Abstract
The Retinal Homeobox (Rx) gene is essential for vertebrate eye development. Rx function is required for the specification and maintenance of retinal progenitor cells (RPCs). Loss of Rx function leads to a lack of eye development in a variety of species. Here we show that Rx function is also necessary during retinal regeneration. We performed a thorough characterization of retinal regeneration after partial retinal resection in pre-metamorphic X. laevis. We show that after injury the wound is repopulated with retinal progenitor cells (RPCs) that express Rx and other RPC marker genes. We used an shRNA-based approach to specifically silence Rx expression in vivo in tadpoles. We found that loss of Rx function results in impaired retinal regeneration, including defects in the cells that repopulate the wound and the RPE at the wound site. We show that the regeneration defects can be rescued by provision of exogenous Rx. These results demonstrate for the first time that Rx, in addition to being essential during retinal development, also functions during retinal regeneration.
Keywords: retinal homeobox, regeneration, transdifferentiation, retinal progenitor cells, shRNA
INTRODUCTION
Retinal regeneration in vertebrates was first demonstrated in urodele amphibians over 100 hundred years ago (Del Rio-Tsonis and Tsonis, 2003; Yoshii et al., 2007). Retinal regeneration has also been documented in frogs, embryonic and post-natal chickens, and fish (Araki, 2007; Bernardos et al., 2007; Del Rio-Tsonis and Tsonis, 2003; Fischer, 2005; Vergara and Del Rio-Tsonis, 2009; Yoshii et al., 2007). The mammalian retina can also initiate regeneration (Karl et al., 2008). The Xenopus laevis tadpole is capable of regenerating its retina after surgical removal of 2/3 of the eye (Ide et al., 1984; Ide et al., 1987). Similarly, studies in Rana catesbiana showed that tadpoles of this species could also regenerate the retina after damage induced by devascularization and severing the optic nerve (Reh and Nagy, 1987). Additionally, adult R. temporaria and X. laevis can also regenerate the retina following partial resection (Levine, 1981; Lombardo, 1969). Recently, it was demonstrated that both tadpoles and adult X. laevis have the capacity to regenerate its retina even after complete retinectomy (Vergara and Del Rio-Tsonis, 2009; Yoshii et al., 2007).
In salamanders and newts, retinal regeneration occurs mostly through transdifferentiation of the retinal pigment epithelium (RPE) (Del Rio-Tsonis and Tsonis, 2003). RPE transdifferentiation is also a source of regenerating cells in embryonic chicks (Spence et al., 2007; Spence et al., 2004). Regeneration is also possible in post-natal chickens (Fischer and Reh, 2001). After neurotoxic damage, chickens can regenerate the retina by transdifferentiation of Müller glia (Fischer and Reh, 2001). Müller glia can also transdifferentiate and give rise to new photoreceptors after light-induced damage in fish (Bernardos et al., 2007). Similar to regeneration in newts, RPE transdifferentiation is considered to be a major source of regenerating cells in frogs. Transplantation of RPE into the eye showed that RPE could undergo metaplasia and produce new retinal tissue (Sologub, 1975) (Arresta et al., 2005). RPE can differentiate into neural retina in post-metamorphic Xenopus laevis as well (Yoshii et al., 2007). The process and molecular details of transdifferentiation of frog RPE into new retinal neurons has not been characterized. Another potential source of regenerating cells in frogs are the retinal progenitor cells (RPCs) located at the ciliary marginal zone (CMZ) (Moshiri et al., 2004; Reh and Fischer, 2001, 2006; Reh and Levine, 1998). These RPCs continually proliferate and give rise to most of the retinal growth that occurs in X. laevis larvae (Hollyfield, 1971).
Regeneration is said to recapitulate embryonic development. The Retinal Homeobox (Rx) gene is one of the earliest genes to be expressed during eye development (Casarosa et al., 1997; Chuang et al., 1999; Deschet et al., 1999; Furukawa et al., 1997; Mathers et al., 1997). It is expressed throughout retinal development, beginning at neural plate (Mathers et al., 1997). In the mature frog retina Rx is expressed in the photoreceptor layer (PRL), inner nuclear layer (INL) and throughout the CMZ (Pan et al., 2006). Loss of Rx function leads to a lack of eye structures in a variety of species including frogs, fish, mice and humans (Andreazzoli et al., 1999; Chen and Cepko, 2002; Chuang and Raymond, 2001; Loosli et al., 2003; Loosli et al., 2001; Mathers et al., 1997; Voronina et al., 2004). Conversely, Rx overexpression results in the formation of extra retinal tissue (Andreazzoli et al., 1999; Chuang and Raymond, 2001; Mathers et al., 1997). Results from loss- and gain-of-function studies in X. laevis suggested that Rx function is essential for the specification and proliferation of RPCs. Subsequent studies then showed that Rx functions to maintain RPCs in a proliferative and multipotent state throughout development (Andreazzoli et al., 2003; Casarosa et al., 2003). Additionally, overexpression of Rx in the developing optic cup does not bias the fate of newly generated cells (Andreazzoli et al., 2003; Casarosa et al., 2003).
The purpose of this study is to characterize retinal regeneration in pre-metamorphic X. laevis both at a morphological and molecular level. Here we show that pre-metamorphic X. laevis fully regenerates the retina by 30 days after surgical resection of 1/4 of the eye. We also show that retinal progenitor cells (RPCs) are induced at the site of resection after 1 week post-resection. Finally, we demonstrate that Rx is necessary for retinal regeneration and that the generation of RPCs during retinal regeneration may require Rx function.
EXPERIMENTAL PROCEDURES
Retinal resection
Xenopus laevis tadpoles reared by in vitro fertilization (Sive et al., 2000) were raised to stage 44 (Nieuwkoop and Faber, 1994) and anesthetized in 0.1% MS-222 (Ethyl-3-aminobenzoate methanesulfonate; Sigma) diluted in 0.1X MMR before resection. Tadpoles were placed in a small rectangular well made in a 2.5% agarose dish for immobilization. The nasal-dorsal quarter of the eye was removed from the right eye of each tadpole using a pair of No.5 forceps and a 271/2-gauge syringe or a Gastromaster. The left eye of the same tadpole was not resected and used for control experiments. Tadpoles were cultured at 16°C and fed (Sera Micron) 6 days a week. Tadpoles in which the eye resorbed or collapsed over the first few days after resection were discarded and not used for further experiments. Under these conditions, tadpoles developed as follows: st 44–day 1; st 45–day 2; st 46–day 3; st 47–day 5; st 48–day 10; st 49–day 15; st 50–day 18; st 51–day 22.
Histological staining and immunohistochemistry
For histology and immunohistochemistry, tadpoles were fixed in MEMPFA [MOPS-EGTA-MgSO4-paraformaldehyde] at different time points after resection during a span of 30 days (Sive et al., 2000), dehydrated in methanol, and embedded in paraffin as previously described (Pan et al., 2006). Eyes were sectioned coronally at 8 μm. Immunohistochemistry was performed as described previously (El-Hodiri et al., 1997). The primary antibodies were used in the following dilutions: mouse anti-rhodopsin (RetP1; Biomeda, Foster City, CA) 1:50; mouse anti-islet 1 (Clone 39.4D5; Developmental Studies Hybridoma Bank [DSHB], University of Iowa) 1:50; rabbit anti-CRALBP (courtesy of Dr. J. Saari), 1:1000; mouse anti-BrdU (Clone G3G4; DSHB), 1:50. For immunofluorescence, we used an Alexa-fluor 488-conjugated goat anti-mouse secondary antibody (Invitrogen/Molecular Probes), diluted 1:1000.
BrdU incorporation
BrdU crystals (Sigma) were diluted to 0.01% in 40% Holtfreter’s from a stock solution of 0.1X Holtfreter’s and injected intra-abdominally. After injection, tadpoles were incubated at 16°C for 2 hr, fixed in MEMPFA for 1 hr and dehydrated in methanol. To analyze the incorporation of BrdU in proliferating cells, embryos were parafinized, and 8 μm sections were prepared and subjected to immunohistochemistry as described above, but with an incubation in 4 M HCl for 7 minutes prior to the blocking step during immunostaining or immunofluorescence.
In situ hybridization of retinal sections
Section in situ hybridization was performed on 8μm retinal sections processed using either digoxygenin or fluorescein-labeled antisense riboprobes as previously described (Shimamura et al., 1994; Viczian et al., 2003). Antisense riboprobes for Rx1A, Pax6, Sox2, Notch1, NeuroD, and Xic1 were generated as previously described (Mathers et al., 1997; Mizuseki et al., 1998; Ohnuma et al., 1999; Pan et al., 2006). Double section in situ hybridization was performed using digoxigenin-labeled Notch1 and fluorescein labeled NeuroD antisense riboprobes as described previously (Martinez-De Luna and El-Hodiri, 2007). Fast Red (Sigma) was used as the second chromogen in the double in situ hybridization experiments.
Transgenesis
Transgenic Xenopus embryos were generated by the intracytosolic sperm injection (ICSI) method (Sparrow et al., 2000). To make the Rx and control shRNA trangenes, the transgene DNA was released from the vector by restriction digestion with BglII, PstI and SalI, and purified from agarose gel using the Gene Clean kit (QBiogene). ICSI was performed as previously described (Sparrow et al., 2000), using snap frozen sperm nuclei. For the transgenesis reaction 400,000 sperm nuclei were incubated with 250 ng of transgene DNA and 2 μl of sperm dilution buffer (SDB) for 15 min at room temperature. The reaction was then diluted in 22.5 μl and 2.5 μl of this mixture was further diluted in 230 μl of SDB for injection. Cysteine dejellied eggs were injected with 10nl of transgenesis reaction in 0.4 X MMR (Marc’s Modified Ringer’s) + 6% Ficoll. Properly dividing embryos were transferred to 0.1 X MMR + 6% Ficoll and changed to 0.1 X MMR after 24 hrs. Embryos were raised in 0.1 X MMR until the appropriate stage. Control and Rx shRNA and mRx rescue transgenes were prepared as described previously (Pan et al., 2010). Transgenic embryos were selected using a fluorescent microscope with a blue-green filter to detect coral GFP (cGFP) fluorescence derived from the cGFP cassette present in the transgene vector.
Counts of retinal progenitor cells
We counted RPCs using digital images of sectioned regenerating retinas stained with hematoxylin and eosin as described above. RPCs were identified and counted in electronic images of sections through the center of the wound site. Examples are shown in Figure S1. RPCs were identified by shape and stain color. Abnormally-shaped RPCs, often observed in Rx shRNA transgenic tadpoles, were included in our counts. RPCs were counted from 5 different tadpoles (one section each) in each group. Counts were averaged and compared using a 2-tailed Student’s t-test using Prism software (GraphPad, Inc.).
RESULTS
Progression of retinal regeneration in Xenopus laevis
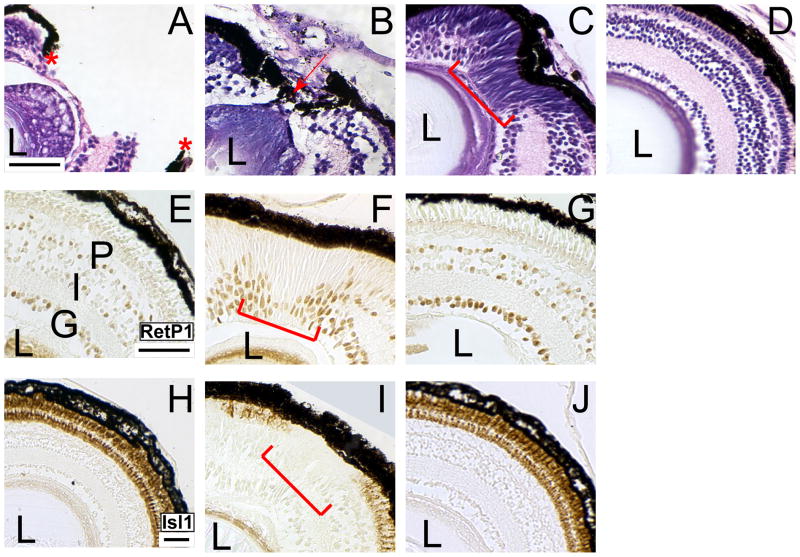
We began our studies of X. laevis tadpole retinal regeneration with histological and molecular characterization of retinal regeneration. To determine the time course of regeneration, we performed histology on regenerating retinas from 1 to 30 days after resection. We found that the retina is essentially regenerated by 30 days post-resection as evidenced by the reorganization of the RPE and the retinal laminae (Figure 1). On day 1, resection of the nasal-dorsal quarter is quite evident because retinal lamination and RPE integrity are disrupted (Figure 1A; asterisks). By 3 days post-resection the RPE begins to wrap around the wound and the wound begins to close (Figure 1B; red arrow). Retinal lamination is still disorganized at this stage (Figure 1B; red arrow). During the second week post-resection (days 8–15), retinal lamination is still incomplete, although the RPE has completely reorganized around the wound (Figure 1C). Interestingly, by this time a group of spindle-shaped cells have repopulated the wound (Figure 1C, red bracket). These cells have the morphology characteristics of retinal progenitor cells (RPCs) that reside in the ciliary marginal zone (CMZ) (Straznicky and Gaze, 1971). The retina appears completely regenerated by 30 days post-resection (Figure 1D). At this point, the regenerated retina is essentially indistinguishable from a control retina with respect to size, morphology, and histology.
Figure 1. The retina is essentially regenerated 30 days after resection.
(A–D) The progress of regeneration was analyzed by hematoxylin and eosin staining. (A) The retina after resection of the nasal-dorsal quarter on day 1. The site of resection is evidenced by the disruption of the retinal lamination and RPE (red asterisks). (B) On post-resection day 3 the RPE has re-assembled around the site of resection (red arrow) and cells have begun to fill in the wound. (C) On post-resection day 13 the RPE has closed around the wound (red arrow) and RPCs have repopulated the wound. (D) On post-resection day 30 the lamination of the retina is completely restored and the resection site is no longer evident. (E J) Analysis of regeneration progress using markers of differentiated neural cell types. Immunolabeling for Islet-1 (E – G) and Rhodopsin (H – J) in control retinas (E, H) and regenerating retinas at 15 days (F, I) and 30 days post-resection (G, J). Control retinas shown in panels E and H are from sibling embryos to those shown in panels G and J, respectively. At 15 days post-resection, the putative RPCs are still present at the site of resection (F, I; red bracket). The putative RPCs are not immunoreactive to Islet 1 (F; red bracket) or Rhodopsin (I; red bracket) antibodies. At 30 days post-resection, the putative RPCs are absent from the nasal-dorsal quarter of the retina and complete retinal lamination is observed by immunoreactivity to Islet-1 (G) and Rhodopsin (J). Uninjured retinas lack putative RPCs in the nasal-dorsal quarter and show Islet-1 and Rhodopsin immunoreactivities (E, H). L-lens; G-ganglion cell layer, I-inner nuclear layer; P-photoreceptor layer. Scale bar = 50 μ.
We then proceeded to confirm the completion of regeneration by immunolabeling retinal sections with Islet-1 and Rhodopsin antibodies at 15 and 30 days post-resection. At 15 days post-resection, the putative RPCs that repopulated the wound are still visible at the resection site, indicating that the retina is not completely re-laminated and that regeneration is incomplete (Figure 1F, I; red bracket). At 30 days post-resection the putative RPCs are no longer observed and the site of resection is not discernible (Figure 1G, J). In addition, both Islet-1 and Rhodopsin immunoreactivities are detected in the nasal-dorsal quarter of the retina where resection was performed, thus showing similar immunoreactivities to both markers in the control retinas (compare Figures 1G and J to 1E and H, respectively).
The putative RPCs that repopulate the wound are actively proliferating and express typical RPC markers
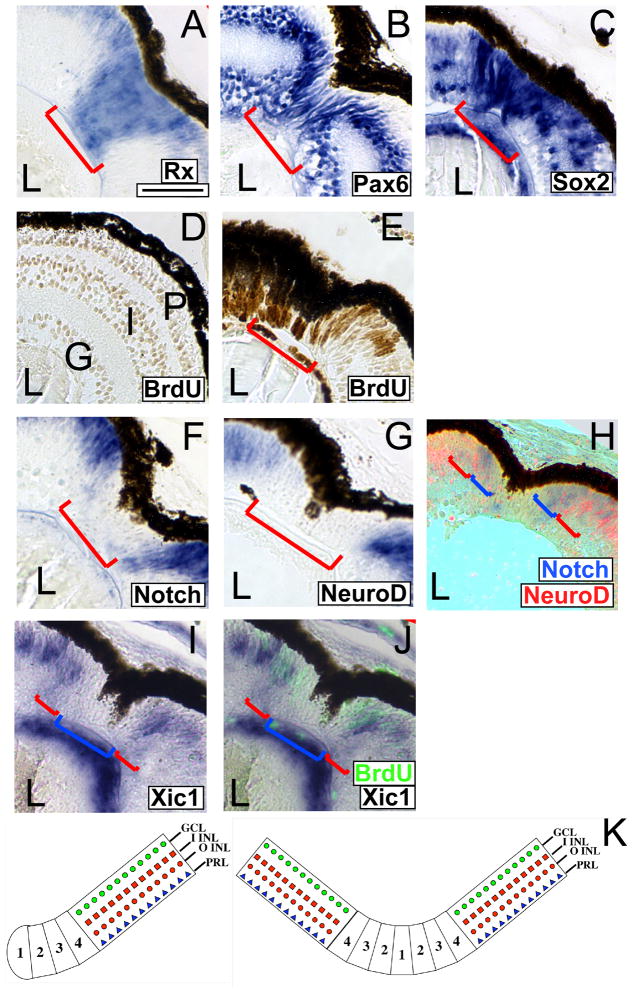
As discussed above, the regenerating retina contains spindle-shaped cells, similar to RPCs, during the second week after resection. RPCs can be identified by their expression of specific markers, including Rx, Pax6 and Sox2 (Casarosa et al., 1997; Hirsch and Harris, 1997; Mathers et al., 1997; Perron et al., 1998; Van Raay et al., 2005). We found that the cells repopulating the wound strongly express Rx1A (Figure 2A; red bracket), Pax6 (Figure 2B; red bracket) and Sox2 (Figure 2C; red bracket). Additionally the RPC-like cells repopulating the wound incorporate BrdU, indicating that they are proliferative (Figure 2E; red bracket). These BrdU positive cells are absent from the nasal-dorsal quarter in uninjured retinas (Figure 2D). Taken together, these results suggest that the cells repopulating the wound are RPCs.
Figure 2. The regenerating wound is populated by retinal progenitor cells and is organized similarly to the CMZ.
(A–C) In situ hybridization performed using retinal sections of embryos at 9 days post-resection. Cells filling the regenerating wound express pan-RPC markers Rx1A (A), Pax6 (B), and Sox2 (C). (D, E) Cells filling the regenerating wound are proliferating. Immunolabeling of regenerating retinas at 9 days post-resection with anti-BrdU antibody. The putative RPCs incorporate BrdU and are immunoreactive to the anti-BrdU antibody (E, red bracket). The nasal-dorsal quarter of an uninjured retina lacks proliferating RPCs (D). (F, G) In situ hybridization performed on sections of embryos at 9 days post-resection with riboprobes for Notch1 (F) or NeuroD (G). (H) Double in situ hybridization for Notch1 (blue) and NeuroD (red). Different subsets of the RPCs (red) express Notch1 and NeuroD. Notch is expressed closer to center of the wound (H; blue brackets) than NeuroD (H; red brackets) confirming that the expression of these two markers begins in different subsets of the RPCs that repopulate the wound. (I, J) The cyclin-dependent kinase inhibitor Xic1 is expressed at the extreme periphery of the regenerating region. (I) In situ hybridization for Xic1 (red brackets) demonstrates expression at the periphery of the regenerating wound and not in the center (blue bracket). (J) Overlay of BrdU incorporation (fluorescent green) and Xic1 in situ hybridization from (I). Proliferating cells are largely in the center of the regenerating wound (blue bracket), with little overlap with cells expressing Xic1 (red brackets). (K) Left - Model of normal CMZ (adapted from (Perron et al., 1998). Right - Model of the CMZ formed in the regenerating wound. Scale bar = 50 μ.
The RPCs repopulating the wound are organized similarly to the CMZ
The CMZ can be divided into four zones based on expression of molecular markers (Perron et al., 1998). In this model, the most stem cell-like progenitors are located in zone 1 and the most determined cells are found in zone 4 (Perron et al., 1998). Rx and Pax6 are expressed throughout the CMZ of the tadpole retina and we observed their expression throughout the regenerating portion of the retina (Figure 2A, B). The RPCs in the regenerating retina also expressed Notch1, NeuroD, and Xic1 (Figure 2F, G, I), markers of CMZ zones 2, 3, and 4 respectively (Perron et al., 1998). None of these markers were expressed in the RPCs at the center of the wound. Further, NeuroD was absent from a region of the regenerating wound that expressed Notch1 (Figure 2H). Xic1 was expressed at the periphery of the wound and was largely excluded from proliferating cells at the center of the wound (Figure 2J). This organization was reminiscent of the organization of the CMZ at the retinal periphery, where Notch is expressed in zones 2–4 and NeuroD is expressed in zones 3–4, and Xic1 is primarily expressed in zone 4 (Perron et al., 1998). It is not surprising that there was some overlap between BrdU-positive cells and Xic1-expressing cells, as it has recently been demonstrated that Xic1 is expressed in some proliferating RPCs (Bilitou and Ohnuma, 2010). These results suggest that the RPCs are organized into zones, similar to the endogenous CMZ, at this stage of retinal regeneration (Figure 2K).
Reduction of Rx expression impairs retinal regeneration in Xenopus laevis
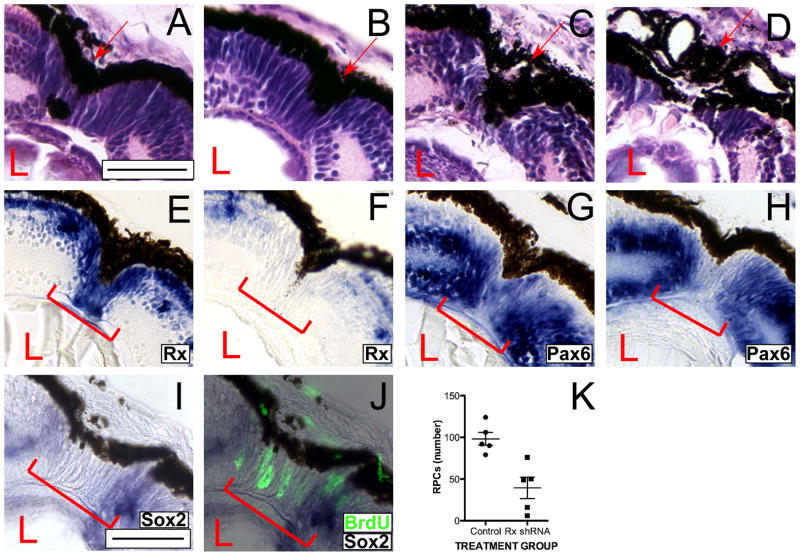
To investigate the involvement of Rx in retinal regeneration, we used a transgenic shRNA approach to knock down Rx expression (Pan et al., 2010). Previously, we demonstrated that Rx expression is knocked down 50–90% in Rx shRNA transgenics but that the eye develops with apparently normal morphology through st 41. To address the function of Rx during retinal regeneration, we induced regeneration in Rx shRNA transgenic tadpoles. We found that regeneration is impaired in Rx shRNA transgenics (Figures 3C,D) as compared to nontransgenic controls (Figure 3A) and control shRNA transgenics (Figure 3B). The wound is disorganized at 9 days post-resection (Figure 3C, D). In some tadpoles the cells repopulating the wound do not appear to be normal RPCs. The cells have a rounder morphology than the typical spindle-shaped RPCs found at the CMZ (Figures 3C and S1). In some cases, the cells repopulating the wound lack the columnar organization we had previously observed in the RPCs that repopulate the wound by 9 days post-resection. Others have both defects in RPE reformation and RPC repopulation of the wound. We did not find tadpoles in which only the RPE regeneration at the wound site was defective. On the other hand, in some tadpoles, the RPE is either not completely reformed at the wound site or is disorganized (Figure 3C, D). To quantify our observations we developed a classification of the regeneration defects we found in Rx shRNA transgenic tadpoles (Table 1). Based on the regeneration defects, regenerating embryos were classified into 3 categories, defined by morphological criteria. Using this classification system we found that 72% (p < 0.0001 compared to non-transgenic controls) of the scored Rx shRNA tadpoles had abnormal retinal regeneration (Table 1 and Figure 4I). Of these, 52% of the Rx shRNA tadpoles were classified in category 2 and 20% were classified in category 3. Essentially all nontransgenic controls and control shRNA transgenic embryos were classified in category 1.
Figure 3. Retinal regeneration is abnormal in Rx knockdown tadpoles.
(A–D) Histological staining of regenerating retinas of a control non-transgenic tadpole (A), a control shRNA transgenic tadpole (B), and Rx shRNA transgenic tadpoles scored at 9 days post-resection (C, D). Rx shRNA transgenic tadpoles display shorter and/or rounder RPCs that are sometimes disorganized (C) and incompletely re-formed, disorganized RPE (D). (E–H) Rx (E, F) and Pax6 (G, H) expression are markedly reduced in the cells that repopulate the wound in Rx shRNA transgenic tadpoles. In situ hybridization on retinal sections of regenerating retinas from Rx shRNA transgenic tadpoles (F, H) and control non-transgenic tadpoles (E, G). Rx expression is markedly reduced in the cells that repopulate the wound in Rx shRNA transgenic tadpoles, but is not reduced in the Rx expressing cells at the INL (F). Pax6 expression is also reduced in the cells repopulating the wound in Rx shRNA transgenic tadpoles, but not in the INL or GCL (H). (I, J) Expression of Sox2 is also markedly reduced in the cells that repopulate the wound (I, red bracket). (J) Overlay of panel I with BrdU incorporation visualized by immunofluorescence (fluorescent green color). Arrow indicates RPE at the wound site; bracket indicates RPCs at the wound site. Scale bar = 50 μ. (K) Number of RPCs in the wound sites of regenerating retinas from control nontransgenic or Rx shRNA transgenic tadpoles. Each dot represents the RPC count from a single regenerating retina. Horizontal bar represents average of the 5 counts shown; vertical bar represents standard deviation from the mean for each group.
Table 1.
Classification of regeneration defects observed in Rx shRNA transgenic embryos.
| Classification | Regeneration defect | Regeneration phenotype | Non-transgenic control1 | Control shRNA2 | Rx shRNA3 | Rescue4 |
|---|---|---|---|---|---|---|
| Category 1 | No defect | Elongated RPCs that span all retinal layers at the wound site | 21/22 95.4% |
16/16 100% |
7/25 28% |
7/13 53.8% |
| Category 2 | Defective RPCs | RPCs are shorter and/or rounder and sometimes disorganized at wound site | 0/22 0% |
0/16 0% |
13/25 52% |
5/13 38.4% |
| Category 3 | Defective RPCs and RPE | RPCs are defective as described above and the RPE is disorganized; not completely reformed around the wound | 1/22 4.5% |
0/16 0% |
5/25 20% |
1/13 7.7% |
Morphological defects in retinal regeneration were scored at 9 days post-resection and the defects were assigned to each category
Non-transgenic control-wild type tadpoles
Control shRNA - tadpoles transgenic for the control shRNA
Rx shRNA- tadpoles transgenic for the Rx shRNA
Rescue- tadpoles co-transgenic for the Rx shRNA and the mRx transgene
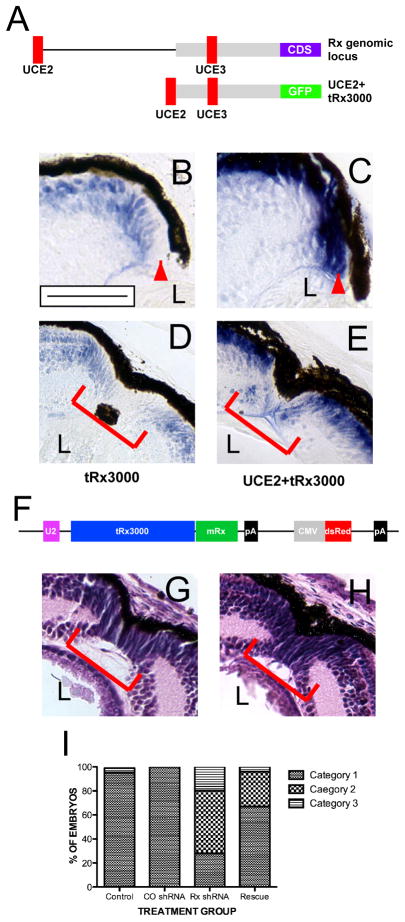
Figure 4. The effects of Rx knockdown on regeneration can be rescued by mouse Rx.
(A) Upper construct: Schematic of the X. tropicalis Rx (tRx) genomic locus showing the relative positions of ultraconserved genomic elements UCE2 and 3 (red) within the tRx regulatory region (gray). The Rx coding region (CDS) is indicated (blue). Lower construct: transgene containing a 3 kb portion of the X. tropicalis Rx locus (tRx3000), UCE2, and a GFP expression cassette (green). (B – E) In situ hybridization using a GFP antisense riboprobe using sections of uninjured (B, C) or regenerating transgenic tadpoles (D, E). The tRx3000/GFP transgene is not expressed in the in the RPCs at the distal tip of the CMZ (B, red arrowhead) or RPCs at the center of the regenerating wound (D). Addition of UCE2 drives transgene expression in RPCs throughout the CMZ (C) and the regenerating wound (E). (F) Schematic of mRx rescue construct, containing X. tropicalis Rx transcriptional regulatory elements as shown in (A) and the mouse Rx coding region (green). (G, H) Hematoxylin and eosin staining of retinal sections from a non-transgenic tadpole (G) and a Rx shRNA+ rescue tadpole (H) at day 9 post-resection. (I) Quantification of regeneration impairment in Rx shRNA transgenic tadpoles relative to nontransgenic controls, control (CO) shRNA transgenic tadpoles, and tadpoles co-transgenic for mRx. Categories of phenotype severity are defined in Table 1. Scale bar = 50 μ.
We previously demonstrated that Rx knockdown tadpoles lose visual function at st 50, at which point photoreceptors degenerate (Pan et al., 2010). We carried out our regeneration experiments to 30 days, the point at which regeneration appears to be complete (Figure 1D, G, J), and the tadpoles develop well past st 50. These tadpoles exhibited failed regeneration at the same frequency described above (data not shown), consistent with our observations at day 9. Most of the cases exhibiting failed regeneration also lacked photoreceptor outer segments or photoreceptors entirely (data not shown), consistent with our previous observations (Pan et al., 2010).
We also observed fewer RPCs in the wound site of Rx shRNA transgenic tadpoles as compared to control tadpoles. We counted the number of RPCs in the wound sites of 5 shRNA transgenic tadpoles and 5 control tadpoles and found that there an average of 98.2 ± 17.3 RPCs per section (range: 79–124 RPCs per section) in the wound sites of control nontransgenic tadpoles and 39.4 ± 28.3 RPCs per section (range: 6–76 RPCs per section) in the wound sites of Rx shRNA transgenic tadpoles (Figure 3K). There were significantly fewer RPCs in the wound sites of Rx shRNA transgenic tadpoles (p < 0.0012). Based on these results, we concluded that reduction of Rx expression levels results in impaired retinal regeneration.
Expression of RPC markers are reduced in the wound of Rx shRNA transgenics
We had previously established that the RPCs that repopulate the wound have the molecular profile of RPCs, expressing Rx, Pax6 and Sox2 (Figure 2). We similarly analyzed cells repopulating the wound in Rx shRNA transgenics (Figure 3). We observed that Rx expression is reduced overall in Rx shRNA transgenic tadpoles and essentially undetectable in the cells that repopulate the wound (Figure 3F; red bracket). As we have seen before, Rx is strongly expressed in the cells that repopulate the wound in control non-transgenic tadpoles (Figure 3E).
We found that Pax6 expression is also reduced in the cells that repopulate the wound (Figure 3H; red bracket). Despite the marked reduction of Pax 6 in the cells repopulating the wound, normal Pax6 expression is observed in the INL and GCL (Figure 3H). Pax6 is strongly expressed in the RPCs that repopulate the wound in control non-transgenic tadpoles (Figure 3G; red bracket). These results are in agreement with the failure of other EFTFs to be upregulated in the ventral neuroectoderm of Rx deletion mice (Zhang et al., 2000). Finally, the cells that repopulate the wound also express diminished levels of Sox2 (Figure 3I), although they continue to proliferate (Figure 3J). From these results we conclude that the cells repopulating the wound in Rx shRNA transgenic tadpoles lack the molecular profile of RPCs.
The regeneration defect in Rx shRNA transgenic tadpoles can be rescued by introduction of a mouse Rx transgene
We previously demonstrated that the effects of the Rx shRNA are specific to knock down of Rx expression since the developmental effects of the Rx shRNA can be rescued by a transgene expressing mouse Rx (mRx) under the control of Rx regulatory elements (Pan et al., 2010). The rescue transgene contained 3 kb of the X. tropicalis Rx regulatory region (tRx3000) and an ultraconserved genomic element (UCE) we termed UCE2 (Figure 4A). tRx3000 directs expression of a GFP reporter in a similar pattern as the endogenous Rx gene, but is notably lacking from the distal CMZ (Figure 4B) (Pan et al., 2010). Addition of UCE2 to the tRx3000 results in transgene expression throughout the entire CMZ (Figure 4C). Similarly, we found that tRx3000/GFP is not expressed in the RPCs at the center of the regenerating wound (Figure 4D). Addition of UCE2 drives expression of the transgene throughout the regenerating wound at 9 days post-resection (Figure 4E). From this analysis, we concluded that UCE2 is necessary for Rx promoter activity in retinal stem cells during retinal regeneration.
We found that the mRx transgene (Figue 4F) also rescues the regeneration defects observed in the retina of Rx shRNA transgenic embryos at 9 days post-resection (Figure 4G – I). The RPE was completely reformed at the wound site and morphologically normal RPCs repopulate the wound by 9 days post-resection in rescue transgenics (Figure 4H). Using our regeneration classification system, we found that 67% (n=24) of the rescue transgenic tadpoles lacked regeneration defects at 9 days post-resection and were classified in category 1 (p=0.0186), 29.1% (n=7) of the rescue transgenic tadpoles appeared to have defects in generation of RPC at the wound (category 2), and 4.2% (n=1) had both RPC and RPE defects (Figure 4I). Our results are consistent with rescue of the regeneration defects by co-expression of mRx, suggesting that Rx is specifically required for retinal regeneration.
DISCUSSION
In this paper we investigated the cellular and molecular mechanisms underlying retinal regeneration in pre-metamorphic X. laevis. Previous studies demonstrated that the tadpole retina regenerates and establishes retinotectal neural connections after resection of up to two-thirds of the retina in pre-metamorphic X. laevis (Ide et al., 1984; Ide et al., 1987), but largely did not investigate the molecular and cellular details of regeneration. Our study is the first to provide a histological and molecular characterization of regeneration in pre-metamorphic X. laevis. We found that regeneration is essentially complete by 30 days after resection and that regeneration occurs, involving repopulation of the wound by RPCs. Additionally, little is known about the molecular events that underlying retinal regeneration. It has been established that Rx function is essential for eye development. In the present work, we show that Rx function is also necessary during retinal regeneration. Reduction of Rx expression levels resulted in a lack of RPC generation at the wound site of the regenerating retina. We propose that Rx may be necessary for recruitment of RPCs during retinal regeneration.
Retinal regeneration in pre-metamorphic X. laevis is mediated by the induction of RPCs organized as in the CMZ
We found that RPCs are induced at the wound site after resection and that they are organized into a CMZ-like structure. A similar CMZ-like structure was observed as a new proliferative zone in the central retina of R. catesbiana tadpoles (Reh and Nagy, 1987). This proliferative zone seemed to give rise to new retina and it was discontinuous with the RPE-derived regenerate (Reh and Nagy, 1987). The formation and organization of a CMZ-like structure in our regeneration model is in line with the concept that regeneration recapitulates development.
The X. laevis retina CMZ has been systematically classified into zones according to RPC maturity and marker gene expression (Perron et al., 1998). We found that the CMZ-like structure induced during regeneration is organized in a similar fashion to the endogenous CMZ. First, all of the repopulating RPCs express Rx and Pax6. Additionally, consistent with a CMZ-like organization, Notch1 and NeuroD are only expressed in RPCs outside the center of the wound. Finally, Notch1 is expressed closer to the center of the wound than NeuroD. These results suggest that the center of the CMZ-like structure corresponds to zone 1 of the endogenous CMZ, contains retinal stem cells, and flanked by zones 2–4, arranged sequentially from the center of the regenerating wound outwards (Figure 2K). However, there are differences between the CMZ-like structure generated during regeneration and the endogenous CMZ that develops at the periphery of the neural retina. First, we observed that Sox2 is expressed throughout the CMZ-like structure of the regenerating retina, including the RPCs at the center. Additionally, we observed that all of the repopulating RPCs rapidly incorporate BrdU after a short pulse. Normally the retinal stem cells at the periphery of the CMZ divide slowly and express Rx and Pax6 (Perron et al., 1998) but not Sox2 (Van Raay et al., 2005). Nevertheless, it appears that the RPCs at the site of regeneration are organized essentially as a CMZ, as illustrated in Figure 2K.
Reduced levels of Rx expression impair retinal regeneration and change the identity of the cells repopulating the wound
In the present study we show that significantly reduced Rx expression levels impaired retinal regeneration in Rx shRNA transgenic tadpoles. The RPE at the wound site is disorganized and the cells repopulating the wound are rounder and shorter than the RPCs that repopulate the wound in wild type embryos. Regeneration involves either transdifferentiation of a mature, post-mitotic cell or proliferation of intrinsic stem cells (Del Rio-Tsonis and Tsonis, 2003). During transdifferentiation, differentiated cells give rise to an undifferentiated neuroepithelium from which all retinal cell types are specified and generated (Del Rio-Tsonis and Tsonis, 2003). Intrinsic stem cells at the CMZ in X. laevis constantly proliferate and add new cells to the retinal margin (Straznicky and Gaze, 1971). RPE transdifferentiation as well as addition of cells from the CMZ contribute to retinal regeneration in adult X. laevis after complete retinectomy (Yoshii et al., 2007). In either case, an immature neuroepithelium forms at the wound site and acts as a source of regenerated retinal neurons. Thus, it is possible that the regeneration defects observed in the Rx shRNA retina are due to incomplete specification of RPCs. Just as the RPC markers Six3, Otx2 and Pax6 are not upregulated in the presumptive optic cup primordium of Rx null mice (Zhang et al., 2000), Pax6 and Sox2 expression are markedly reduced in the cells that repopulate the wound in Rx shRNA tadpoles. This result suggests that regenerating RPCs perhaps are not properly specified in Rx shRNA transgenic tadpoles.
Alternatively, Rx knockdown could impair regeneration by leading to a drastic reduction in proliferation. Previous studies have demonstrated that Rx regulates the proliferation of retinal progenitors (Casarosa et al., 2003). Overexpressing Rx leads to an increase in the production of retinal cells, while expression of dominant negative form of Rx has the opposite effect (Casarosa et al., 2003). Since proliferation is required during regeneration for the production of retinal tissue, severe reduction in Rx expression levels could lead to regeneration defects. Although we did not test whether proliferation was reduced in the Rx shRNA retina, our histological analysis suggests that fewer cells appear to repopulate the wound. It would be interesting to examine whether fewer cells are indeed produced and whether this results in the morphology changes we observed in the repopulating cells of Rx shRNA tadpoles.
mRx rescues retinal regeneration in Rx shRNA transgenic tadpoles
We found that mRx can rescue the regeneration defects observed in Rx knockdown tadpoles, even though mice (and other higher vertebrates) exhibit extremely limited retinal regeneration capacity. This result indicates that expression of mRx under the control of X. tropicalis transcriptional regulatory elements is sufficient to rescue the effects of the Rx shRNA, indicating that the effects of the Rx shRNA are specific to reduction of Rx expression. Notably, tadpoles transgenic for both mRx and Rx shRNA develop morphologically normal RPCs at the regeneration site, reinforcing the finding that Rx expression is necessary for recruitment of RPCs during retinal regeneration. Further, this result demonstrates that mRx is capable of functioning to promote RPC development in the regenerating retinas. It is interesting to speculate that the lack of regenerative capability of higher vertebrates may be due, at least in part, to an inability to activate Rx expression in response to retinal damage. Activation of Rx expression is necessary for the formation of RPCs in our tadpole retinal regeneration model and is necessary for the formation of RPCs in embryonic development of many, if not all, vertebrates. Perhaps the lack of retinal regeneration in higher vertebrates stems, at least in part, to an inability to activate Rx and form RPCs in response to retinal injury.
Supplementary Material
Examples demonstrating identification of RPCs in sections of retinas from regenerating control nontransgenic or Rx shRNA transgenic tadpoles (as labeled). The Rx shRNA section shown here is the same section shown in Figure 3C. Only some cells are highlighted in each panel to demonstrate RPC identification. In the lower panel, we show examples of normal (N) and abnormal (A) RPCs, both included in our counts.
Acknowledgments
We thank Natalia Vergara for help with the BrdU incorporation procedure, Milan Jamrich, Teri Belecky-Adams, Andy Fischer, Katia Del Rio-Tsonis and their labs for helpful comments and criticisms, and Amy Sater for critical reading of the manuscript. This work was supported by NIH/NEI grant EY015480 to H.M.E. and a Young Investigators Student Fellowship Award for Female Scholars in Vision Research from Prevent Blindness Ohio to R.I.M. Antibodies to BrdU and Islet-1 (G3G4 and 39.4D5, respectively) developed by S.J. Laufman and T. Brenner-Morton/T.M. Jessell (respectively) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Andreazzoli M, Gestri G, Angeloni D, Menna E, Barsacchi G. Role of Xrx1 in Xenopus eye and anterior brain development. Development. 1999;126:2451–2460. doi: 10.1242/dev.126.11.2451. [DOI] [PubMed] [Google Scholar]
- Andreazzoli M, Gestri G, Cremisi F, Casarosa S, Dawid IB, Barsacchi G. Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate. Development. 2003;130:5143–5154. doi: 10.1242/dev.00665. [DOI] [PubMed] [Google Scholar]
- Araki M. Regeneration of the amphibian retina: role of tissue interaction and related signaling molecules on RPE transdifferentiation. Dev Growth Differ. 2007;49:109–120. doi: 10.1111/j.1440-169X.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- Arresta E, Bernardini S, Bernardini E, Filoni S, Cannata SM. Pigmented epithelium to retinal transdifferentiation and Pax6 expression in larval Xenopus laevis. J Exp Zoolog A Comp Exp Biol. 2005;303:958–967. doi: 10.1002/jez.a.219. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilitou A, Ohnuma S. The role of cell cycle in retinal development: cyclin-dependent kinase inhibitors co-ordinate cell-cycle inhibition, cell-fate determination and differentiation in the developing retina. Dev Dyn. 2010;239:727–736. doi: 10.1002/dvdy.22223. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Amato MA, Andreazzoli M, Gestri G, Barsacchi G, Cremisi F. Xrx1 controls proliferation and multipotency of retinal progenitors. Mol Cell Neurosci. 2003;22:25–36. doi: 10.1016/s1044-7431(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Andreazzoli M, Simeone A, Barsacchi G. Xrx1, a novel Xenopus homeobox gene expressed during eye and pineal gland development. Mech Dev. 1997;61:187–198. doi: 10.1016/s0925-4773(96)00640-5. [DOI] [PubMed] [Google Scholar]
- Chen CM, Cepko CL. The chicken RaxL gene plays a role in the initiation of photoreceptor differentiation. Development. 2002;129:5363–5375. doi: 10.1242/dev.00114. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech Dev. 1999;84:195–198. doi: 10.1016/s0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Raymond PA. Zebrafish genes rx1 and rx2 help define the region of forebrain that gives rise to retina. Dev Biol. 2001;231:13–30. doi: 10.1006/dbio.2000.0125. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- Deschet K, Bourrat F, Ristoratore F, Chourrout D, Joly JS. Expression of the medaka (Oryzias latipes) Ol-Rx3 paired-like gene in two diencephalic derivatives, the eye and the hypothalamus. Mech Dev. 1999;83:179–182. doi: 10.1016/s0925-4773(99)00037-4. [DOI] [PubMed] [Google Scholar]
- El-Hodiri HM, Shou W, Etkin LD. xnf7 functions in dorsal-ventral patterning of the Xenopus embryo. Dev Biol. 1997;190:1–17. doi: 10.1006/dbio.1997.8692. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24:161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U S A. 1997;94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. J Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- Hollyfield JG. Differential growth of the neural retina in Xenopus laevis larvae. Dev Biol. 1971;24:264–286. doi: 10.1016/0012-1606(71)90098-4. [DOI] [PubMed] [Google Scholar]
- Ide CF, Reynolds P, Tompkins R. Two healing patterns correlate with different adult neural connectivity patterns in regenerating embryonic Xenopus retina. J Exp Zool. 1984;230:71–80. doi: 10.1002/jez.1402300110. [DOI] [PubMed] [Google Scholar]
- Ide CF, Wunsh LM, Lecat PJ, Kahn D, Noelke EL. Healing modes correlate with visuotectal pattern formation in regenerating embryonic Xenopus retina. Dev Biol. 1987;124:316–330. doi: 10.1016/0012-1606(87)90485-4. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL. La regenerescence de la retine chez Xenopus laevis. Dev Can Biol. 1981;40:19–27. [Google Scholar]
- Lombardo F. Regeneration of the neural retina in adult anurian amphibians. Arch Ital Anat Embriol. 1969;74:29–44. [PubMed] [Google Scholar]
- Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, Baier H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4:894–899. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F, Winkler S, Burgtorf C, Wurmbach E, Ansorge W, Henrich T, Grabher C, Arendt D, Carl M, Krone A, Grzebisz E, Wittbrodt J. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 2001;128:4035–4044. doi: 10.1242/dev.128.20.4035. [DOI] [PubMed] [Google Scholar]
- Martinez-De Luna RI, El-Hodiri HM. The Xenopus ortholog of the nuclear hormone receptor Nr2e3 is primarily expressed in developing photoreceptors. Int J Dev Biol. 2007;51:235–240. doi: 10.1387/ijdb.062236rm. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48:1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Garland Publishing, Inc; New York: 1994. [Google Scholar]
- Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- Pan Y, Martinez-De Luna RI, Lou CH, Nekkalapudi S, Kelly LE, Sater AK, El-Hodiri HM. Regulation of photoreceptor gene expression by the retinal homeobox (Rx) gene product. Dev Biol. 2010;339:494–506. doi: 10.1016/j.ydbio.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Nekkalapudi S, Kelly LE, El-Hodiri HM. The Rx-like Homeobox Gene (Rx-L) Is Necessary for Normal Photoreceptor Development. Invest Ophthalmol Vis Sci. 2006;47:4245–4253. doi: 10.1167/iovs.06-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199:185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001;58:296–305. doi: 10.1159/000057571. [DOI] [PubMed] [Google Scholar]
- Reh TA, Fischer AJ. Retinal stem cells. Methods Enzymol. 2006;419:52–73. doi: 10.1016/S0076-6879(06)19003-5. [DOI] [PubMed] [Google Scholar]
- Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36:206–220. [PubMed] [Google Scholar]
- Reh TA, Nagy T. A possible role for the vascular membrane in retinal regeneration in Rana catesbienna tadpoles. Dev Biol. 1987;122:471–482. doi: 10.1016/0012-1606(87)90311-3. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hirano S, McMahon AP, Takeichi M. Wnt-1-dependent regulation of local E-cadherin and alpha N-catenin expression in the embryonic mouse brain. Development. 1994;120:2225–2234. doi: 10.1242/dev.120.8.2225. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Sologub AA. Metaplastic transformation of the tissue of the eye in tadpoles and adult Xenopus laevis frogs. Ontogenez. 1975;6:563–571. [PubMed] [Google Scholar]
- Sparrow DB, Latinkic B, Mohun TJ. A simplified method of generating transgenic Xenopus. Nucleic Acids Res. 2000;28:E12. doi: 10.1093/nar/28.4.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Aycinena JC, Del Rio-Tsonis K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol Vis. 2007;13:57–65. [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K. The hedgehog pathway is a modulator of retina regeneration. Development. 2004;131:4607–4621. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The growth of the retina in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1971;26:67–79. [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Vergara MN, Del Rio-Tsonis K. Retinal regeneration in the Xenopus laevis tadpole: a new model system. Mol Vis. 2009;15:1000–1013. [PMC free article] [PubMed] [Google Scholar]
- Viczian AS, Vignali R, Zuber ME, Barsacchi G, Harris WA. XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus retina. Development. 2003;130:1281–1294. doi: 10.1242/dev.00343. [DOI] [PubMed] [Google Scholar]
- Voronina VA, Kozhemyakina EA, O’Kernick CM, Kahn ND, Wenger SL, Linberg JV, Schneider AS, Mathers PH. Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Hum Mol Genet. 2004;13:315–322. doi: 10.1093/hmg/ddh025. [DOI] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol. 2007;303:45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28:135–142. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples demonstrating identification of RPCs in sections of retinas from regenerating control nontransgenic or Rx shRNA transgenic tadpoles (as labeled). The Rx shRNA section shown here is the same section shown in Figure 3C. Only some cells are highlighted in each panel to demonstrate RPC identification. In the lower panel, we show examples of normal (N) and abnormal (A) RPCs, both included in our counts.