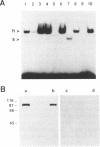
Abstract
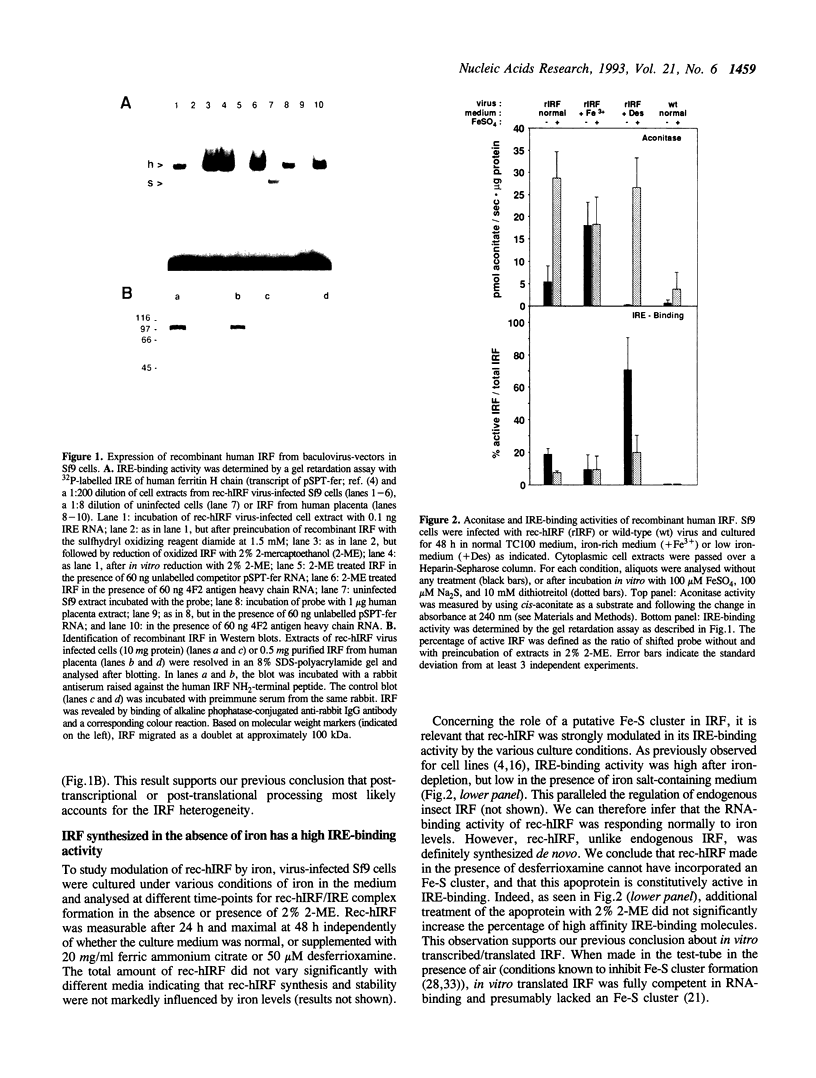
Iron regulatory factor (IRF) is a cytoplasmic mRNA-binding protein that coordinates post-transcriptionally the expression of several important proteins in iron metabolism. Binding of IRF to iron-responsive elements (IRE) in the 5' untranslated region (UTR) of ferritin and erythroid 5-aminolevulinic acid-synthase mRNAs inhibits their translation, whereas binding to IREs in the 3' UTR of transferrin receptor (TfR) mRNA prevents the degradation of this mRNA. IRF binds RNA strongly after iron deprivation, but is inactive, yet present, under conditions of high cellular iron supply. Recently, IRF was also shown to have aconitase activity indicating the existence of an Fe-S cluster in the protein. In the current study we expressed human IRF in insect cells from recombinant baculovirus and analysed IRE-binding and aconitase activities under various culture conditions. Newly made apoprotein, synthesized in the absence of iron, was fully active in IRE-binding, but showed no aconitase activity. In contrast, IRF made by cells grown in high iron medium bound RNA poorly, but exhibited high aconitase activity with a Km of 9.2 microM for cis-aconitate. Apo-IRF was converted in vitro to active aconitase by Fe-S cluster-generating conditions, and under the same conditions lost its RNA-binding capacity. These results indicate that the two activities are mutually exclusive and controlled through formation of the Fe-S cluster.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton H. A., Eisenstein R. S., Bomford A., Munro H. N. Determinants of the interaction between the iron-responsive element-binding protein and its binding site in rat L-ferritin mRNA. J Biol Chem. 1990 Apr 25;265(12):7000–7008. [PubMed] [Google Scholar]
- Beinert H., Kennedy M. C. 19th Sir Hans Krebs lecture. Engineering of protein bound iron-sulfur clusters. A tool for the study of protein and cluster chemistry and mechanism of iron-sulfur enzymes. Eur J Biochem. 1989 Dec 8;186(1-2):5–15. doi: 10.1111/j.1432-1033.1989.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable A., Quick S., Gray N. K., Hentze M. W. Modulation of the RNA-binding activity of a regulatory protein by iron in vitro: switching between enzymatic and genetic function? Proc Natl Acad Sci U S A. 1992 May 15;89(10):4554–4558. doi: 10.1073/pnas.89.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Martin A., May B. K. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991 Jul;10(7):1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., Hentze M. W. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991 Jul;10(7):1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson C. P., Cleland W. W. Purification and kinetic studies of beef liver cytoplasmic aconitase. J Biol Chem. 1967 Sep 10;242(17):3833–3838. [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hirling H., Emery-Goodman A., Thompson N., Neupert B., Seiser C., Kühn L. C. Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Res. 1992 Jan 11;20(1):33–39. doi: 10.1093/nar/20.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Beinert H. The state of cluster SH and S2- of aconitase during cluster interconversions and removal. A convenient preparation of apoenzyme. J Biol Chem. 1988 Jun 15;263(17):8194–8198. [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kühn L. C., Hentze M. W. Coordination of cellular iron metabolism by post-transcriptional gene regulation. J Inorg Biochem. 1992 Aug 15;47(3-4):183–195. doi: 10.1016/0162-0134(92)84064-t. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Guo B. Iron-dependent regulation of ferritin and transferrin receptor expression by the iron-responsive element binding protein. Annu Rev Nutr. 1992;12:345–368. doi: 10.1146/annurev.nu.12.070192.002021. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Rothenberger S., Müller A. M., Kühn L. C. In vivo and in vitro modulation of the mRNA-binding activity of iron-regulatory factor. Tissue distribution and effects of cell proliferation, iron levels and redox state. Eur J Biochem. 1992 Sep 15;208(3):597–605. doi: 10.1111/j.1432-1033.1992.tb17224.x. [DOI] [PubMed] [Google Scholar]
- Neupert B., Thompson N. A., Meyer C., Kühn L. C. A high yield affinity purification method for specific RNA-binding proteins: isolation of the iron regulatory factor from human placenta. Nucleic Acids Res. 1990 Jan 11;18(1):51–55. doi: 10.1093/nar/18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino M. M., Walden W. E. Cloning of a functional cDNA for the rabbit ferritin mRNA repressor protein. Demonstration of a tissue-specific pattern of expression. J Biol Chem. 1992 Sep 15;267(26):19011–19016. [PubMed] [Google Scholar]
- Philpott C. C., Rouault T. A., Klausner R. D. Sequence and expression of the murine iron-responsive element binding protein. Nucleic Acids Res. 1991 Nov 25;19(22):6333–6333. doi: 10.1093/nar/19.22.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C., Artymiuk P. J., Guest J. R. The aconitase of Escherichia coli. Nucleotide sequence of the aconitase gene and amino acid sequence similarity with mitochondrial aconitases, the iron-responsive-element-binding protein and isopropylmalate isomerases. Eur J Biochem. 1992 Mar 1;204(2):599–609. doi: 10.1111/j.1432-1033.1992.tb16673.x. [DOI] [PubMed] [Google Scholar]
- Rothenberger S., Müllner E. W., Kühn L. C. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990 Mar 11;18(5):1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira S., Di Grandi S., Kühn L. C. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987 Jul 15;262(20):9574–9580. [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Yu Y., Radisky E., Leibold E. A. The iron-responsive element binding protein. Purification, cloning, and regulation in rat liver. J Biol Chem. 1992 Sep 15;267(26):19005–19010. [PubMed] [Google Scholar]
- Zheng L., Andrews P. C., Hermodson M. A., Dixon J. E., Zalkin H. Cloning and structural characterization of porcine heart aconitase. J Biol Chem. 1990 Feb 15;265(5):2814–2821. [PubMed] [Google Scholar]