. M. Chemical synthesis of oligodeoxynucleotide dumbbells. Biochemistry. 1991 Mar 19;30(11):2927–2933. doi: 10.1021/bi00225a028. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Becker A., Hurwitz J. The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc Natl Acad Sci U S A. 1967 Jul;58(1):240–247. doi: 10.1073/pnas.58.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc Natl Acad Sci U S A. 1967 Jan;57(1):148–155. doi: 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryaznov S. M., Letsinger R. L. Synthesis and properties of oligonucleotides containing aminodeoxythymidine units. Nucleic Acids Res. 1992 Jul 11;20(13):3403–3409. doi: 10.1093/nar/20.13.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N. K., Ohtsuka E., Weber H., Chang S. H., Khorana H. G. Studies on polynucleotides. LXXXVII. The joining of short deoxyribopolynucleotides by DNA-joining enzymes. Proc Natl Acad Sci U S A. 1968 May;60(1):285–292. doi: 10.1073/pnas.60.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya E., Yanagawa H. Template-directed polymerization of oligoadenylates using cyanogen bromide. Biochemistry. 1986 Nov 18;25(23):7423–7430. doi: 10.1021/bi00371a026. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke K. J., Dervan P. B. Nonenymatic ligation of double-helical DNA by alternate-strand triple helix formation. Nucleic Acids Res. 1992 Jun 25;20(12):3005–3009. doi: 10.1093/nar/20.12.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor R., Gilham P. T. Studies on some interactions and reactions of oligonucleotides in aqueous solution. Biochemistry. 1966 Aug;5(8):2722–2728. doi: 10.1021/bi00872a032. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purmal A. A., Shabarova Z. A., Gumport R. I. A new affinity reagent for the site-specific, covalent attachment of DNA to active-site nucleophiles: application to the EcoRI and RsrI restriction and modification enzymes. Nucleic Acids Res. 1992 Jul 25;20(14):3713–3719. doi: 10.1093/nar/20.14.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Shabarova Z. A. Chemical development in the design of oligonucleotide probes for binding to DNA and RNA. Biochimie. 1988 Oct;70(10):1323–1334. doi: 10.1016/0300-9084(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Sokolova N. I., Ashirbekova D. T., Dolinnaya N. G., Shabarova Z. A. Chemical reactions within DNA duplexes. Cyanogen bromide as an effective oligodeoxyribonucleotide coupling agent. FEBS Lett. 1988 May 9;232(1):153–155. doi: 10.1016/0014-5793(88)80406-x. [DOI] [PubMed] [Google Scholar]
- Wu T., Orgel L. E. Disulfide-linked oligonucleotide phosphorothioates: novel analogues of nucleic acids. J Mol Evol. 1991;32:274–277. doi: 10.1007/BF02102183. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., Ramsing N. B., Germann M. W., Elhorst W., Kalisch B. W., von Kitzing E., Pon R. T., Clegg R. C., Jovin T. M. Parallel stranded DNA. Science. 1988 Jul 29;241(4865):551–557. doi: 10.1126/science.3399890. [DOI] [PubMed] [Google Scholar]


