Abstract
Oxytocin (OT) is a nonapeptide essential for maternal care. The development of the OT neuroendocrine system is a multi-step process dependent on the action of many transcription factors, but upstream signaling molecules regulating this process are still poorly understood. In this study, we examined if fibroblast growth factor 8 (FGF8), a signaling molecule critical for forebrain development, is essential for the proper formation of the OT system. Using immunohistochemistry, we showed a significant reduction in the number of neurons immunoreactive for the mature OT peptide in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) in the hypothalamus of homozygous (HOMO) FGF8 hypomorphic mice compared to wild-type mice. The number of neurons positive for oxyphysin prohormone in the SON but not the PVN was also significantly reduced in FGF8 HOMO hypomorphs. However, steady-state mRNA levels of the oxyphysin prohormone were not significantly different between FGF8 hypomorphs and WT mice. These data suggest that a global reduction in FGF8 signaling leads to an overall reduction of mature OT and oxyphysin prohormone levels that may have resulted from defects in multiple stages of the hormone-synthesis pathway. Since proper hormone synthesis is a hallmark of mature OT neurons, this study suggests that FGF8 signaling may contribute to the phenotypic maturation of a neuroendocrine system that originates within the diencephalon.
Keywords: Fibroblast growth factors (FGF), FGF8, Oxytocin, Oxyphysin, Neurophysin, Development
Introduction
One of the most well-characterized endocrine functions of the oxytocin (OT) system is to stimulate mammary myoepithelial contraction, which leads to milk letdown. OT-knockout mice produce milk but cannot nurse their pups, demonstrating a critical role of this neurohormone in maternal care [1]. OT acting within the brain has also been implicated in the onset of maternal behaviors such as nesting, sniffing and exploration of pups, pup retrieval, licking, and grooming [2]. Defects in the formation or maturation of this system could lead to disruption of behaviors critical for offspring survival.
OT neuron development involves a number of steps. Magnocellular neuroendocrine OT neurons are derived from the neuroepithelium lining the third ventricle by embryonic day 10.5 (E10.5). OT neurons then migrate either laterally to form the paraventricular nucleus (PVN) or ventro-laterally to form the supraoptic nucleus (SON) in the hypothalamus (HYPO) from E10.5 to E14.5 [3–5]. After migration, OT neurons target their axons to the pars nervosa of the neurohypophysis, where the mature nonapeptide is secreted into the circulation. The proper formation and maturation of OT neurons are guided by precise spatial and temporal signaling. Many studies have shown that transcription factors such as Otp, the POU transcription factor Brn2, and the PAS domain transcription factors Sim1 and Arnt2 [6–8] are critical for the development of the PVN and SON. However, to our knowledge, no studies to date have examined the role of upstream signaling molecules in the formation of OT system in mice.
We hypothesize that members of the fibroblast growth factor (FGF) family may serve as signaling molecules for the maturation of OT neurons. FGFs are widely active in the developing central nervous system. This family of 22 ligands and 4 transmembrane receptors regulates neuronal growth, survival, migration, and patterning [9]. One of the FGFs, FGF8, has early developmental effects on central tissues such as the midbrain/hindbrain junction [10, 11] and the telencephalon [12]. Further, FGF8 is indispensable for the development of a peripheral structure, the olfactory placode, which gives rise to important neuroendocrine neurons [13, 14].
Less clear is the role of FGF8 in the development of neuroendocrine systems, such as the OT system, that originate in the HYPO. However, indirect evidence suggests such a role of FGF8 is possible. First, FGF8 is expressed in the ventral diencephalon and infundibulum during development [15]. Second, FGF receptors are present on magnocellular SON and PVN neurons [16, 17]. Lastly, FGF8 expression in the infundibulum modulates the induction of Rathke’s pouch and differentiation of dorsal cell types of the adenohypophysis [18–20], thereby implicating FGF8 in the development of the pituitary. It is therefore possible that FGF8 participates in the development of OT neurons that originate in the presumptive hypothalamic region adjacent to the FGF8-expressing infundibulum.
In this study, we investigated the role of FGF8 signaling in the development of OT neurons. The evidence that FGF8 is important for the development of the numerous structures, including the pituitary, led to our hypothesis that a reduction in FGF8 signaling may have deleterious effects on the development of OT neurons. Using the techniques of immunohistochemistry (IHC) and quantitative reverse transcriptase polymerase chain (qRT-PCR) reaction, we found significant defects in the OT system of mice deficient in FGF8 signaling, suggesting that FGF8 is involved in the phenotypic maturation of OT neurons.
Results
OT-immunoreactive neurons
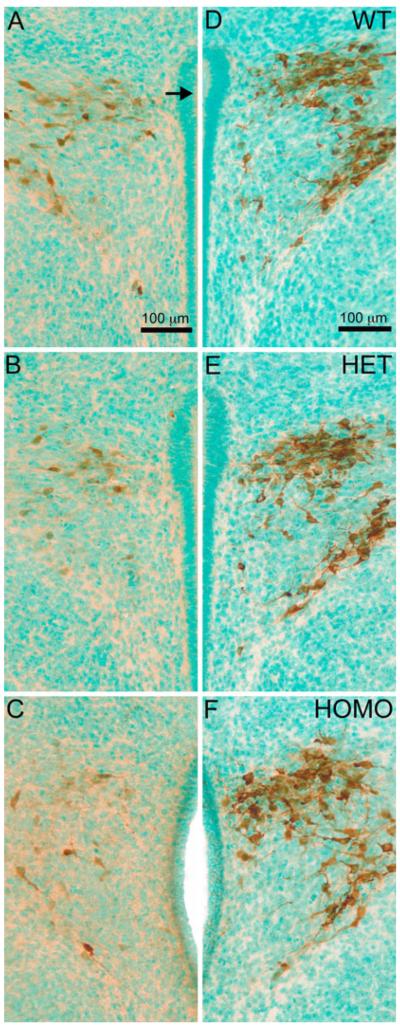
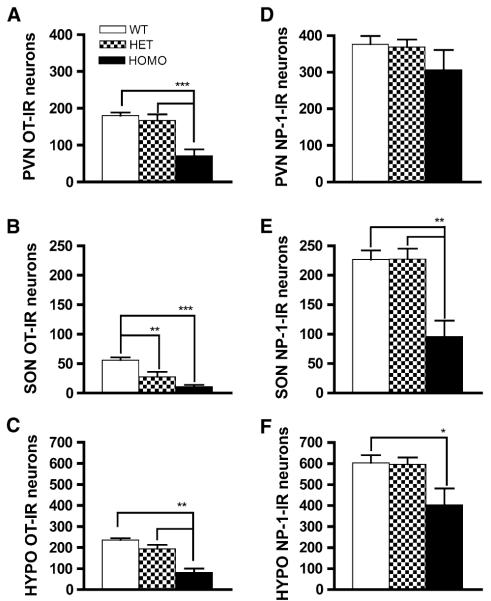
In order to determine whether FGF8 signaling is critical for the normal presence of the OT system, IHC was performed to detect mature OT peptide-containing neurons in the PVN and SON of postnatal day (PN) 0 wild-type (WT), heterozygous (HET), and homozygous (HOMO) FGF8 hypomorphic mice. Representative photomicrographs of the PVN and SON of each genotype are shown in Figs. 1a–c and 2a–c, respectively. OT-immunoreactive (IR) neurons were quantified in all PVN- and SON-containing sections from half of the section series (Fig. 3a–c). The mean PVN OT neuron counts ± SEM for each genotype were 180 ± 9 in WT, 167 ± 17 in HET, and 71 ± 17 in HOMO. OT-IR neurons in the PVN of HOMO mice were significantly different between HOMO and WT, as well as between HOMO and HET (Fig. 3a). The mean SON OT neuron counts ± SEM for each genotype were 56 ± 5 in WT, 28 ± 8 in HET, and 11 ± 3 in HOMO. OT-IR neurons in the SON of WT mice were significantly different between WT and HET, as well as between WT and HOMO (Fig. 3b). There was also a significant reduction in total OT-IR neurons in the HYPO (the sum of neurons in SON and PVN) in HOMO mice compared to WT and HET (Fig. 3c). During the course of neuron counting, we did not notice any unusual ectopic accumulation of OT-IR neurons in the preoptic/hypothalamic regions of either HET or HOMO compared to WT (data not shown).
Fig. 1.
Representative photomicrographs of OT-IR (a–c) and NP-1-IR (d–f) neurons in the PVN of PN0 WT (a, d), HET (b, e), and HOMO (c, f) FGF8 hypomorphs. Brown color represents OT immunostaining. Green color represents methyl green nuclear counterstain. Note the visual reduction of OT-IR neurons in (b) and (c) compared to a. Arrow points toward the third ventricle. Scale bar = 100 μm. (Color figure online)
Fig. 2.
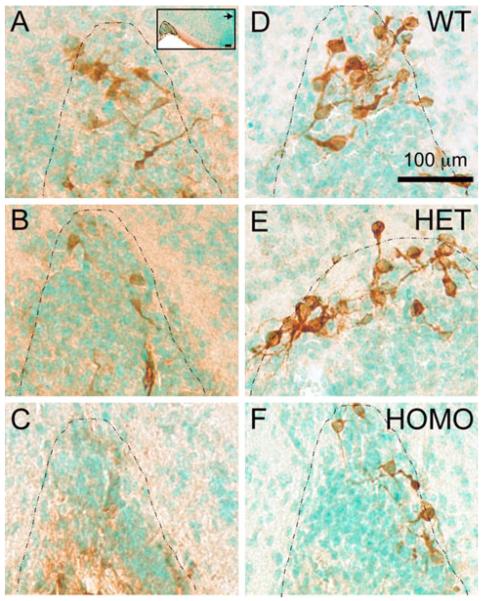
Representative photomicrographs of OT-IR (a–c) and NP-1-IR (d–f) neurons in the SON of PN0 WT (a, d), HET (b, e), and HOMO (c, f) FGF8 hypomorphs. The boundary of SON is delineated by the dotted line. Brown color represents OT immunostaining. Green color represents methyl green nuclear counterstain. Inset in (a) is a low-magnification photomicrograph of the same section, with the SON delineated by dotted line. Note the visual reduction of OT-IR neurons in (b) and (c) compared to (a), and the reduction of NP-1-IR neurons in (f) compared to (d) and (e). Arrow points toward the third ventricle. Scale bars = 100 μm. (Color figure online)
Fig. 3.
Quantification of OT-IR (a–c) and NP-1-IR (d–f) neurons in WT, HET, and HOMO FGF8 hypomorphic mice. Data are represented as mean ± SEM. N = 6–7 per genotype. Significant difference between groups is indicated by brackets above bars. * P < 0.05, ** P < 0.01, *** P < 0.001
NP-1-IR neurons
The peptides NP-1 and OT are cleaved from the same prohormone. Whereas the anti-OT antiserum detects specifically the mature OT nonapeptide, the NP-1 antiserum can detect the unprocessed prohormone as well as the cleaved NP-1. This makes NP-1 an ideal marker for detecting oxytocinergic neurons regardless of whether the prohormone has been processed into mature OT. IHC using a NP-1 antibody was performed to examine if a reduction in OT-IR neurons in HOMO mice is accompanied by a parallel decrease in the NP-1-IR neurons. Representative photomicrographs of the PVN (Fig. 1d–f) and SON (Fig. 2d–f) and the quantification of NP-1-IR neurons in the PVN and SON of each genotype are shown in Fig. 3d–f. Sections used for NP-1 IHC represent half of the section series derived from the same animals used for OT IHC above. The mean PVN NP-1 neuron counts ± SEM were 376 ± 23 in WT, 369 ± 20 in HET, and 307 ± 53 in HOMO. No significant differences in NP-1-IR neurons were found among the genotypes in the PVN (Fig. 3d). The mean SON neuron counts ± SEM were 227 ± 15 in WT, 227 ± 18 in HET, and 96 ± 27 in HOMO. There was a significant reduction of NP-1-IR neurons in the SON of HOMO between HOMO and WT and HOMO and HET (Fig. 3e). There was also a significant reduction in total NP-1-IR neurons in the HYPO of HOMO mice when compared to WT (Fig. 3f). During the course of neuron counting, we did not notice any unusual ectopic accumulation of NP-1-IR neurons in the preoptic/hypothalamic regions of either HET or HOMO compared to WT (data not shown).
In order to verify that the lower number of OT-IR neurons compared to NP-1-IR neurons in pups of all genotypes was due to a developmental delay in peptide processing instead of an IHC artifact, we performed OT and NP-1 IHC in an adult animal. As expected, the number of OT-IR neurons in the PVN was comparable to that of NP-1-IR neurons in the adult brain (data not shown).
Oxyphysin prohormone mRNA
In order to examine whether the reduction in OT- and NP-1 neurons in FGF8 hypomorphic mice was caused by reduced oxyphysin prohormone mRNA levels, qRT-PCR was performed on cDNA from the hypothalamus of PN0 mice. One-way ANOVA did not reveal significant differences (P = 0.4360) in hypothalamic oxyphysin prohormone mRNA levels among genotypes. The mean copy numbers (×106) of oxyphysin prohormone mRNA per microgram total RNA were 3.9 ± 0.31 in WT, 3.4 ± 0.19 in HET, and 3.5 ± 0.19 in HOMO (Fig. 4).
Fig. 4.
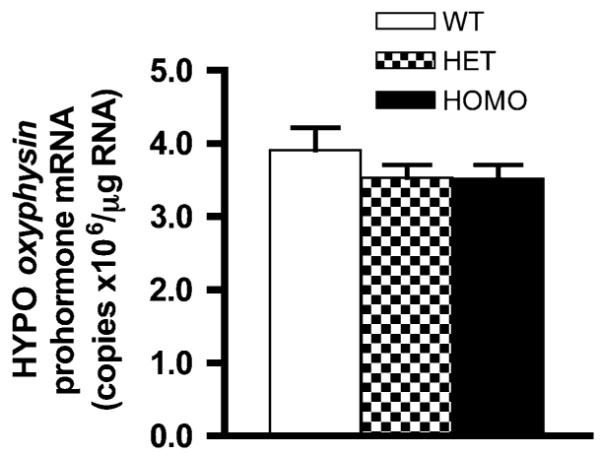
Hypothalamic oxyphysin prohormone mRNA levels are not significantly different between genotypes. Data are represented as mean ± SEM. N = 6 per genotype
Discussion
The maturation of OT neurons involves a number of steps ranging from progenitor proliferation, migration, and axon path finding to mRNA transcription, prohormone synthesis and processing, and vesicular transport. These steps eventually culminate in a vesicular pool of releasable mature OT nonapeptide [21]. Dysregulation of any these steps could alter the functionality of the OT system. By demonstrating that FGF8 hypomorphs had reduced OT-IR and NP-1-IR neurons in hypothalamic regions, this study suggests that FGF8 signaling deficiency impairs the formation of the OT system. Both the PVN and SON are affected by FGF8 hypomorphy, but the impact appears more severe in the SON. The levels of hypothalamic oxyphysin prohormone mRNA do not differ among genotypes, suggesting FGF8 deficiency does not significantly alter the specification of OT neuronal fate. These data are the first to demonstrate that FGF8 is required for a normal population of OT neurons.
As detection of mature OT at PN0 is limited [22, 23], we used NP-1 IHC to examine whether FGF8 hypomorphy led to reduced number of neurons able to synthesize the oxyphysin prohormone. Further, we performed IHC to examine whether the oxyphysin-synthesizing neurons acquired the ability to synthesize the mature OT, a process that primarily takes place after birth [21, 24]. We found that FGF8 deficiency caused an overall reduction of NP-1-IR neurons in the HYPO of HOMO FGF8 hypomorphs. As expected, this reduction was accompanied by a parallel reduction in OT neurons in the HYPO. However, the reduction of OT neurons in the SON of HET (Fig. 3a) and PVN of HOMO (Fig. 3b), neither of which exhibited reduced NP-1-IR neurons, raised the possibility that several post-transcriptional steps leading to the formation of mature OT may be affected by FGF8 signaling deficiency.
At present, the most parsimonious explanation for our data is that [1] the translation of oxyphysin transcript into the oxyphysin prohormone and [2] the processing of oxyphysin prohormone into the mature OT peptide are both impaired in FGF8 hypomorphs. We observed normal oxyphysin transcript levels in all genotypes, but the total number of neurons positive for NP-1 (also indicative of neurons positive for oxyphysin prohormone) was reduced in HYPO of FGF8 hypomorphs, suggesting decreased efficacy of translation. After translation, OT is cleaved from NP-1 by a prohormone convertase. The resulting products are a three amino acid C-terminally extended OT (OT-Gly-Lys-Arg) and NP-1. The terminally extended OT is then cleaved sequentially by an endopeptidase and a carboxypeptidase to remove the remaining three amino acids. The resultant peptide is considered mature after the C-terminal amidation [25, 26]. In our study, normal numbers of NP-1-IR neurons in PVN of HOMO and SON of HET were accompanied by reduced OT-IR neurons, suggesting that the efficacy of this post-translational processing is also reduced. The finding that FGF signaling might influence post-translational processing events is not unprecedented. Like OT, gonadotropin-releasing hormone (GnRH) peptide is also cleaved from a larger prohormone. FGF2 treatment was reported to alter GnRH prohormone processing by increasing the proportion of C-terminally extended intermediate products [27].
In rats, the detection of mature OT lags behind that of oxyphysin prohormone during development. In fact, the C-terminally extended OT peptide is the prominent form of OT in the fetal brain with the final amidated peptide not becoming evident until birth [22, 24]. These results suggest that the capacity to process the oxyphysin prohormone into OT is associated with the phenotypic maturation of the neurons [22, 23] and can be used as a marker to gauge the maturity of these neurons. Our current data that NP-1-IR cells in all genotypes are consistently more numerous and intensely stained at PN0 than OT-IR neurons suggest murine OT neurons undergo similar phenotypic maturation around the time of birth. The lower efficacy of posttranslational processing in FGF8 hypomorphs could therefore be a consequence of impaired or delayed phenotypic maturation of OT neurons. It would be ideal to examine whether this is a permanent defect or only a transient delay. However, our HOMO mice do not survive past PN0, thus other models of FGF8 deficiency will need to be explored. At present, a prohormone processing defect is the most parsimonious explanation for the mismatch between NP-1-IR and OT-IR neuron numbers, but other possibilities such as disruption in the stability, folding, or transport of the mature OT peptide in FGF8 hypomorphs cannot be ruled out.
An alternative hypothesis for the cause of reduced OT neuronal population in FGF8 hypomorphs is that FGF8 could promote the migration of OT neurons out of their birthplace, the subventricular zone. Due to the greater distance of SON from the birthplace, the arrival of these neurons in SON may be impacted more than PVN when FGF8 is deficient. Supporting this, FGF8 is important for the organization of the fibers that guide GnRH neuronal migration from the olfactory placode to the hypothalamus [28]. Further, FGF8 is expressed near the presumptive SON in optic stalks, optic nerves, and chiasmatic area until E14.5 [15]. FGF8 expression in these zones coincides temporally with the arrival of OT neurons in the SON, suggesting an involvement of FGF8 in the proper positioning of these neurons. However, if cells destined for the SON do not receive the proper signal to migrate, one might expect them to become arrested in or near the PVN. The lack of ectopic accumulation of OT neurons as well as the normal number of NP-1-IR neurons in the PVN did not support this hypothesis.
In this study, we demonstrated that FGF8 deficiency decreased the number of OT-IR and NP-1-IR neurons. The pattern of neuronal loss was similar between PVN and SON in that the significant decrease in OT-IR neurons was accompanied by the seemingly less affected NP-1-IR neurons in both nuclei. Although the precise mechanism underlying this neuronal loss is unclear, reduced efficacy in translational and post-translational events that ultimately decreases hormone production is the most plausible explanation. Further, the disruption in this OT synthesis pathway could be a consequence of impaired or delayed OT neuronal maturation resulting from FGF8 deficiency.
Materials and methods
Animals
FGF8 hypomorphic mice [29] (129p2/OlaHsd* CD-1; obtained from Mouse Regional Resource Centers, Davis, CA, USA) kept on a 12-h light and 12-h dark cycle and fed water and rodent chow ad libitum were bred in our animal care facility. These animals contain a neomycin-resistance element between Exons 1 and 2 of the FGF8 gene. This element contains a false splice site which leads to a ~55% reduction in the level of functional FGF8 transcript under HOMO condition [29]. Possibly due to the absence of olfactory bulbs [13] and inability to suckle, HOMO hypomorphs do not survive past the first day of birth. Therefore, pups were killed and genotyped on PN0. WT, HET, and HOMO animals are represented in this study. The brains were processed for IHC or qRT-PCR for the quantification of oxyphysin prohormone message. All animal procedures complied with the protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado.
Immunohistochemistry (IHC)
Tissue preparation
For IHC, the brains were dissected and immersion-fixed in 5% acrolein for 5 h at room temperature and then cryoprotected in 30% sucrose until sectioning.
IHC
Two peptides, OT and neurophysin-1 (NP-1), are derived from the post-translational cleavage of the oxyphysin prohormone. In order to visualize neurons IR for these two peptides, 40-μm coronal frozen sections through the hypothalamus were cut and used for IHC. One half of these sections were incubated with a mouse monoclonal anti-OT antibody (1:10,000, 4°C, 3 nights) (Millipore, Billerica, MA, USA) and the other half with a goat polyclonal anti-NP-1 antibody (1:500, 4°C, 3 nights) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The anti-OT antibody detects only the mature OT, whereas the anti-NP-1 antibody detects both NP-1 and oxyphysin prohormone. All sections were then incubated sequentially with a biotinylated secondary antibody (1:500), avidin–biotin complex (Vector Labs, Burlingame, CA, USA), and reacted with diaminobenzidine for color detection. Sections were then mounted on slides, counter-stained with methyl green, cleared with Histoclear (National Diagnostics, Atlanta, GA, USA), and coverslipped. The negative controls included sections incubated with OT primary antibody pre-adsorbed with 10 μM OT and without primary antibodies. The best antibody concentrations were chosen based on pilot studies conducted with three serial dilutions (1:5000, 1:10000, and 1:20000) of the primary antibodies.
Quantification of neurons and data analysis
An investigator blind to the identities of the slides quantified OT-IR and NP-1-IR neurons in PVN and SON. The total number of OT neurons in the PVN and SON were combined and called HYPO. In mice, it is also not possible to differentiate between the magnocellular and parvocellular OT neurons [30], thus all OT-positive neurons were scored. All data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Differences were considered significant when P < 0.05.
Reverse transcriptase quantitative polymerase chain reaction
Tissue preparation
Pups were killed by decapitation and the brains were dissected and blocked to obtain the hypothalamus, which encompasses the brain region between the optic chiasm and caudal mammillary body. Hypothalamic blocks were immediately placed on dry ice and kept frozen at −70°C until processed.
RNA isolation and cDNA synthesis
Tissues were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and RNA was isolated according to manufacturer’s instructions. The concentration and quality of RNA were evaluated by spectrophotometric reading at wavelengths of 260 and 280 nm. 1 μg of total RNA was used for the synthesis of cDNA using Superscript III First-Strand cDNA Synthesis kit (Invitrogen) following manufacturer’s instructions.
qRT-PCR of oxyphysin prohormone transcript
For each PCR reaction, FastStart SYBR Green Master mix (Roche, Indianapolis, IN, USA) was combined with primers and sample cDNA or standard to achieve a final volume of 22 μl. The standard used was a 433-bp oxyphysin prohormone cDNA inserted into pT7T3D-Pac vector (I.M.A.G.E. cDNA Clones, Invitrogen). Reactions were run in a 96-well plate in duplicates. The final reaction mixture consists of 4 μl cDNA template, 18 μl SYBR Green Master mix, and 0.25 μM of forward and reverse primers each. The primer sequences were 5′-CAGGGCGAAGGCAGGTAGTT-3′ for the forward primer, and 5′-GTCTCGCTTGCTGCCTGCTT-3′ for the reverse primer (Integrated DNA Technologies, San Diego, CA, USA). The reaction was performed by a Mastercycler® ep realplex* using a three-step PCR amplification program. The following program was used: hold at 95°C for 10 min prior to amplification, the denaturing (95°C), annealing (65°C), and elongation (72°C) phases were held at their respective temperatures for 30 s each and were repeated for 40 cycles. After amplification, a melting curve was included to verify amplification of the correct product.
Quantification and data analysis
The threshold of the Mastercycler was set to ten times the standard deviation above the noise of the automatic baseline. The data points of the exponential phase of the amplification curves were then used to calculate sample CT values. The software used linear regression of the standard curve to determine the mean copies/well of the samples. An investigator blind to the sample identity excluded those that did not have any product in the melting curve, or whose melting curve profile did not match the standard amplicon. The mean copies/well for each sample was then normalized by the amount of starting RNA used for cDNA synthesis. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Differences were considered significant when P < 0.05.
Acknowledgment
This study was supported by NIH R01 HD042634 to PST and Endocrine Society Summer Fellowship to L.R.B.
References
- 1.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl Acad. Sci. USA. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: a detailed descriptive analysis. Physiol. Behav. 2006;89:320–328. doi: 10.1016/j.physbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. Development of the diencephalon in the rat. II. Correlation of the embryonic development of the hypothalamus with the time of origin of its neurons. J. Comp. Neurol. 1978;182:973–993. doi: 10.1002/cne.901820512. [DOI] [PubMed] [Google Scholar]
- 4.Karim MA, Sloper JC. Histogenesis of the supraoptic and paraventricular neurosecretory cells of the mouse hypothalamus. J. Anat. 1980;130:341–347. [PMC free article] [PubMed] [Google Scholar]
- 5.Nakai S, Kawano H, Yudate T, Nishi M, Kuno J, Nagata A, Jishage K, Hamada H, Fujii H, Kawamura K, et al. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev. 1995;9:3109–3121. doi: 10.1101/gad.9.24.3109. [DOI] [PubMed] [Google Scholar]
- 6.Simeone A, D’Apice MR, Nigro V, Casanova J, Graziani F, Acampora D, Avantaggiato V. Orthopedia, a novel homeobox-containing gene expressed in the developing CNS of both mouse and Drosophila. Neuron. 1994;13:83–101. doi: 10.1016/0896-6273(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 7.Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Simeone A. The role of Otx and Otp genes in brain development. Int. J. Dev. Biol. 2000;44:669–677. [PubMed] [Google Scholar]
- 8.Jo YH, Chua S., Jr. Transcription factors in the development of medial hypothalamic structures. Am. J. Physiol. Endocrinol. Metab. 2009;297:E563–E567. doi: 10.1152/ajpendo.00064.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dono R. Fibroblast growth factors as regulators of central nervous system development and function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R867–R881. doi: 10.1152/ajpregu.00533.2002. [DOI] [PubMed] [Google Scholar]
- 10.Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 11.Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 12.Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 13.Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- 14.Ford-Perriss M, Abud H, Murphy M. Fibroblast growth factors in the developing central nervous system. Clin. Exp. Pharmacol. Physiol. 2001;28:493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- 15.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 16.Burbach JP. Genetic pathways in the developmental specification of hypothalamic neuropeptide and midbrain catecholamine systems. Eur. J. Pharmacol. 2000;405:55–62. doi: 10.1016/s0014-2999(00)00541-0. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez AM, Logan A, Ying W, Lappi DA, Berry M, Baird A. Fibroblast growth factor in the hypothalamic-pituitary axis: differential expression of fibroblast growth factor-2 and a high affinity receptor. Endocrinology. 1994;134:2289–2297. doi: 10.1210/endo.134.5.8156932. [DOI] [PubMed] [Google Scholar]
- 18.Norlin S, Nordstrom U, Edlund T. Fibroblast growth factor signaling is required for the proliferation and patterning of progenitor cells in the developing anterior pituitary. Mech. Dev. 2000;96:175–182. doi: 10.1016/s0925-4773(00)00393-2. [DOI] [PubMed] [Google Scholar]
- 19.Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- 20.Herzog W, Sonntag C, von der Hardt S, Roehl HH, Varga ZM, Hammerschmidt M. FGF3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development. 2004;131:3681–3692. doi: 10.1242/dev.01235. [DOI] [PubMed] [Google Scholar]
- 21.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 22.Altstein M, Gainer H. Differential biosynthesis and posttranslational processing of vasopressin and oxytocin in rat brain during embryonic and postnatal development. J. Neurosci. 1988;8:3967–3977. doi: 10.1523/JNEUROSCI.08-11-03967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitnall MH, Key S, Ben-Barak Y, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. II. Immunocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J. Neurosci. 1985;5:98–109. doi: 10.1523/JNEUROSCI.05-01-00098.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainer H, Altstein M, Whitnall MH. The cell biology and development of vasopressinergic and oxytocinergic neurons. Prog. Brain Res. 1987;72:153–161. doi: 10.1016/s0079-6123(08)60204-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris M, Castro M, Rose JC. Alterations in oxytocin prohormone processing during early development in the fetal sheep. Am. J. Physiol. 1992;263:R738–R740. doi: 10.1152/ajpregu.1992.263.3.R738. [DOI] [PubMed] [Google Scholar]
- 26.Burbach JP, Lebouille JL. Proteolytic conversion of arginine-vasopressin and oxytocin by brain synaptic membranes. Characterization of formed peptides and mechanisms of proteolysis. J. Biol. Chem. 1983;258:1487–1494. [PubMed] [Google Scholar]
- 27.Wetsel WC, Hill DF, Ojeda SR. Basic fibroblast growth factor regulates the conversion of pro-luteinizing hormone-releasing hormone (Pro-LHRH) to LHRH in immortalized hypothalamic neurons. Endocrinology. 1996;137:2606–2616. doi: 10.1210/endo.137.6.8641215. [DOI] [PubMed] [Google Scholar]
- 28.Chung WC, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4997–5003. doi: 10.1210/en.2007-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers EN, Lewandoski M, Martin GR. An FGF8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 30.de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm. Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]