Abstract
This study presents an extended children-of-twins model, allowing us to test the direction of the association between parenting and child adjustment. Three mechanisms can be examined: direct phenotypic influence of parenting on child behavior (controlling for both parental and child genotype), passive genotype-environment correlation and evocative genotype-environment correlation. This model has been tested using Monte-Carlo simulations. We generated datasets consisting of 1000 twin parent pairs together with their children, and 1000 twin children pairs together with their parents. These simulated datasets were then used to estimate the model and the procedure was repeated 1000 times. The simulation results showed that our model recovered the true values of parameters with high precision. The model was also applied to an observed dataset to analyze, as a first example, the association between maternal emotional overinvolvement and child internalizing problems. The results showed that this association was best explained by evocative genotype-environment correlation.
Keywords: children-of-twins model, parenting, child adjustment, genotype-environment correlation
Introduction
Understanding the interplay between genes and the environment is crucial for advancing our understanding of the development of individuals. One facet of gene-environment interplay that has received somewhat less attention than genotype × environment interaction (GxE) is genotype-environment correlation (rGE) yet it may be more pervasive throughout development. A number of recent reviews have focused on how to distinguish types of GxE interaction from rGE with the common caution that, if not considered, rGE may masquerade as GxE (e.g., Jaffee & Price, 2007; Kendler, 2005; Rutter, Moffitt, & Caspi, 2006; Rutter & Silberg, 2002). Most of the recent explosion of publications in the area of gene-environment interplay have focused on GxE (e.g., Button, Scourfield, Martin, Purcell, & McGuffin, 2005; Feinberg, Button, Neiderhiser, Reiss, & Hetherington, 2007; Tuvblad, Grann, & Lichtenstein, 2006). These studies have typically found that heritability varies as a function of an environmental measure and have helped to advance our understanding of the complex interplay of genes and the environment. Extensive studies are also needed to understand the role of rGE in development. However, as of today there exist very few easily applicable statistical models to directly evaluate the existence of rGE and even fewer models to differentiate between the different kinds of rGE.
Genotype-environment correlation (rGE) occurs when “genotypes are selectively exposed to different environments” (Plomin, DeFries, & Loehlin, 1977). Typically, three types of rGE are distinguished: passive, evocative, and active (Moffitt, Caspi, & Rutter, 2005; Scarr & McCartney, 1983). Passive rGE is a result of parents providing their children with both genes and with environments that are correlated with genetically-influenced characteristics of parents. For example, parents with internalizing problems may express their anxiety by becoming emotionally overinvolved towards their children. Thus, the children will not only inherit a propensity for internalizing problems, but also experience an environment that enhances their likelihood of developing such problems. Evocative rGE appears when a child’s inherited characteristics evoke a response from their environment. Using the same example of internalizing problems and parental overinvolvement, children with internalizing problems, who are inwardly focused or anxious, may elicit emotional overinvolvement from their parents. Here, the emotional overinvolvement is still correlated with the child’s internalizing problems – which are genetically influenced – but in this case the parent is reacting to the child. Active rGE refers to children actively selecting their environments for genetically influenced reasons. This type of rGE is unlikely to occur in parent-child relationships as parents and children do not select one another. Because the current report is focused on parenting and adolescent adjustment, we will not consider active rGE here in detail.
It is equally important to learn that rGE might not be present. That is, the environment affecting the behavior of children can be uncorrelated with the genes contributing to either parental or child characteristics. This would support a hypothesis that parenting has a direct environmental causal effect on child behavior (Moffitt, 2005; Neiderhiser et al., 2004) suggesting that parents by their behavior directly influence the behavior of their children.
There have been a number of studies that have found evidence of genetic influences on parenting (see McGuire, 2003 or Maccoby, 2000, for reviews). For example, studies of adult twins have shown that parental behaviors like physical discipline, warmth and positivity, control and parental overprotection all can be explained, in part, by genetic influences (Kendler, 1996; Wade & Kendler, 2000; Losoya, Callor, Rowe, & Goldsmith, 1997; Neiderhiser et al., 2004; Perusse, Neale, Heath, & Eaves, 1994). The rest of the variance is mainly explained by non-shared environmental effects (nongenetic factors that make family members different). Studies of twin children present similar findings on parenting: most parental behaviors examined show evidence of genetic influence (indicating the role of genetic factors in the twin children), with the remaining variance due to varying degrees of both shared and non-shared environmental influences (Elkins, McGue, & Iacono, 1997; Kendler, 1996; McGue, Elkins, Walden, & Iacono, 2005; Neiderhiser, Reiss, Lichtenstein, Spotts, & Ganiban, 2007; Neiderhiser et al., 2004; Plomin, McClearn, Pedersen, Nesselroade, & Bergeman, 1989; Rowe, 1981; Wade & Kendler, 2000). One exception is parental control, which has been found to be influenced primarily by shared and non-shared environmental influences (Plomin et al., 1989; Rowe, 1981).
The heritability of parenting can be interpreted in several ways. First, as suggested by studies on adult twins, parenting styles might partially overlap with heritable parents’ personality traits (Spinath & O’Connor, 2003). The influence of parents on children may be a result of shared genetic effects, which might be expressed as passive rGE. Second, heritability of parenting can also reflect the response to child’s genetically influenced characteristics (McGuire, 2003). This interpretation corresponds to evocative rGE.
A few studies have directly examined the presence of rGE in parenting behavior (e.g., Caspi et al., 2004; Deater-Deckard & Petrill, 2004; Ge et al., 1996; Lynch et al., 2006; Neiderhiser et al., 2007; Neiderhiser et al., 2004; O’Connor, Deater-Deckard, Fulker, Rutter, & Plomin, 1998). The conclusions about rGE have converged on findings that vary somewhat by the parenting construct. For example, parental conflict and negativity showed the most consistent evidence for evocative rGE, especially in studies that have examined children’s antisocial behavior as an outcome. On the other hand, parental warmth and positivity appears to be influenced by both passive and evocative rGE.
A recent study by Button, Lau, Maughan & Eley (2008) has revealed that not only rGE but also GxE was operating in the association between parental punitive discipline and the development of depressive symptoms in adolescents. Depending on their genetic liability for depressive symptoms, adolescents seemed to be differently exposed (rGE) as well as differently sensitive (GxE) to parental punitive discipline.
Because of the methodological specificity of conventional twin studies evocative and passive rGE can not be clearly distinguished. Specifically, for telling apart passive and evocative rGE, we have to be able to separate between genetic and shared environmental effects for children and parents. Traditional twin studies alone can not achieve this because genetic information is only available for either the parent or the child. In other words, a twin study of parenting will examine parents who are twins or children who are twins, but does not typically include both parent and child pairs who vary in degree of genetic relatedness. Thus, in such studies, evocative and passive rGE are confounded with genetic and shared environmental effects, respectively (Silberg & Eaves, 2004). Some studies have attempted to address this issue by comparing findings from studies of twin parents and studies of twin children in an effort to distinguish between these two types of rGE (Neiderhiser et al., 2007; Neiderhiser et al., 2004). Although such approaches are a step in the right direction, the findings are still approximations as the samples are not nested.
A more elegant approach to evaluate parental influences on children includes assessments of child adjustment within the framework of the powerful children-of-twins model (Rutter, Pickles, Murray, & Eaves, 2001; Silberg & Eaves, 2004). Children of MZ twins share half of their genes with both their own parent and the parent’s co-twin (the child’s aunt or uncle) while children of DZ twins share half of their genes with their parent and approximately 25% of their genes with their parent’s co-twin (like any niece/nephew-aunt/uncle pair). The rearing environment, on the other hand, is distinct for each child. The environmental effect of parenting on children can thus be estimated by controlling for the genetic correlation between parents and children (Rutter et al., 2001). In this way, the children-of-twins (CoT) model provides an effective strategy for disentangling the direct environmental influences of parenting from passive rGE (e.g., D’Onofrio et al., 2003; Lynch et al., 2006). However, even in this design the power for detecting evocative rGE is relatively weak. This is due to the fact that the children of twins are cousins who are, on average, 25% or 12.5% genetically similar, for children of MZ and DZ twins, respectively. Given such a modest variation in the degree of genetic relatedness, the effects of the children’s genotypes influencing parenting behavior – evocative rGE – are difficult to detect. A novel extension of the CoT design is to enhance the power of this design by including information on the same measured constructs provided by a companion study of twin children and their parents. Similar strategies have been used in the past, where a single study design has not been powerful enough to disentangle the complex gene-environment interplay that shapes human behavior (Heath, Kendler, Eaves, & Markell, 1985; Neiderhiser et al., 2004; Reiss, Neiderhiser, Hetherington, & Plomin, 2000). In the companion study the high contrast of genetic similarity between the twin children (100% for MZ and 50% for DZ) in combination with the cousin pairs allows the estimation of the effects of parents’ and children’s genes influencing each construct. Thus, passive and evocative rGE as well as direct environmental influences of parenting on child adjustment may be distinguished.
To illustrate the strength of this model we can compare it separately to simple children-of-twins model and twin-children based designs. In studies of adult twins and their children we can examine avuncular correlations (i.e., correlations between aunt/uncle and niece/nephew) between parenting and child behavioral outcomes among MZ and DZ twins. Higher correlations between children of MZ twins and their aunts/uncles would indicate the genetic effects involved in the relationship between parenting and child behavior. This can then be interpreted as an example of passive rGE, although it could also be a result of evocative rGE, not detected because of low power. On the other hand, findings of evocative rGE in studies of parenting of twin children may be just a manifestation of passive rGE. By pooling these two study designs, our extended CoT model provides us an opportunity to examine both types of rGE simultaneously.
In the current report, we have extended the children-of-twins model to include data from a comparable sample of twin children and their parents. The combination of a children-of-twins design with a twin-children and parents design increases our power to resolve passive and evocative rGE, and direct environmental influences of parenting on children. We evaluate this model with a series of simulations. Finally, to examine the performance of this model with real data, we apply it to observed data on maternal emotional overinvolvement and internalizing problems in children.
Method
Extended Children-of-Twins Model (ECOT)
The model we present in this report is based on a children-of-twins model suggested in Silberg and Eaves (2004). We extend their model in two ways. First, instead of using the full children-of-twins model, which includes both the twin parents and their spouses, we employ a reduced version of the model (Rutter et al., 2001), where only twin parents and their children are included. Second, the model consists of two parts: one that describes twin parents and their children, while the other defines twin children and their parents. In such a way, the extended children-of-twins model includes a sufficient number of genetic and environmental sources of variation for the traits studied, and thus can be identified and provide unbiased parameter estimates (Heath et al., 1993). The data for model estimation can be obtained from two different studies. The samples should be matched on measures, marital status of parents, and age of children as well as on prevalence of the observed phenotypes.
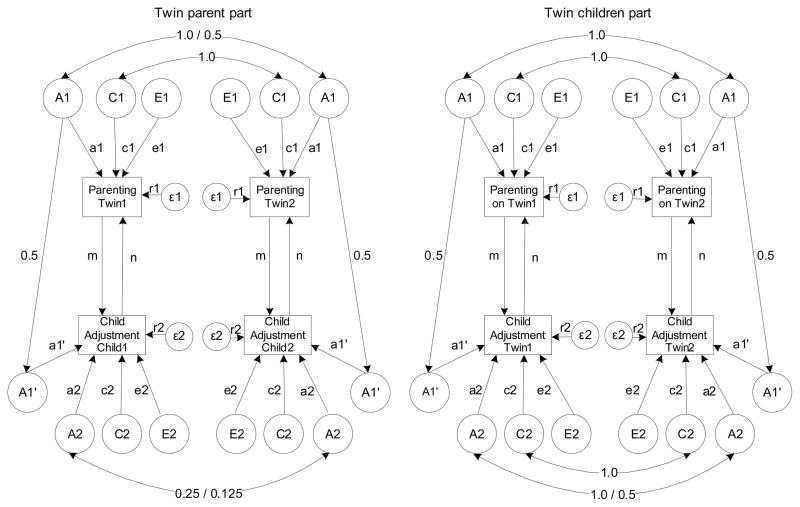
The ECOT model includes two phenotypes: one describing parental practices and one reflecting child adjustment (labeled Parenting and Child Adjustment, respectively in Figure 1). Each phenotype is influenced by genetic (A), shared environmental (C) and non-shared environmental (E) influences. Parenting is influenced by genetic (A1), shared environmental (C1) and non-shared environmental (E1) effects. Child Adjustment is similarly affected by genetic (A2), shared environmental (C2) and non-shared environmental (E2) factors. Factor A1′ represents genetic effects on Child Adjustment that are due to parental genetic influences on Parenting. Since children shares half of their segregating genes with each parent, the path leading from factor A1 to factor A1′ is fixed to 0.5. Path a1′ denotes parental genetic contributions to Child Adjustment that are shared with parents, while path a2 reflects child-specific genetic effects on the phenotype.
Figure 1.
Extended children-of-twins model. The model is described in two parts: for twin parents and for twin children. Phenotypes Parenting and Child Adjustment are denoted in rectangles. Genetic (A) and environmental (C, E) influences are depicted in circles. Parenting phenotype is influenced by genetic (A1), shared (C1), and non-shared environment (E1), while Child Adjustment is influenced by genetic (A1′ and A2), shared (C2), and non-shared environmental effects (E2). Measurement error (ε1 and ε2) contributes directly to the variance of both phenotypes. In twin parents part, the genetic effects correlate by 1.0 or 0.5, depending on the twin zygosity. Shared environment (C1) correlated perfectly for both MZ and DZ twins. Genetic effects for children, or cousins, correlate by 0.25 or 0.125, depending on the zygosity of the parents. Shared environmental effects are uncorrelated since the cousins do not share the family. In twin children part, genetic and shared environmental effects correlated perfectly for Parenting phenotype, because there was always the same parent rating both twins. For children, genetic effects correlated by 1.0 or 0.5 for MZ and DZ twins, respectively, and shared environmental effects correlated perfectly for both zygosity groups. Paths m and n denote reciprocity in the relationship between the phenotypes. Path m reflects direct environmental effect of Parenting on Child Adjustment, while path n denotes evocative processes in the relationship. Significant paths m, a1′ and a1 will indicate passive rGE, while evocative rGE will be suggested by significant n, a1′ and/or a2.
The most interesting and important part of the model are the reciprocity paths, m and n. Path m denotes direct phenotypic influence of Parenting on Child Adjustment. Thus, if significant, this path would support the hypothesis that parents directly affect the behavior of their children via environmental mechanisms. Passive rGE is defined by parents contributing both genes and environment to their children. Therefore, if the m path as well as both paths a1 and a1′ are significant, the presence of passive rGE would be suggested. On the other hand, evocative rGE occurs when children’s inherited characteristics evoke particular parent behavior and is supported when paths n, a1′ and/or a2 are significant (Silberg & Eaves, 2004). It is important to note that a significant m path indicates direct environmental influence but does not provide sufficient evidence for passive rGE. Similarly, passive rGE is not supported by a significant a1′ path alone, because this path denotes genetic effects that are shared between parents and children. A nonsignificant m path suggests that neither direct environmental influence nor passive rGE contribute significantly to the association.
In contrast to the usual univariate or bivariate twin models, measurement error is estimated as separate parameter in a reciprocal causation model. Otherwise parameter estimates might be biased (Heath et al., 1993). If we can assume equal error variances for both traits, a reciprocal causation model will be identified and provide negligibly biased estimates, given that at least four sources of variance are available for both traits together (Heath et al., 1993). There are six sources of variances present in the ECOT model and therefore the model is identified. Measurement error terms (ε1 and ε2 in Figure 1) contribute directly to variance in the phenotypes.
Twin parent part of the model
Parents that are MZ twins share all their segregating genes. Therefore, the correlation between parents’ genetic effects (A1) is equal to 1. On the other hand, DZ twins share on average only half of their segregating genes, and thus the correlation is set to 0.5. Shared environment is, by definition, all nongenetic influences that make family members similar, thus the correlation between shared environmental (C1) effects is set to 1. The correlation between the A2 paths is set to 0.25 for children of MZ twins and 0.125 for children of DZ twins as this indicates their degree of genetic relatedness. The correlation between shared environmental effects (C2) for children of twins is set to zero since they were not sharing the same rearing environment.
Twin child part of the model
For parents, correlations between genetic (A1) and shared environment (C1) effects are set to 1.0 for both twin children because the same parent is rating his/her parenting of both children. In other words, there is no variance in these factors for the parent of the twin children. Since the parent reports about each child separately, some aspects of parental behavior will be distinct for each child. Therefore, non-shared environment parameters (E1) are left uncorrelated. For twin children, the genetic correlation was set to 1 for MZ twins and to 0.5 for DZ twins. The correlation between shared environmental influences (C2) is set to 1.0 for both types of twin children because they are being reared in the same household.
The total estimated phenotypic variance for each trait is calculated as follows (Heath et al, 1993):
| (1) |
| (2) |
Simulations
Procedure
We verified the performance of the ECOT model by running series of Monte Carlo simulations. The simulations test the performance of a model of interest by applying it to randomly generated samples with suitable parameters. In general, the simulation procedure in this study included the following steps: 1) the true values of variance components were decided; 2) using information from step 1, the values of traits were generated; 3) the ECOT model was applied and the estimated variance components were recorded; 4) steps 1–3 were repeated 1000 times; 5) the average of variance components across the 1000 simulations were calculated and compared to the true values of parameters. If the model performs reasonably well, the estimated and true values of variance components should be close to each other. In more detail, for each of 1000 replications we generated data for 500 pairs of MZ and 500 pairs of DZ twin parents together with their children, as well as equally many pairs of MZ and DZ twin children together with their parents. Thus, the total number of twins included in each analysis was 2000, with an additional inclusion of either children or parents.
When both traits are influenced by genetic and shared environmental effects this type of models has limited power to reject false causality hypothesis, unless very large data samples are obtainable (Heath et al., 1993). Also, previous research on twin who are parents has shown that parenting style tends not to be influenced by their shared childhood environment (Perusse et al., 1994; Wade & Kendler, 2000). Therefore, for these simulations we included only genetic (A1) and non-shared environmental effects (E1) for Parenting. Further, since in TOSS and TCHAD studies (see descriptions below) reliability of measures of different phenotypes for parents and children usually varied from approximately 0.75 to 0.90, we assumed that 20% of the observed variance was due to measurement error.
The sets of true values of model parameters for generating phenotypes were calculated depending on the assumed variance structure of phenotypes. In general, our interest was to test the performance of the model depending on different heritability of the traits studied. Three possible variance structures were chosen: 1) the phenotypes were influenced equally by genetic and environmental effects (50% to 50%); 2) both the phenotypes were predominantly influenced by genetic effects (70%), while environmental influences accounted for 30% of total variance; 3) both the phenotypes were mainly affected by environmental influences (70%) and genetic effects explained the remaining 30% of the total variance. Knowing the approximate variance structure of a phenotype, it is possible to calculate the exact size of each variance component by applying equations (1) and (2). Findings from earlier studies suggest that parenting traits usually are not explained by shared environment. Therefore, environmental effects for Parenting were generated as non-shared environmental influences. Child Adjustment, on the other hand, was influenced by both shared and non-shared environmental effects, generated of roughly equal proportions.
It was also important to test how well the model performs when different types of rGE are present. Thus, under each of three variance structure alternatives, we assumed five scenarios of rGE existence, ranging from absence of passive or evocative rGE to equal influences of both. In Table 1, we present different sets of true variance component values, depending on the variance structure and existence of passive or evocative rGE.
Table 1.
Sets of true parameter values depending on the variance structure of the phenotypes and presence of gene-environment correlation (rGE)
| Presence of rGE | a1 | a1′ | a2 | e1 | e2 | c2 | m | n |
|---|---|---|---|---|---|---|---|---|
|
Equal genetic and environmental effects
| ||||||||
| - no evocative rGE | 0.70 | 0.65 | 0.00 | 0.70 | 0.45 | 0.45 | 0.20 | 0.00 |
| - no passive rGE | 0.65 | 0.55 | 0.20 | 0.65 | 0.45 | 0.45 | 0.00 | 0.20 |
| - small evocative rGE | 0.60 | 0.43 | 0.43 | 0.60 | 0.45 | 0.45 | 0.30 | 0.10 |
| - small passive rGE | 0.63 | 0.25 | 0.60 | 0.54 | 0.45 | 0.45 | 0.10 | 0.30 |
| - equal passive and evocative rGE | 0.70 | 0.00 | 0.65 | 0.70 | 0.50 | 0.50 | 0.25 | 0.25 |
|
| ||||||||
|
Genetic predominant
| ||||||||
| - no evocative rGE | 0.79 | 0.72 | 0.00 | 0.55 | 0.30 | 0.35 | 0.20 | 0.00 |
| - no passive rGE | 0.75 | 0.60 | 0.15 | 0.50 | 0.35 | 0.35 | 0.00 | 0.20 |
| - small evocative rGE | 0.70 | 0.50 | 0.50 | 0.40 | 0.30 | 0.35 | 0.30 | 0.10 |
| - small passive rGE | 0.74 | 0.20 | 0.75 | 0.45 | 0.40 | 0.40 | 0.10 | 0.30 |
| - equal passive and evocative rGE | 0.79 | 0.00 | 0.75 | 0.55 | 0.45 | 0.45 | 0.25 | 0.25 |
|
| ||||||||
|
Environment predominant
| ||||||||
| - no evocative rGE | 0.55 | 0.55 | 0.00 | 0.80 | 0.50 | 0.55 | 0.20 | 0.00 |
| - no passive rGE | 0.50 | 0.40 | 0.15 | 0.80 | 0.50 | 0.55 | 0.00 | 0.20 |
| - small evocative rGE | 0.50 | 0.30 | 0.30 | 0.70 | 0.50 | 0.55 | 0.30 | 0.10 |
| - small passive rGE | 0.50 | 0.20 | 0.50 | 0.75 | 0.60 | 0.60 | 0.10 | 0.30 |
| - equal passive and evocative rGE | 0.55 | 0.00 | 0.55 | 0.80 | 0.60 | 0.60 | 0.25 | 0.25 |
Passive rGE is suggested when paths m, a1′ and a1 are significant. Under all simulation scenarios where passive rGE was modeled to be present, the parental phenotypes were generated to be heritable (a1) and contributing to child phenotypes (a1′). Consequently, the main effect of passive rGE becomes reflected solely by the parameter m, which in our simulation study will be referred to as the non-evocative path. Similarly, the effect of evocative rGE can be indicated by the evocative path n.
For simulations we have used both R (R Development Core Team, 2003) and Mx (Neale, Boker, Xie, & Maes, 2003) software packages. The precision of recovery of true sample parameters was evaluated by calculating means and standard deviation of all estimates.
Results
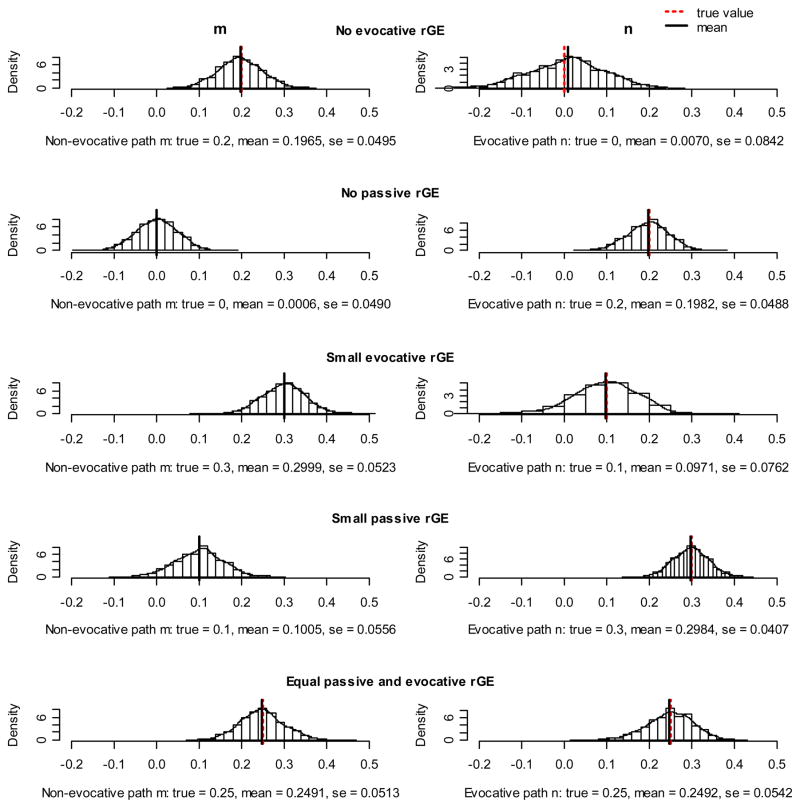
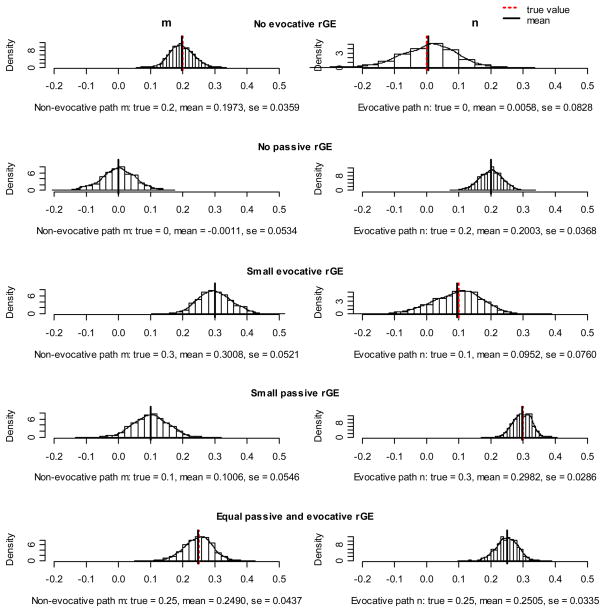
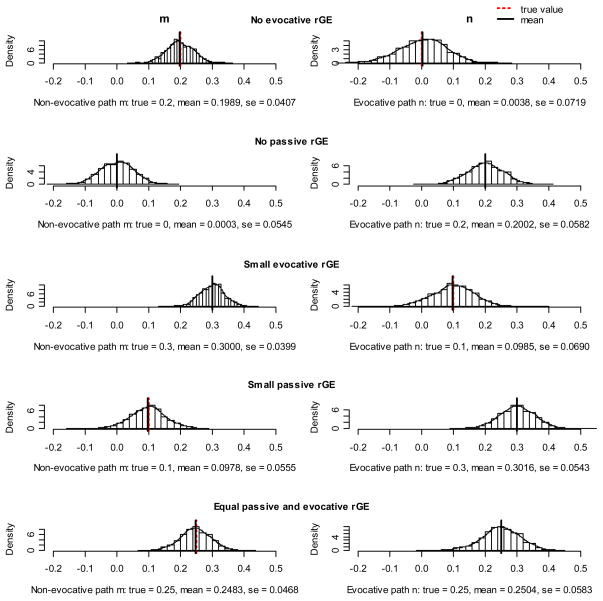
Simulation results are presented as histograms in Figures 2a, b, and c. Every figure represents a different variance structure of the phenotypes. In Figure 2a equal genetic and environmental influences on phenotypes were assumed, Figures 2b and 2c reflect predominantly genetic and predominantly environmental effects on phenotypes, respectively. Since we were most interested in the rGE, which is reflected in the reciprocity parameters m (passive rGE) and n (evocative rGE), we only present histograms of these estimates. Ideally, the values of true and estimated values should overlap and the histogram should consist of only one bar. This would indicate that the model recovered the true sample parameter perfectly at every simulation. A good model would provide estimates that are symmetrically and narrowly distributed around the mean value, which is also close to the true value of parameters.
Figure 2.
Figure 2a. Results of simulations: the phenotypes were influenced equally by genetics and environment. The dashed line indicates the true parameter value and the solid line denotes the mean of all parameter estimates. Every row of pictures represents the simulation with different values of m and n paths, reflecting different scenarios of rGE presence.
Figure 2b. Results of simulations: genetic predominant variance structure of the phenotypes.
Figure 2c. Environment predominant variance structure of the phenotypes.
In general, we can see that all histograms in the figures were symmetric, with one distinct peak, other values being distributed tightly around. This suggests that the ECOT model is stable, indicated also by low standard errors ranging from 0.01 to 0.08.
The performance of the model did not seem to depend on the variance structure of the phenotypes. For all alternatives – equal genetic and environmental effects, genetic predominant, and environment predominant - the reciprocity parameters m and n were recovered very accurately, with only small deviations.
The parameters for genetic and environmental effects were also recovered properly. Standard errors of estimates varied between 0.01–0.09, 0.02–0.25, and 0.01–0.11 for equal genetic and environmental effects, genetic predominant and environmental predominant variance structures, respectively. Detailed results of the simulations are available in the online appendix.
We have also run additional simulations to test the model more extensively (detailed results are available in the online appendix). First, we repeated the simulations by generating a larger sample size, 4,000 individuals in total. In these simulations the parameter estimates more closely approximated the true value of the parameter, which suggests that the estimates provided by ECOT model are consistent.
Second, we evaluated the model by including phenotypes that were different for parents and children in their variance structure. The phenotypes were again generated to be influenced either equally by genetic and environmental effects or genetic/environmentally predominant. But this time we chose the phenotype of one variance structure for parents and of another variance structure for children. For example, there could be genetic predominant phenotype for parents and environment predominant phenotype in children. Eventually, we ended-up with nine different simulation scenarios. The results showed the same tendency as when including both parental and children phenotypes of equal inheritance mode: the model recovered the true values of parameters with high precision.
Third, we also investigated whether the ECOT model was tenable when the parental phenotypes also were influenced by shared environment. We have therefore repeated the simulation procedure with the model including the shared environment parameter for parents (C1). That is, in this scenario both parental and child phenotypes were affected by shared environment. The precision of recovery of reciprocity parameters was lower, revealed primarily by higher standard deviations (range from 0.03 to 0.26). This suggests that our chosen sample size was not large enough. Heath et al. (1993) has shown that when both genetic and shared environmental effects are present, the reasonable study sample should range from ~4000 to ~27,000 individuals, depending on the size of the true direct environmental effect.
Model application to observed data
Next, for demonstration purposes, we applied the model to real data. In this case, we analyzed the relationship between maternal emotional overinvolvement and child internalizing problems. Studies have suggested that parental emotional overinvolvement is related to internalizing problem behavior in children (Hirshfeld, Biederman, Brody, Faraone, & Rosenbaum, 1997). However, the mechanisms underlying this association have not been well studied in genetically informative samples.
Internalizing problems in children comprise withdrawn behavior, anxiety, depression, and somatic complaints (Achenbach, 1991). The longitudinal studies suggest that anxious or depressed children tend to experience major depression later in adolescence as well as generalized anxiety and major depression disorders in adulthood (Moffitt et al., 2007; Roza, Hofstra, van der Ende, & Verhulst, 2003). There are two well established predictors of internalizing problems: child temperament and family environment, both acting independently and in interaction (Leve, Kim, & Pears, 2005). Higher risk groups include children that are fearful, shy, or emotional reactive/inhibitive, especially in combination with experienced maternal depression, psychological control, or parental conflict (Leve et al., 2005; Morris et al., 2002).
Emotional overinvolvement (EOI) is a component of the expressed emotion construct and refers to overprotective and self-sacrificing parent behavior targeted towards a child (Asarnow, Tompson, Woo, & Cantwell, 2001; Hirshfeld et al., 1997). The EOI has been showed to be a state-related measure, meaning that parents get more emotionally overinvolved as a response to their child’s illness (Schreiber, Breier, & Pickar, 1995). In several studies high EOI was related to the increase in anxiety disorders in children (e.g., Asarnow et al., 2001; Hirshfeld et al., 1997; Stubbe, Zahner, Goldstein, & Leckman, 1993). For example, Hirshfeld et al. (1997) have demonstrated that maternal EOI was significantly associated with child separation anxiety in the at-risk sample (i.e., sample of children at risk for anxiety disorders). The authors suggested that either EOI could be more influential on vulnerable children or child anxiety could evoke the overprotective behavior in mothers that were themselves anxious. In terms of rGE, these findings are consistent with passive rGE, where children of anxious mothers both tend to inherit anxious traits and be treated more overprotectively.
A similar study was performed by Brennan, Le Brocque and Hammen (2003). The authors examined whether the effect of EOI on “resilient outcomes” in youth differed among depressed and nondepressed mothers. Resilient outcome here was defined as no evidence for a number of behavior problems, including internalizing problems. The results suggested that emotionally overinvolved but not depressed mothers increased the levels of “resilient outcomes” in youth. Depressed mothers with higher levels of emotional overinvolvement, on the contrary, were associated with lower levels of resilient outcomes (Brennan et al., 2003). This latter finding can be explained by passive rGE: depressed mothers not only convey a genetic liability for depression to their children, but also inflate the risk of internalizing behavior through their high emotional overinvolvement. On the other hand, the protective effect of emotional overinvolvement amongst non-depressed mothers may reflect evocative rGE: mothers could react to their children’s behavior problems and, by getting more involved, eventually diminish them.
Previous twin studies on the development of internalizing problems in 10 year old twins showed that genetic, shared environmental, and non-shared environmental effects are approximately of equal importance (e.g., Bartels et al., 2004). The findings of genetic and shared environmental factors can be confounded by evocative or passive rGE, respectively. By applying the suggested ECOT model we tested whether the association between maternal emotional overinvolvement and adolescent internalizing problems was regulated by passive or evocative rGE, or whether a direct environmental influence from maternal emotional overinvolvement to child internalizing problems was present. The Mx program script is presented in Appendix.
The data for this report come from two different studies. The Twin and Offspring Study in Sweden (TOSS) is a two-cohort study of twin parents, one adolescent child and the spouse/partner. Detailed measures of parent-child relationships, marital relationships, personality, and the mental health of all study participants were collected (Neiderhiser et al., 2007; Reiss et al., 2001). For the purposes of this study, we used data from 254 MZ and 285 DZ female twin pairs and one of their biological adolescent children aged 11–20 years (average age = 15.9 ±2.5 years). The second study, the Twin study of CHild and Adolescent Development (TCHAD), is an ongoing Swedish longitudinal child-based twin study of health and behavior in children and adolescents (Lichtenstein, Tuvblad, Larsson, & Carlstrom, 2007). Twins and their parents have been contacted four times since the twins were 8–9 years old. In the present study, we included 250 MZ and 181 DZ male pairs as well as 258 MZ and 185 DZ female pairs of 16–17 years old female twins, together with their mothers. The average age of the adolescents was 16.7 (±0.42) years. This age of assessment for TCHAD was selected as the age of the children was most comparable to TOSS and the same measures were collected in both studies.
Maternal emotional overinvolvement (EOI) was measured by parent report on the Emotional Overinvolvement subscale of the Expressed Emotion measure (EE) (Hansson & Jarbin, 1997). The subscale includes 8 items concerning the level parents sacrifice themselves for their children or are preoccupied with their behavior. Reliability was acceptable with Cronbach’s alphas of 0.70 for TOSS and 0.78 for TCHAD. Adolescent internalizing problems were assessed by adolescent’s self report on the Youth Self-Report version of the CBCL, which measures behavioral and emotional problems in children and adolescents (Achenbach, 1991). For our analyses we used the Internalizing scale, which combines the subscales Anxious/Depressed, Withdrawn, and Somatic complains. Cronbach’s alphas were 0.86 for TOSS and 0.88 for TCHAD.
Analyses
In twin studies, preliminary estimates of genetic and environmental effects for phenotypes are calculated by comparing intraclass correlations between MZ and DZ twins (Plomin, 1997). Higher correlations among MZ twins indicate genetic influences, while approximately equal correlations for both zygosity groups suggest shared environmental influences. If DZ twin correlations are greater than half of the MZ twin correlation, the phenotype is influenced by both genetic and shared environmental effects. Finally, non-shared environmental influences, along with measurement error, are indicated by correlations less than 1.0 for MZ twins. By definition, non-shared environmental influences are all nongenetic influences that make family members different, including error of measurement.
When twins and their children are studied, it can be useful to calculate correlations across generations and across or within families. The cross-generation within-family correlations do not provide information on genetic or environmental influences because the genetic relatedness between parents and children is the same (50%) for both MZ and DZ twins (D’Onofrio et al., 2003). Cross-generation cross-family correlations, in this case between the child of the twin and their aunt for TOSS, can be used to approximate the nature of intergenerational transmission. If the intergenerational transmission is heritable, the correlation between MZ parents and their niece/nephew will be higher compared to DZ parents and their niece/nephew. Again, equal intergenerational correlations between MZ and DZ families will indicate shared environmental effects.
Results
Descriptive statistics of TOSS and TCHAD samples are presented in Table 2. It appears that mean levels of phenotypes are similar in both samples. Because the data were slightly skewed to the left, we transformed the data by taking the logarithm of the scores. Also, age and sex corrections were made by computing standardized partial residuals from the regression of scores on these variables (McGue & Bouchard, 1984). Phenotypic correlations between Emotional Overinvolvement and child Internalizing problems were 0.21 (p<0.001) in TOSS and 0.25 (p<0.001) in TCHAD.
Table 2.
Descriptive statistics of TOSS and TCHAD samples
| Measure (respondent) | MZ | DZ | ||
|---|---|---|---|---|
| n of individuals | mean (SD) | n of individuals | mean (SD) | |
| TOSS mothers | ||||
| Emotional Overinvolvement (twin parents) | 503 | 16.6 (3.9) | 558 | 16.7 (4.3) |
| Internalizing problems (children of twins) | 501 | 8.6 (7.1) | 552 | 9.2 (6.7) |
| TCHAD mothers | ||||
| Emotional Overinvolvement (parents of twins) | 591 | 14.9 (4.9) | 414 | 14.5 (4.9) |
| Internalizing problems (twin children) | 609 | 7.8 (7.0) | 435 | 8.3 (7.5) |
Intraclass correlations as well as correlations between mothers and their children are presented in Table 3, for TOSS (in which mothers are twins) and TCHAD (in which the children are twins) separately. The intraclass correlations were generally lower in TOSS, especially among the children of twins (cousin pairs). The intraclass correlations for EOI in TOSS reached 0.31 and 0.14, for MZ and DZ mothers, while in TCHAD the corresponding correlations were 0.82 and 0.61, when mothers rated MZ and DZ children, respectively. Internalizing problems between children of MZ and DZ twins in TOSS were 0.07 and −0.01, and in TCHAD, the correlations were 0.52 and 0.38, for MZ and DZ twin children, respectively. In both studies, intraclass correlations (in bold) were consistently higher for MZ twins compared to DZ twins, indicating that both Emotional Overinvolvement and Internalizing Problems are genetically influenced. Specifically, for EOI, intraclass correlations for DZ twins (in TOSS sample) were slightly less than half of the MZ twin correlations. Shared environmental effects are therefore not indicated for this parenting measure. On the other hand, for children’s Internalizing Problems in the TCHAD study, intraclass correlations for DZ twins were greater than half of MZ correlations, signifying both genetic and shared environmental effects. According to the pattern of intraclass correlations among mothers in TCHAD, genetic and shared environmental influences are also suggested for EOI. However, since the twins studied in this sample are children, the genetic and shared environmental effects reflect the importance of these factors in the child generation.
Table 3.
Intraclass correlations for maternal emotional overinvolvement and child internalizing problems
| Measure (respondent) | Twin mother 1 | Twin mother 2 | Child 1 | Child 2 |
|---|---|---|---|---|
| TOSS mothers | ||||
|
| ||||
| Emotional Overinvolvement (twin mother 1) | 0.31* | 0.16* | −0.02 | |
| Emotional Overinvolvement (twin mother 2) | 0.14* | 0.01 | 0.14* | |
| Internalizing problems (child 1) | 0.22* | 0.03 | 0.07 | |
| Internalizing problems (child 2) | 0.04 | 0.24* | −0.01 | |
|
| ||||
| TCHAD mothers | Mother 1 | Mother 2 | Twin child 1 | Twin child 2 |
|
| ||||
| Emotional Overinvolvement (mother 1) | 0.82* | 0.24* | 0.22* | |
| Emotional Overinvolvement (mother 2) | 0.61* | 0.22* | 0.24* | |
| Internalizing problems (twin child 1) | 0.28* | 0.19* | 0.52* | |
| Internalizing problems (twin child 2) | 0.19* | 0.28* | 0.38* | |
Note. MZ and DZ twins are displayed above and below the diagonal, respectively;
p<0.05
Intergenerational transmission can be assessed only in the TOSS sample. Correlations between mothers and their nieces/nephews were almost equal among MZ and DZ twins, suggesting that intergenerational transmission is less likely to be influenced by genetic effects, that is, by passive rGE.
Model fitting
Univariate analyses showed that shared environmental effects were not large for the EOI in TOSS (a2=0.29, c2=0.01, e2=0.70). Therefore, this factor was excluded from further estimation of ECOT model. Conventional univariate analysis of EOI for TCHAD data is not very informative in our case because we had one parent evaluating both twins and in the ECOT model we assume perfect correlations between the genetic effects, for MZ and DZ twins. Assuming the same correlations in the univariate analysis would result in approximately equal genetic and shared environmental effects.
First, we fitted a saturated model which estimates all the means, variances and the covariances between all variables. This model fits the data best, but it is not very useful for making further predictions and generalizations. A more parsimonious model with fewer estimated parameters is preferred. Therefore, we have further fitted the full ECOT model and then constrained ECOT models that excluded the reciprocity parameters m and n.
Model fit is evaluated by comparing reduced models to the saturated model using different fit indices. We present the model comparison results using log-likelihood ratio test, Akaike’s Information Criterion (AIC), and Bayesian Information Criterion (BIC). The lowest value of the two last criterions indicates the best-fitting model. Since the latter two criterions evaluate the parsimony of the model, we primarily refer to these tests.
Table 4 presents the results of the model comparisons using different tests. The AIC value was lowest for the saturated model, while according to BIC the best-fitting model was the constrained ECOT model with the dropped direct environmental path, m (i.e., it had 0 effect size). The BIC measure takes sample size into account and is therefore most suitable for our data. According to it, the constrained ECOT model seemed to explain the data best in the most parsimonious way. The significant results of the log-likelihood ratio test might depend on its sensitivity to large size samples.
Table 4.
Model fit comparison
| Model | −2log L | df | ΔChi2 | Δ df | p-value | AIC | BIC |
|---|---|---|---|---|---|---|---|
| Saturated | 8965.910 | 4095 | - | - | - | 775.910 | −9855.822 |
| Full ECOT | 9159.638 | 4140 | 193.728 | 45 | 0.00 | 879.638 | −9916.526 |
| Dropped m | 9159.638 | 4141 | 0 | 1 | - | 877.638 | −9920.028 |
| Dropped n | 9162.768 | 4141 | 3.130 | 1 | 0.08 | 880.768 | −9918.463 |
| Dropped m & n | 9182.183 | 4142 | 22.544 | 2 | 0.00 | 898.183 | −9912.257 |
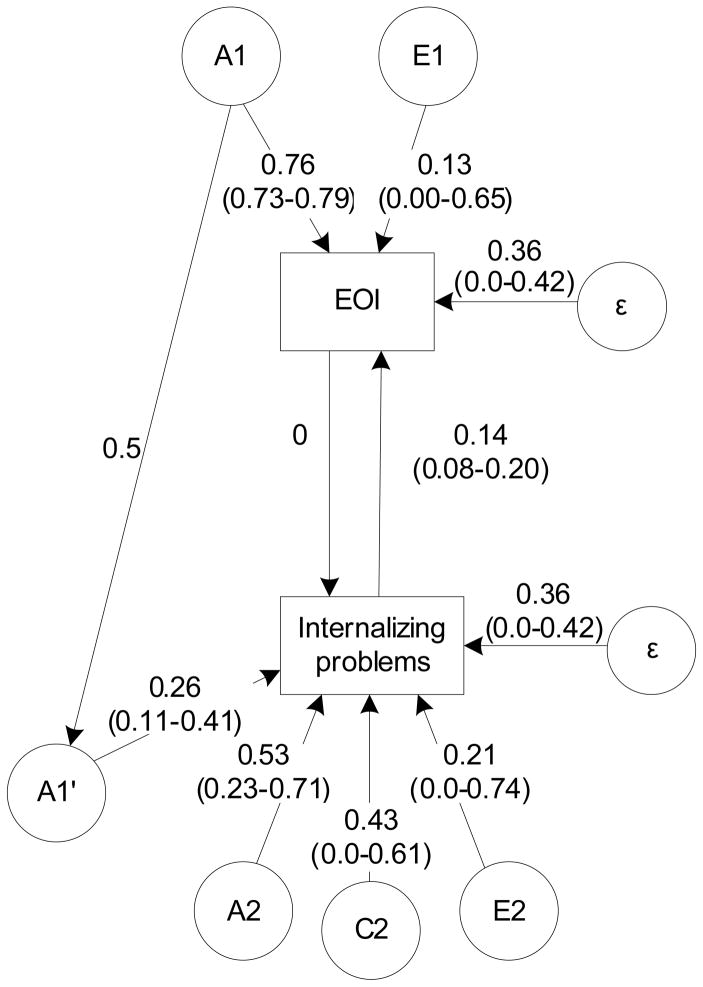
The standardized and unsquared parameters for the best-fitted estimated ECOT model are presented in Figure 3. Following path tracing rules, squared parameter estimates provide the amount of variance accounted for (Loehlin, 1998). These findings suggest that Emotional Overinvolvement is heritable (a12=0.762=0.58) and influenced by non-shared environmental effects (e12=0.132=0.02). The total estimated variance of Emotional Overinvolvement also included the error term (ε=0.36, 95% CI (0.0–0.42)) and is calculated following the (1) equation. Internalizing problems were significantly influenced by both genetic effects contributed by mothers (a1′2=−0.262=0.07) and child specific effects (a22=0.532=0.28). Shared environmental effects explained 18% (c22 = 0.432 = 0.28), while non-shared environment contributed by 4% (e22=0.212=0.04) of the phenotypic variance. The total phenotypic variance of Internalizing problems again includes the error term (ε=0.36), and is calculated by applying the (2) equation.
Figure 3.
Estimated children-of-twins model for maternal emotional overinvolvement and child internalizing problems. Note. * p < 0.05.
The evocative path n was significant, while the direct causality path m could be dropped from the model. It is therefore unlikely that maternal emotional overinvolvement has any direct environmental effect on the internalizing problems of adolescent children. Even if the parental genetic influences (a1′) contributed significantly to the child phenotype, the nonsignificant m path suggests that passive rGE is likely to be less influential in the development of the relationship. Thus, internalizing problems of adolescent children seem to evoke emotional overinvolvement in their mothers.
Discussion
In this study we presented an extension of the children-of-twins model, which can be used to study genotype-environment correlation. Because the ECOT model combines data from two different studies the model can be utilized to make maximal use of studies with moderate samples sizes, which are more common. More importantly, the ECOT model allows us to examine three possible mechanisms underlying associations between parent-child relationships and child adjustment: 1) parenting affects the adjustment of children directly through the mechanisms that are independent of the parents or child’s genotype; 2) passive genotype-environment correlation; 3) evocative genotype-environment correlation.
The results of simulations showed that the ECOT model was stable and recovered the true parameter values with high precision. The model was robust to the variance structure and was able to adequately recover both the presence and absence of the evocative and non-evocative paths when passive or evocative rGE was absent or present. It also performed very well when small effects of rGE were present.
When the model was applied to the observed data, the results suggested that the association between maternal emotional overinvolvement and internalizing problems in adolescents was likely to primarily be regulated by evocative rGE. That is, mothers, experiencing their children as anxious, withdrawn or depressed, tended to get more emotionally overinvolved in their parenting. Our findings are consistent with the results of a study by Reitz, Dekovic and Meijer (2006) where adolescents showing higher levels of internalizing problems were parented with higher involvement, which further increased the problem behavior one-year later. Evocative rGE could also explain the results of Brennan et al. (2003), where nondepressed and emotionally overinvolved mothers seemed to have, in contrast, a protective effect for child behavior problems (see above).
Another finding of the same study of Brennan et al. (2003) was consistent with passive rGE: depressed and emotionally overinvolved mothers were associated with decreased levels of resilient outcomes in youth. That is, problem behavior in children could depend on both inherited genetic liability to depression as well as overprotective parenting. Similarly, passive rGE can explain the results of Hirshfeld et al. (1997), where high maternal EOI was associated with separation anxiety disorder only in children of mothers with history of anxiety disorders. Comparing these findings with the ones of our population-based study, one may expect that the type of rGE acting in the association between EOI and child internalizing problems differs depending on mother’s psychopathology. This hypothesis should be tested in a future study, where information on maternal depression and/or other disorders is taken into account.
The direct causality path was nonsignificant and could be excluded from the model. This suggests that maternal EOI does not affect the level of internalizing problems in adolescents in a direct environmental way. However, a study by Reitz et al. (2006) has shown that parental involvement alone decreased, although insignificantly, internalizing problem behavior measured in adolescents one-year later. The difference in findings between these two studies may depend on different measures of parental involvement as well as to the different designs of the studies.
Finding evidence for evocative rGE has been a common pattern in studies of associations between negative parent-child relationships and antisocial behavior (Burt, McGue, Krueger, & Iacono, 2005; O’Connor et al., 1998). According to the results of our study, the same pattern seems to be valid for internalizing problems, too. This is in line with earlier findings in research of development trajectories of child problem behavior. Specifically, a number of studies have suggested that internalizing and externalizing problems often appear and develop together (e.g., Capaldi, 1991 or Gilliom & Shaw).
A study by Lau and Eley (2008) has examined the co-occurrence of rGE and GxE between maternal punitive discipline and adolescent depressive symptoms. The findings revealed that both rGE and GxE were operating in the development of the association. It is possible that our findings of evocative rGE may also, fully or partly, mask the existence of GxE. The ECOT model alone is unable to test for these processes simultaneously because it employs cross-sectional data. Future longitudinal data studies are therefore needed to further examine this issue.
The main concern when applying the ECOT model is the need to combine two distinct samples. A number of difficulties can arise in attempting to find comparable samples. Even though studies can be matched on a number of characteristics of participants and measurements, differences in, for example, size of correlations still may occur, as was seen in the TOSS and TCHAD samples. Such discrepancies may mainly be the result of different genetic designs of the studies, which does not appear to affect the strength of association between the measures. However, in model application, this may result in larger residuals, leading to a worsened model fit. In our study, large residuals could explain the significant differences between the saturated and full ECOT model. The sample dissimilarity may also have caused the estimates of measurement error to be greater than expected from earlier studies.
Lower intraclass correlations among cousins in TOSS, compared to children in TCHAD, may also depend on the wider age range of the participants. Low correlations among cousins, in comparison to twin children, might result in the underestimated passive rGE. In this study, we have controlled the data for child’s sex and age and therefore believe the possible underestimation of passive rGE, if any, should be negligible.
The ECOT model assumes that passive rGE is present if genes affecting the parental trait contribute to the child adjustment, too. However, it should be noted that because genes might act differently throughout development, it is possible that genes influencing the parental phenotype in adulthood and those important for the child phenotype in childhood are not the same. The effect of passive rGE could therefore be underestimated. One way to deal with this problem would be to include information on child adjustment in parents. However, such extensive developmental studies are difficult to accomplish.
The ECOT as well as general children-of-twins models are estimated by applying cross-sectional data. The conclusions we can draw reflect therefore only a particular time point in the development of parent-child relationships. For the traits with heritability changing over time (e.g., antisocial behavior), the ECOT model can serve only as the first step in explaining the development of the relationships in a family. On the other hand, if no sample size restrictions are present, a cross-sectional children-of-twins model could be extended to a longitudinal model, which would capture the changes over time.
This study has several strengths. The ECOT model can be used to evaluate the type of genotype-environment correlation even when information on only one parent is employed, as is common in many existing twin studies where the children are twins. Also, the ECOT model can be used in studies with commonly used sample sizes. Together with a possibility to combine different study designs, this makes the model particularly useful and relatively easy to apply. The findings on the processes underlying the association between maternal emotional overinvolvement and internalizing problems in children are, to our knowledge, reported for the first time.
However, there are several noteworthy limitations. First, children-of-twins models require large sample sizes for detailed analyses of family relationships. In our study, the data were corrected for the age and sex of the child. Separate analyses on the relationships between mothers and their sons or daughters would shed more light on the potentially different processes involved depending on the sex of the child. Our sample size was not, however, large enough to perform such detailed analyses.
Second, children-of-twins models including only one parent should be applied carefully to study dyadic parental phenotypes (e.g. divorce). A report by Eaves et al. (2006) has demonstrated that the model is not reliable for resolving the direct environmental effect from associations due to genetic effects when studying dyadic parental treatment measures. This is not the case for parental treatments affected only by one of the parents (e.g., depression). Although emotional overinvolvement is not directly defined as a dyadic parental measure (i.e. involving both parents), we cannot exclude that it is not affected by the other parent’s behavior. Therefore, the results of our application study should be replicated by including both parents into analyses.
Third, the ECOT model can only be identified when the measurement error is assumed to be equal for both phenotypes. The model should therefore be applied with caution when measurement errors of the phenotypes differ considerably.
Fourth, although power and sample size analyses for reciprocity model have been performed in previous studies (e.g., Heath et al., 1993), it is important to test the exact power of the ECOT model. This would let to specify the exact sample size requirements for further application studies. However, because of the extensity of calculations, power analyses are not within the scope of the current report.
Fifth, results of the simulations showed that the ECOT model performs less well when we include parental traits influenced also by shared environment (C1). This makes the model mainly applicable to study parental phenotypes that are not influenced by shared environmental contributions. It is worth noting here that the shared environmental influences on parental phenotypes refer to shared rearing experiences of the twin parents who are now adults and/or to current shared experiences that increase the similarity of the twin parents.
Finally, since the ECOT model excludes the spouse of the parent, assortative mating cannot be taken into account. This should be further tested by employing an extended twin kinship model (Maes et al., 2006).
In sum, the current study presents an extended children-of-twins model. This model was designed to advance our understanding of the development of parent-child relationships. More specifically, the model allows a direct test of the presence of genotype-environment correlation, even when only samples of moderate sizes are available. This study also finds that the association between maternal emotional overinvolvement and internalizing problems in children is primarily accounted for by evocative rGE.
Acknowledgments
Jurgita Narusyte, Department of Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden; Jenae M. Neiderhiser, Department of Psychology, The Pennsylvania State University, USA; Brian D’Onofrio, Department of Psychological and Brain Sciences, Indiana University, USA; David Reiss, Child Study Center, Yale University, USA; Erica L. Spotts, National Institute of Aging/National Institutes of Health, Bethesda, USA; Jody Ganiban, Department of Psychology, George Washington University, USA; Paul Lichtenstein, Department of Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
This project was supported by grant R01MH54610 from the National Institute of Mental Health. We would like to thank Andrew C. Heath for his assistance with generating the initial model as well as Michael C. Neale and Hermine H. Maes for their comments regarding the model specification.
Appendix (Mx script)
Description
The Mx program script for the extended children-of-twins model. The script is written by using RAM approach (McArdle & Goldsmith, 1990) and is organized as follows: the first 4 groups describe every twin group (twin parents or twin children) depending on the zygosity. The last group summarizes the results and calculates the standardized solution.
Data type
Continuous, raw. Data is controlled for child’s age and sex
Participants
Twin mothers and their children; twin children and their mothers



Contributor Information
Jurgita Narusyte, Karolinska Institutet, Sweden.
Jenae M. Neiderhiser, The Pennsylvania State University, USA
Brian M. D’Onofrio, Indiana University, USA
David Reiss, Yale University, USA.
Erica L. Spotts, National Institute on Aging/National Institutes of Health, Bethesda, USA
Jody Ganiban, George Washington University, USA.
Paul Lichtenstein, Karolinska Institutet, Sweden.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Asarnow JR, Tompson M, Woo S, Cantwell DP. Is expressed emotion a specific risk factor for depression or a nonspecific correlate of psychopathology? Journal of Abnormal Child Psychology. 2001;29(6):573–583. doi: 10.1023/a:1012237411007. [DOI] [PubMed] [Google Scholar]
- Bartels M, Boomsma DI, Hudziak JJ, Rietveld MJH, van Beijsterveldt TCEM, van den Oord EJCG. Disentangling Genetic, Environmental, and Rater Effects on Internalizing and Externalizing Problem Behavior in 10-year-old Twins. Twin Research. 2004;7:162–175. doi: 10.1375/136905204323016140. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Le Brocque R, Hammen C. Maternal depression, parent-child relationships, and resilient outcomes in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(12):1469–1477. doi: 10.1097/00004583-200312000-00014. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development and Psychopathology. 2005;17(1):145–165. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behavior Genetics. 2005;35(2):115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Capaldi DM. Co-occurrence of conduct problems and depressive symptoms in early adolescent boys: I. Familial factors and general adjustment at Grade 6. Development and Psychopathology. 1991;3(3):277–300. doi: 10.1017/s0954579499001959. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, et al. Maternal expressed emotion predicts children’s antisocial behavior problems: using monozygotic-twin differences to identify environmental effects on behavioral development. Developmental Psychology. 2004;40:149–161. doi: 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Petrill SA. Parent-child dyadic mutuality and child behavior problems: an investigation of gene-environment processes. Journal of Child Psychology and Psychiatry. 2004;45(6):1171–1179. doi: 10.1111/j.1469-7610.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, et al. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44(8):1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33(2):351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Feinberg ME, Button TM, Neiderhiser JM, Reiss D, Hetherington EM. Parenting and adolescent antisocial behavior and depression: evidence of genotype x parenting environment interaction. Archives of General Psychiatry. 2007;64(4):457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger R, Cadoret R, Neiderhiser JM, Yates W, Troughton E, et al. The Developmental Interface Between Nature and Nurture: A Mutual Influence Model of Child Antisocial Behavior and Parent Behaviors. Developmental Psychology. 1996;32(4):574–589. [Google Scholar]
- Gilliom M, Shaw DS. Codevelopment of externalizing and internalizing problems in early childhood. Development and Psychopathology. 2004;16(2):313–333. doi: 10.1017/s0954579404044530. [DOI] [PubMed] [Google Scholar]
- Hansson K, Jarbin H. New self-rating questionnaire in Swedish for measuring expressed emotion. Nordic Journal of Psychiatry. 1997;51:287–297. [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: informativeness of different relationships. Behavior Genetics. 1985;15(5):439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional family data. Behavior Genetics. 1993;23(1):29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Biederman J, Brody L, Faraone SV, Rosenbaum JF. Associations between expressed emotion and child behavioral inhibition and psychopathology: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):205–213. doi: 10.1097/00004583-199702000-00011. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Parenting: a genetic-epidemiologic perspective. American Journal of Psychiatry. 1996;153(1):11–20. doi: 10.1176/ajp.153.1.11. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Psychiatric genetics: a methodologic critique. American Journal of Psychiatry. 2005;162(1):3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- Lau JY, Eley TC. Disentangling gene-environment correlations and interactions on adolescent depressive symptoms. Journal of Child Psychology and Psychiatry. 2008;49(2):142–150. doi: 10.1111/j.1469-7610.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Leve LD, Kim HK, Pears KC. Childhood Temperament and Family Environment as Predictors of Internalizing and Externalizing Trajectories from Ages 5 to 17. Journal of Abnormal Child Psychology. 2005;33(5):505–520. doi: 10.1007/s10802-005-6734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD-study. Twin Research and Human Genetics. 2007;10(1):67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. Latent variable models: an introduction to factor, path, and structural analysis. 3. Mahwah, N.J.: Lawrence Erlbaum; 1998. [Google Scholar]
- Losoya SH, Callor S, Rowe DC, Goldsmith HH. Origins of familial similarity in parenting: a study of twins and adoptive siblings. Developmental Psychology. 1997;33(6):1012–1023. doi: 10.1037//0012-1649.33.6.1012. [DOI] [PubMed] [Google Scholar]
- Lynch SK, Turkheimer E, D’Onofrio BM, Mendle J, Emery RE, Slutske WS, et al. A genetically informed study of the association between harsh punishment and offspring behavioral problems. Journal of Family Psychology. 2006;20(2):190–198. doi: 10.1037/0893-3200.20.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby EE. Parenting and its Effects on Children: On Reading and Misreading Behavior Genetics. Annual Review of Psychology. 2000;51(1):1–27. doi: 10.1146/annurev.psych.51.1.1. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Kendler KS, Martin NG, Heath AC, Eaves LJ. Genetic and cultural transmission of smoking initiation: an extended twin kinship model. Behavior Genetics. 2006;36(6):795–808. doi: 10.1007/s10519-006-9085-4. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behavior Genetics. 1990;20(5):569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ., Jr Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14(4):325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Walden B, Iacono WG. Perceptions of the parent-adolescent relationship: a longitudinal investigation. Developmental Psychology. 2005;41(6):971–984. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- McGuire S. The heritability of parenting. Parenting: Science and Practice. 2003;3(1):73–94. [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131(4):533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ, Melchior M, Goldberg D, et al. Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychological Medicine. 2007;37(3):441–452. doi: 10.1017/S0033291706009640. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Sessa FM, Avenevoli S, Essex MJ. Temperamental Vulnerability and Negative Parenting as Interacting Predictors of Child Adjustment. Journal of Marriage and Family. 2002;64(2):461–471. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modelling. 6. Richmond, VA: Department of Psychiatry, Medical College of Virginia; 2003. [Google Scholar]
- Neiderhiser JM, Reiss D, Lichtenstein P, Spotts EL, Ganiban J. Father-adolescent relationships and the role of genotype-environment correlation. Journal of Family Psychology. 2007;21(4):560–571. doi: 10.1037/0893-3200.21.4.560. [DOI] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Pedersen NL, Lichtenstein P, Spotts EL, Hansson K, et al. Genetic and environmental influences on mothering of adolescents: a comparison of two samples. Developmental Psychology. 2004;40:335–351. doi: 10.1037/0012-1649.40.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype-environment correlations in late childhood and early adolescence: antisocial behavioral problems and coercive parenting. Developmental Psychology. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- Perusse D, Neale MC, Heath AC, Eaves LJ. Human parental behavior: evidence for genetic influence and potential implication for gene-culture transmission. Behavior Genetics. 1994;24(4):327–335. doi: 10.1007/BF01067533. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Plomin R, McClearn GE, Pedersen NL, Nesselroade JR, Bergeman CS. Genetic influence on adults’ ratings of their current family environment. Journal of Marriage and Family. 1989;51(3):791–803. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing; 2003. [Google Scholar]
- Reiss D, Cederblad M, Pedersen NL, Lichtenstein P, Elthammar O, Neiderhiser JM, et al. Genetic probes of three theories of maternal adjustment: II. Genetic and environmental influences. Family Process. 2001;40(3):261–272. doi: 10.1111/j.1545-5300.2001.4030100261.x. [DOI] [PubMed] [Google Scholar]
- Reiss D, Neiderhiser JM, Hetherington E, Plomin R. The relationship code: Deciphering genetic and social influences on adolescent development. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Reitz E, Dekovic M, Meijer AM. Relations between parenting and externalizing and internalizing problem behaviour in early adolescence: child behaviour as moderator and predictor. Journal of Adolescence. 2006;29(3):419–436. doi: 10.1016/j.adolescence.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Rowe DC. Environmental and genetic influences on dimensions of perceived parenting: A twin study. Developmental Psychology. 1981;17(2):203–208. [Google Scholar]
- Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. American Journal of Psychiatry. 2003;160(12):2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves L. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127(3):291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Rutter M, Silberg J. Gene-environment interplay in relation to emotional and behavioral disturbance. Annual Review of Psychology. 2002;53:463–490. doi: 10.1146/annurev.psych.53.100901.135223. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schreiber JL, Breier A, Pickar D. Expressed emotion. Trait or state? British Journal of Psychiatry. 1995;166(5):647–649. doi: 10.1192/bjp.166.5.647. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Eaves LJ. Analysing the contributions of genes and parent-child interaction to childhood behavioural and emotional problems: a model for the children of twins. Psychological Medicine. 2004;34:347–356. doi: 10.1017/s0033291703008948. [DOI] [PubMed] [Google Scholar]
- Spinath FM, O’Connor TG. A behavioral genetic study of the overlap between personality and parenting. Journal of Personality. 2003;71(5):785–808. doi: 10.1111/1467-6494.7105004. [DOI] [PubMed] [Google Scholar]
- Stubbe DE, Zahner GE, Goldstein MJ, Leckman JF. Diagnostic specificity of a brief measure of expressed emotion: a community study of children. Journal of Child Psychology and Psychiatry. 1993;34(2):139–154. doi: 10.1111/j.1469-7610.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: Gene-environment interaction. Journal of Child Psychology and Psychiatry. 2006;47(7):734–743. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- Wade TD, Kendler KS. The genetic epidemiology of parental discipline. Psychological Medicine. 2000;30(6):1303–1313. doi: 10.1017/s0033291799003013. [DOI] [PubMed] [Google Scholar]