Abstract
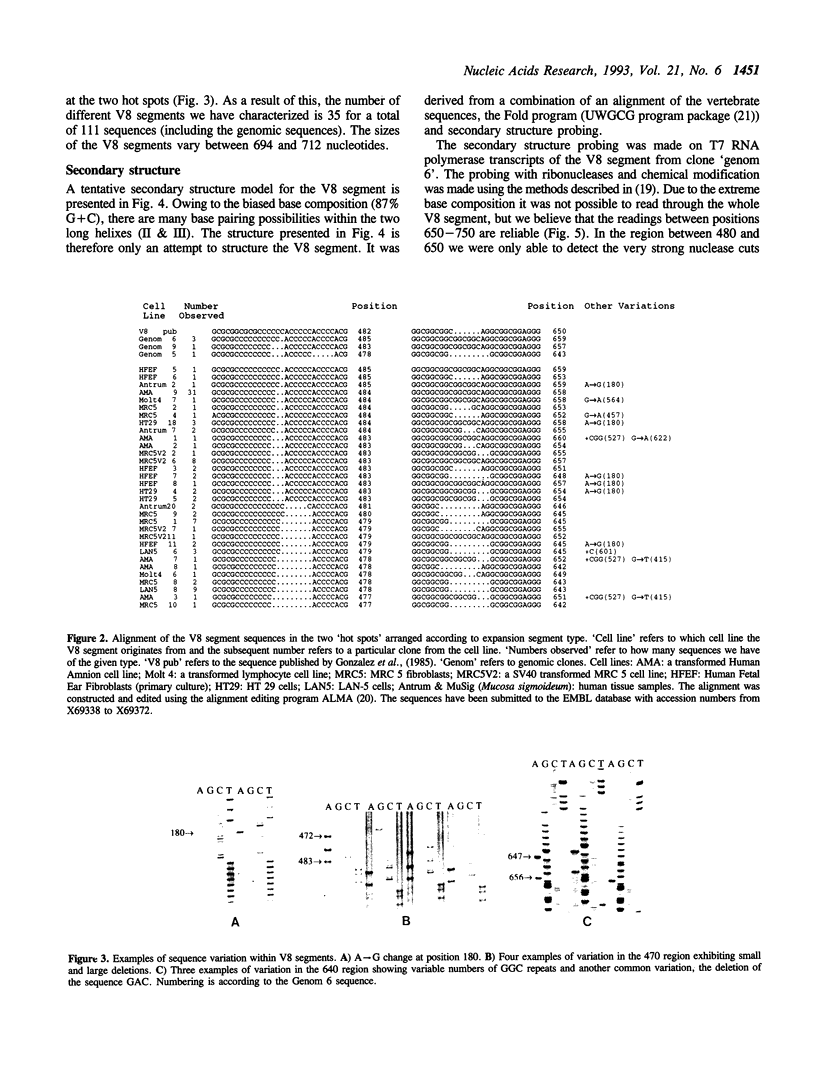
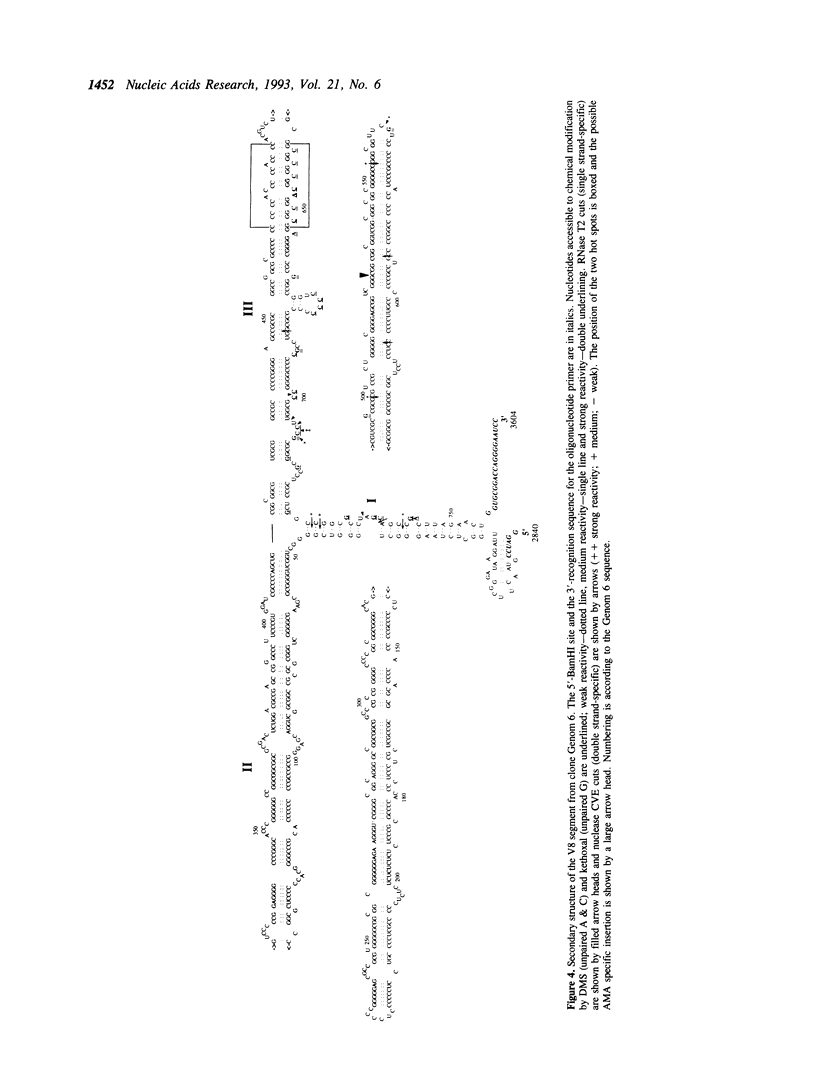
The primary structure of 28S ribosomal RNA constitutes a conserved core which is similar among most 23S-like rRNAs and expansion segments which occur at specific positions in the sequence. The expansion segments account for most of the size difference between prokaryotic (archaeal and eubacterial) and eukaryotic rRNAs and they exhibit a sequence variation which is unique among rRNAs. We have investigated the sequence variation of one of the expansion segments, V8, by sequencing a total of 111 V8 segments from 9 different human cell lines and tissues and have found 35 different variants. The variation occur mainly at two 'hot spots' which are separated by 170 nucleotides in the primary sequence but are neighbours in the secondary structure. The sequence of V8 segments varies both within and between human cell lines and tissues. The implications for the evolution of the eukaryotic 28S rRNA are discussed together with possible functions of the expansion segments. We also present a secondary structure model for the V8 segment based on comparative sequence analysis and chemical and enzymatic foot printing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W., Barker R. System for DNA sequencing with resolution of up to 600 base pairs. J Biochem Biophys Methods. 1984 Mar;9(1):33–47. doi: 10.1016/0165-022x(84)90064-2. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross K., Krone W. On the number of ribosomal RNA genes in man. Humangenetik. 1972;14(2):137–141. doi: 10.1007/BF00273298. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. G., Tague B. W., Ware V. C., Gerbi S. A. Xenopus laevis 28S ribosomal RNA: a secondary structure model and its evolutionary and functional implications. Nucleic Acids Res. 1984 Aug 10;12(15):6197–6220. doi: 10.1093/nar/12.15.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conconi A., Widmer R. M., Koller T., Sogo J. M. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell. 1989 Jun 2;57(5):753–761. doi: 10.1016/0092-8674(89)90790-3. [DOI] [PubMed] [Google Scholar]
- Degnan B. M., Yan J., Hawkins C. J., Lavin M. F. rRNA genes from the lower chordate Herdmania momus: structural similarity with higher eukaryotes. Nucleic Acids Res. 1990 Dec 11;18(23):7063–7070. doi: 10.1093/nar/18.23.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J., Leffers H., Christensen A., Andersen H., Garrett R. A. Structure and accessibility of domain I of Escherichia coli 23 S RNA in free RNA, in the L24-RNA complex and in 50 S subunits. Implications for ribosomal assembly. J Mol Biol. 1987 Jul 5;196(1):125–136. doi: 10.1016/0022-2836(87)90515-8. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Sulston J. E., Coulson A. R. The rDNA of C. elegans: sequence and structure. Nucleic Acids Res. 1986 Mar 11;14(5):2345–2364. doi: 10.1093/nar/14.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Nielsen H. Complete sequence of the extrachromosomal rDNA molecule from the ciliate Tetrahymena thermophila strain B1868VII. Nucleic Acids Res. 1990 Dec 11;18(23):6915–6919. doi: 10.1093/nar/18.23.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev O. I., Nikolaev N., Hadjiolov A. A., Skryabin K. G., Zakharyev V. M., Bayev A. A. The structure of the yeast ribosomal RNA genes. 4. Complete sequence of the 25 S rRNA gene from Saccharomyces cerevisae. Nucleic Acids Res. 1981 Dec 21;9(24):6953–6958. doi: 10.1093/nar/9.24.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez I. L., Gorski J. L., Campen T. J., Dorney D. J., Erickson J. M., Sylvester J. E., Schmickel R. D. Variation among human 28S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7666–7670. doi: 10.1073/pnas.82.22.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez I. L., Sylvester J. E., Schmickel R. D. Human 28S ribosomal RNA sequence heterogeneity. Nucleic Acids Res. 1988 Nov 11;16(21):10213–10224. doi: 10.1093/nar/16.21.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. L., Gonzalez I. L., Schmickel R. D. The secondary structure of human 28S rRNA: the structure and evolution of a mosaic rRNA gene. J Mol Evol. 1987;24(3):236–251. doi: 10.1007/BF02111237. [DOI] [PubMed] [Google Scholar]
- Gubler U. A one tube reaction for the synthesis of blunt-ended double-stranded cDNA. Nucleic Acids Res. 1988 Mar 25;16(6):2726–2726. doi: 10.1093/nar/16.6.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L., Wollett G., Hollingdale M., de la Cruz V. F., Waters A. P., McCutchan T. F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- Hancock J. M., Dover G. A. 'Compensatory slippage' in the evolution of ribosomal RNA genes. Nucleic Acids Res. 1990 Oct 25;18(20):5949–5954. doi: 10.1093/nar/18.20.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassouna N., Michot B., Bachellerie J. P. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 1984 Apr 25;12(8):3563–3583. doi: 10.1093/nar/12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G. E., Orlowski M. Primary and secondary structure of the 25S rRNA from the dimorphic fungus Mucor racemosus. Curr Genet. 1990 Jun;17(6):499–506. doi: 10.1007/BF00313078. [DOI] [PubMed] [Google Scholar]
- Kiss T., Kis M., Solymosy F. Nucleotide sequence of a 25S rRNA gene from tomato. Nucleic Acids Res. 1989 Jan 25;17(2):796–796. doi: 10.1093/nar/17.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers G., Maroteaux L., Michot B., Herzog M. Dinoflagellates in evolution. A molecular phylogenetic analysis of large subunit ribosomal RNA. J Mol Evol. 1989 Jul;29(1):40–51. doi: 10.1007/BF02106180. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Dent C. L., Farrell T. E., Garde J., McCallum F. S., Wakeman J. A. Clones of human ribosomal DNA containing the complete 18 S-rRNA and 28 S-rRNA genes. Characterization, a detailed map of the human ribosomal transcription unit and diversity among clones. Biochem J. 1987 Sep 1;246(2):519–527. doi: 10.1042/bj2460519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot B., Hassouna N., Bachellerie J. P. Secondary structure of mouse 28S rRNA and general model for the folding of the large rRNA in eukaryotes. Nucleic Acids Res. 1984 May 25;12(10):4259–4279. doi: 10.1093/nar/12.10.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Nomiyama H., Yoshida H., Kukita T., Kuhara S., Sakaki Y. Complete nucleotide sequence of the 26S rRNA gene of Physarum polycephalum: its significance in gene evolution. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3163–3167. doi: 10.1073/pnas.80.11.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Hoshikawa Y., Iida Y., Iwabuchi M. Sequence analysis of the transcribed and 5' non-transcribed regions of the ribosomal RNA gene in Dictyostelium discoideum. Nucleic Acids Res. 1984 May 25;12(10):4171–4184. doi: 10.1093/nar/12.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L. H., Nicoloso M., Bachellerie J. P. A sequence dimorphism in a conserved domain of human 28S rRNA. Uneven distribution of variant genes among individuals. Differential expression in HeLa cells. Nucleic Acids Res. 1991 Mar 11;19(5):1015–1019. doi: 10.1093/nar/19.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathgeber J., Capesius I. Nucleotide sequence of the intergenic spacer and the 18S ribosomal RNA gene from mustard (Sinapis alba). Nucleic Acids Res. 1990 Mar 11;18(5):1288–1288. doi: 10.1093/nar/18.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Linares A., Hancock J. M., Dover G. A. Secondary structure constraints on the evolution of Drosophila 28 S ribosomal RNA expansion segments. J Mol Biol. 1991 Jun 5;219(3):381–390. doi: 10.1016/0022-2836(91)90178-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmickel R. D. Quantitation of human ribosomal DNA: hybridization of human DNA with ribosomal RNA for quantitation and fractionation. Pediatr Res. 1973 Jan;7(1):5–12. doi: 10.1203/00006450-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Sloof P., Van den Burg J., Voogd A., Benne R., Agostinelli M., Borst P., Gutell R., Noller H. Further characterization of the extremely small mitochondrial ribosomal RNAs from trypanosomes: a detailed comparison of the 9S and 12S RNAs from Crithidia fasciculata and Trypanosoma brucei with rRNAs from other organisms. Nucleic Acids Res. 1985 Jun 11;13(11):4171–4190. doi: 10.1093/nar/13.11.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. F., Collings J. C., Schnare M. N., Gray M. W. Multiple spacer sequences in the nuclear large subunit ribosomal RNA gene of Crithidia fasciculata. EMBO J. 1987 Apr;6(4):1063–1071. doi: 10.1002/j.1460-2075.1987.tb04859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Oono K., Iida Y., Sugiura M. The complete nucleotide sequence of a rice 25S.rRNA gene. Gene. 1985;37(1-3):255–259. doi: 10.1016/0378-1119(85)90280-x. [DOI] [PubMed] [Google Scholar]
- Tautz D., Hancock J. M., Webb D. A., Tautz C., Dover G. A. Complete sequences of the rRNA genes of Drosophila melanogaster. Mol Biol Evol. 1988 Jul;5(4):366–376. doi: 10.1093/oxfordjournals.molbev.a040500. [DOI] [PubMed] [Google Scholar]
- Thirup S., Larsen N. E. ALMA, an editor for large sequence alignments. Proteins. 1990;7(3):291–295. doi: 10.1002/prot.340070310. [DOI] [PubMed] [Google Scholar]
- Unfried I., Gruendler P. Nucleotide sequence of the 5.8S and 25S rRNA genes and of the internal transcribed spacers from Arabidopsis thaliana. Nucleic Acids Res. 1990 Jul 11;18(13):4011–4011. doi: 10.1093/nar/18.13.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman J. A., Maden B. E. 28 S ribosomal RNA in vertebrates. Locations of large-scale features revealed by electron microscopy in relation to other features of the sequences. Biochem J. 1989 Feb 15;258(1):49–56. doi: 10.1042/bj2580049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware V. C., Tague B. W., Clark C. G., Gourse R. L., Brand R. C., Gerbi S. A. Sequence analysis of 28S ribosomal DNA from the amphibian Xenopus laevis. Nucleic Acids Res. 1983 Nov 25;11(22):7795–7817. doi: 10.1093/nar/11.22.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A. P., Syin C., McCutchan T. F. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature. 1989 Nov 23;342(6248):438–440. doi: 10.1038/342438a0. [DOI] [PubMed] [Google Scholar]
- de Capoa A., Marlekaj P., Baldini A., Rocchi M., Archidiacono N. Cytologic demonstration of differential activity of rRNA gene clusters in different human tissues. Hum Genet. 1985;69(3):212–217. doi: 10.1007/BF00293027. [DOI] [PubMed] [Google Scholar]