Abstract
High grade gliomas (HGGs) are characterized by resistance to radiotherapy and chemotherapy. Targeting Rad51-dependent homologous recombination repair may be an effective target for chemo- and radiosensitization. In this study we assessed the role of Rad51-dependent repair on sensitivity to radiation and temozolomide (TMZ) as single agents or in combination. Repair protein levels in established glioma cell lines, early passage glioblastoma multiforme (GBM) cell lines, and normal human astrocytes (NHAs) were measured using western blot. Viability and clonogenic survival assays were used to measure the effects of Rad51 knockdown with radiation (XR) and TMZ. Immunocytochemistry was used to evaluate kinetics of Rad51 and γ-H2AX repair foci. Immunohistochemistry was used to assess Rad51 protein levels in glioma specimens. Repair proteins including Rad51 are upregulated in HGG cells compared with NHA. Established glioma cell lines show a dose-dependent increase in Rad51 foci formation after XR and TMZ. Rad51 levels are inversely correlated with radiosensitivity, and downregulation markedly increases the cytotoxicity of TMZ. Rad51 knockdown also promotes more residual γ-H2AX foci 24 h after combined treatment. Newly established GBM cell lines also have high Rad51 levels and are extremely sensitive to Rad51 knockdown. Clinical samples from recently resected gliomas of varying grades demonstrate that Rad51 levels do not correlate with tumor grade. Rad51-dependent repair makes a significant contribution to DNA repair in glioma cells and contributes to resistance to both XR and TMZ. Agents targeting Rad51-dependent repair would be effective adjuvants in standard combination regimens.
Keywords: DNA repair, glioma, radiation, Rad51, temozolomide
High-grade gliomas (HGGs) are amongst the clinically poorest prognosis adult tumors, with median survival even in the best prognosis groups being little over a year. Historically these tumors were treated with maximal resection, followed by external-beam beam radiotherapy, after which local control was rarely achieved despite attempts at significant dose escalation.1 Clinical data have shown that the addition of temozolomide (TMZ) chemotherapy to standard dose radiotherapy improves overall survival, and recently updated 5-year follow-up data suggest that this effect persists with longer follow-up.2 TMZ is an alkylating agent whose main toxicity is mediated through methylation of the 06 position on guanine.3 The 06-methyl guanine methyltransferase (MGMT) repair enzyme is able to remove the damaged base, but cellular activity levels of this enzyme vary. In gliomas, downregulation of MGMT is most commonly due to methylation at the promoter region, which is present in around 40% of tumors at first presentation.4 In MGMT-deficient and mismatch repair (MMR) proficient cells, it is hypothesized that unrepaired methylation of guanine promotes futile rounds of MMR utilizing MutSα proteins MSH2 and MSH6, leading to formation of DSBs during the subsequent S-phase; in vitro data support a delayed damage response that triggers G2 cell cycle block via ataxia telangiectasia-mutated (ATM)/ATM and Rad3-related (ATR) signaling. However, apoptosis may also occur in subsequent cell cycles, presumably due to late activation of MMR or initial lesion tolerance followed by genomic instability.5–7 Other data have suggested that the MMR proteins themselves can also signal directly to an ATR-mediated response to activate ATRIP/Chk1 in response to DNA methylation.8 A significant clinical response to TMZ treatment is likely to be limited to patients whose tumors have normal MMR and downregulation of MGMT. Loss of the MMR protein MSH6 has been shown to be associated with progression during TMZ treatment and may represent an important mechanism of drug resistance at relapse.9 Other repair pathways may also be relevant to TMZ sensitivity, including TP53-dependent signaling, since wtTP53 may be associated with sensitivity to TMZ, independent of MGMT status.10 Interestingly, recent data suggest that repair of alkylating agent–induced DSB may be predominantly through homologous recombination (HR) rather than nonhomologous end joining (NHEJ).11
The mechanisms by which TMZ enhances the effect of radiotherapy treatment are beginning to be defined. There is some evidence that concurrent treatment produces enhanced radiosensitivity in glioma cells with low MGMT activity and that this may be associated with downregulation of DSB repair mechanisms that are dependent on histone phosphorylation.12 However, whether a radiosensitizing effect is observed is cell line dependent, and different investigators have reported differing outcomes in various models when combined treatment is used.10,13–15 We have recently shown that the predominant effect of combination treatment is additive toxicity, which is highly dependent on scheduling of the two agents.16
In repair of DSBs following irradiation in glioma cell lines, the NHEJ pathway utilizing DNA-PKcs is important, as demonstrated by comparing the radiosensitivity of the paired glioma cell lines MO59J and MO59K, which are DNA-PKcs deficient and proficient, respectively, and by the radiosensitizing effect of dominant negative Ku70.17,18 Recent data suggest that the effect of DNA-Pkcs inhibition may be mediated by induction of autophagy.19 In previous publications we have also shown radiosensitization in vitro using DNA-PKcs inhibition to reduce NHEJ in glioma cells and have more recently demonstrated the effects of inhibiting DNA damage signaling through ATM and ATR, which affect mainly checkpoint responses.16,20 Other studies focusing on radiosensitivity of glioma cells have demonstrated the potential influence of specific proteins, including Survivin, that are thought to influence anti-apoptotic pathways.21
Rad51 is the central protein in controlling strand invasion and recombination in human cells during replication and in response to DNA damage. Rad51 foci can be visualized by immunofluorescence after exogenous damage causing double strand breaks and in S-phase at sites of ssDNA at lesions that promote recombination during replication. The control of Rad51 protein levels is partially cell-cycle dependent, with higher levels expressed during G2/S phase in many cell types.22 Recent data elucidating the control of Rad51 foci formation suggest that this is mediated by release of the protein from BRCA2 binding following phosphorylation of BRCA2 at specific sites by CDK1 and the checkpoint signaling protein Chk2.23,24 Rad51 expression can also be induced by DNA damage following chemotherapy treatment and contributes to resistance to doxorubicin.25,26
In many circumstances Rad51-dependent repair may contribute only a small proportion of total DSB repair capacity, contributing mainly to the slow component of DSB repair during the G2 phase at sites of heterochromatin and to repair replication-associated damage.27 Nevertheless we have postulated that Rad51 protein levels may be particularly relevant in influencing radioresistance in TP53 mutated cell lines, with an abrogated G1 checkpoint response following DNA damage, such that they are overreliant on a dose-sensitive G2 checkpoint and therefore on Rad51-dependent repair during G2.28 Other authors have demonstrated previously that glioma cell lines express high Rad51 protein levels and that they can be radiosensitized by targeting Rad 51-dependent repair, with antisense targeting Rad51 or with Gleevec.29–31 In view of these data and the recently published evidence of the role of homologous recombination in repair of alkylating agent–induced DSB, we hypothesized that DSB repair inhibition during G2 may promote enhanced radio- and chemosensitization when radiation (XR) or TMZ is used as a single agent and may convert additive toxicity to a synergistic effect when they are used in combination.
We investigated the role of Rad51-dependent repair following XR and TMZ in a series of established HGG cells and found that these cells demonstrate high protein levels and prominent Rad51 foci formation after XR and TMZ. We found that Rad51 downregulation using RNA interference was associated with enhancement of cell killing after XR and TMZ used as single agents and that the level of knockdown directly influenced the amount of additional toxicity observed. When XR and TMZ were used together in cells with Rad51 knockdown, the major effect was on TMZ cytotoxicity. In these experiments we also documented increased residual DNA damage at 24 h, suggesting that agents that inhibit Rad51-dependent repair would be effective adjuvants in clinical regimens using daily treatment. We also studied 2 newly established glioma cell lines from surgical specimens of glioblastoma multiforme (GBM) that also showed high Rad51 levels. When Rad51 protein levels were knocked down in these cells, there was a dramatic reduction in viability. However, we could not confirm previous data suggesting that Rad51 levels assessed by immunohistochemistry are associated with prognosis in glioma. In our series of clinical specimens of gliomas of various grades, Rad51 expression levels measured by immunohistochemistry did not correlate with grade.
Materials and methods
Cell lines
Four human HGG tumor cell lines were used: A7, U87, and T98G cells were obtained from the European Collection of Animal Cell Cultures (ECACC) in October 1996. U373 cells were donated by Dr J Perlman in August 1995. All cell lines were confirmed mycoplasma free before use. Detailed clonogenic survival data have been published previously.32 All 4 cell lines are radioresistant in vitro with SF2 of 0.7 (T98G) 0.67 (A7), 0.46 (U87), and 0.6 (U373), respectively. Three of these cell lines (T98G, A7, U373) have point mutations in TP53, and U87 is TP53 wild type. T98G cells have high MGMT levels but can be sensitized to TMZ using the MGMT inhibitor benzyl guanine (Bg). U373 and U87 cells have low MGMT levels.10 Normal human astrocytes (NHA, Clonetics Astrocyte cell systems) were supplied by Cambrex Bio Science.
Newly established glioma cell lines were kindly supplied by Professor S. Brandner at the Institute of Neurology, Queen Square. These cell lines were established from recently resected GBM cases as part of a tumor biobank initiative. All patients gave consent for their tumor specimens to be used for research purposes prior to surgery according to local ethics procedures. For cell line establishment, the protocol described by Pollard was used.33 We confirmed that these cells exhibit markers of tumor-derived stem cells, using immunofluorescent staining for CD133 and Nestin.
Flow cytometry
Cells were harvested at various times post-treatment by scraping and then were fixed in ice cold 70% ethanol before staining with propidium iodide (0.45 µg/mL), RNase (0.45 mg/mL), and 0.045% Tween. Resuspended cells were analyzed for DNA content on a Fluorescence Activated Cell Sorter (FACS)Vantage; data were processed with FACS CellQuest software (Becton Dickinson).
Immunofluorescence
Cells were grown in covered slide chambers (Labtech). Following treatment with XR or TMZ, they were fixed with 2% paraformaldehyde in phosphate buffered saline for 10 min, washed in tris buffered saline (TBS), then blocked with TBS containing 0.2% Triton and 1% normal goat serum and then in TBS containing 1% normal goat serum. Primary antibody at dilution 1:1600 (polyclonal Rad 51, Merck Bioscience), 1:400 (monoclonal, serine 139 phospho-H2AX, Upstate) was then added and incubated at 4°C overnight. The slides were then washed with TBS/0.2% Tween and incubated with secondary antibody (Alexofluor 488 at 1:800 dilution for Rad51, 1:400 for H2AX) added for 1 h at room temperature. Slides were then washed in TBS/0.2% Tween, then stained with 4'-6-diamidino-2-phenylindole and mounted. Slides were viewed with a Bio-Rad confocal laser microscope for dual staining by sequentially scanning the two emission channels (488 and 514 nm). For foci, counting cells were viewed under UV illumination using a Nikon inverted microscope and x100 objective. Foci were counted in at least 100 cells per slide, and 3 slides were counted at each dose point. For calculation of proportions of cells with foci, any cell with ≥5 foci was scored positive. Mean and standard errors for foci per cell were calculated for each dose point (JMP statistical software, SAS).
Clonogenic survival
A FACS Vantage (Becton Dickinson) was used to assay clonogenic survival as described previously.32 In this protocol, individual cells are sorted based on cell scattering parameters and counted into petri dishes or culture flasks until an exact predetermined number is reached in a modification of the method originally described by Durand.34 In our experiments, 500–5000 cells were sorted per flask depending on the expected surviving fraction after irradiation, to keep the number of colonies per flask at approximately 200. Three replicates were used at each dose in each experiment so at least 1500 cells were assessed at each dose point. Irradiations were carried out using a 240 kVp Pantak X-ray set. To assess the effects of drug treatment on survival, parallel flasks were included in which cells were exposed to the drug for varying times pre- and postirradiation. In knockdown experiments, cell numbers were calculated using serial dilution and were treated on day 0 with either short-interfering RNA (siRNA) targeting Rad51 or nonspecific sequence control prior to assessing survival after XR treatment as described above.
Sulphorhodamine assay for cytotoxicity
Newly established GBM cell lines at early passage numbers were grown in vitro in attached cultures as described above. The sulphorhodamine (SRB) assay was carried out when cells were in log phase growth, and preliminary experiments were conducted to determine optimal plating densities. Treated or untreated newly established GBM cell lines were processed using SRB colorimetric assay at day 3 post XR or day 9 post TMZ treatment.
Drug treatment
For TMZ treatment, cells were pre-incubated with various concentrations of TMZ with or without 50 µM Bg for 3 h (high MGMT cell line, T98G). Fresh medium with or without Bg was replenished after 3 h and at 48 h. In combination experiments, cells were irradiated on day 3 post TMZ exposure, to ensure that TMZ-induced damage was apparent at the time of irradiation, which mimics the clinical situation when patients are treated continuously with TMZ through radiotherapy.
Western blot
Cell pellets were lysed in 100 µL ice-cold lysis buffer containing protease inhibitor (Sigma).The protein concentration in each sample was determined, and samples were fixed in Laemelli sample buffer (Bio Rad). Equal amounts of protein were loaded onto 10% NuPage gels (Invitrogen) and were subjected to electrophoresis in MOPS (3-(N-morpholino)propanesulfonic acid) running buffer (Invitrogen) at 125 V for 1.5 h. Samples were then transferred to polyvinylidene fluoride membrane (Millipore) for 60 min at 100 V. Membranes were blocked in TBS/Tween containing 5% milk and 1% bovine serum albumin (BSA) for 1 h and then washed and incubated with primary antibodies to MGMT, Rad51, BRCA2, Chk2, and Ku70 (Cell Signalling) and anti-β actin (Abcam) in TBS containing 5% BSA overnight at 4°C. Membranes were then washed and incubated with secondary antibodies (1:30000 anti-mouse horseradish peroxidase (HRP; Dako Cytomation) and Qdot anti-chicken 655 (Invitrogen)) for 1 h at room temperature in TBS containing 2.5% milk. Membranes were washed again. Images were captured using a 4000 MM imaging system (Eastman-Kodak) equipped with 410 excitation and 670 emission filters. Following fluorescence imaging, blots were incubated with ECL Advance (GE Healthcare) and scanned for luminescence.
Protein knockdown by RNA interference
Sequence-specific siRNA targeting Rad51 was supplied by Dharmacon (Lafayete) with a control nonspecific sequence. These oligonucleotides were transfected into T98G cells using Lipofectamine (Invitrogen) or into newly established GBM cell lines using DharmaFECT4 (Thermo Scientific). Following a 24 h transfection period, medium was replaced with standard antibiotic-free growth medium for 24 h before cells were used in clonogenic survival or immunofluorescence assays as described above. Protein knockdown was confirmed using western blot on samples taken at the time of irradiation and by absence of Rad51 foci following irradiation in parallel chamber slides prepared for immunofluorescence.
For graded knockdown, shRNA plasmids supplied by ThermoFisher Scientific were used; 1 mg of plasmid DNA was transfected using Lipofectamine according to manufacturers instructions. Two hours after transfection, the cells were washed with fresh medium 3 times and finally overlaid with fresh medium and allowed to recover overnight. The following plasmids were used in which the hairpin, puromicin resistance, and the green fluorescent protein (GFP) sequence were all linked and under the control of the same promoter: pGIPZ empty vector control, RHS4379 (led to cell line E); pGIPZ nontargeting shRNA control, RHS4371 (led to cell line NT); pGIPZshRNA Rad51, clone 171184 (led to cell line A); pGIPZshRNA Rad51, clone 218486 (led to cell line B); pGIPZshRNA Rad51, clone 239089 (led to cell line C). After 24 h cells were split onto 10 cm dishes and fed with fresh medium supplemented with Puromicin, to allow for selection of cells having stably integrated the plasmid. Selection was deemed complete when all the cells from the untransfected wells had died. To ensure uniformity of hairpin expression, the stable cell lines emerging from puromicin selection were further sorted by FACS according to their GFP brightness (top 50% bright selected).
Immunohistochemistry for Rad51
Formalin fixed, wax embedded specimens were dewaxed in xylene (Surgipath) and then in consecutive 100%, 90%, and 70% industrial methylated spirit (IMS Surgipath) for 2 min each. The slides were then washed and heat-induced antigen retrieval was carried out using 10-mM citric acid (VWR), pH was adjusted to 6 with 2 M sodium hydroxide (Sigma Aldridge), and then slides were heated in this buffer in an 800 W microwave for 4 min. The specimens were washed with TBS, and then peroxidase block (DAKO) was added for 5 min. One in 20 dilution of anti-Rad51 antibody (Abcam) was applied for 60 min at room temperature and then slides were washed twice with TBS. Mouse HRP (DAKO) was applied for 35 min at room temperature and then washed and 3,3'-diaminobenzidine applied at a dilution of 1 in 50. Nuclei were counter-stained in Gills I haematoxylin (Surgipath) for 5 s, then finally washed and dehydrated by immersion in successive 70%, 90%, and 100% IMS for 2 min each, then xylene for 2 min.
Nineteen paraffin embedded gliomas were stained. Human testicular tissue was included in each staining run as a positive control. Samples were provided by Professor Sebastian Brandner, National Hospital for Neurology and Neurosurgery. All patients had undergone informed consent for part of their tumor specimen to be used for research purposes. All specimens were obtained at first surgery, i.e., prior to any cytotoxic treatment. Staining was scored by examining each specimen under 200× magnification. Ten fields were examined per tumor,and each specimen was categorized into one of four groups: 0, <10, 10–50, and >50% of cells positive for Rad51 staining.
Results
Repair protein levels in glioma cell lines
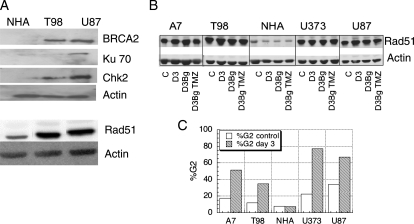
Figure 1A shows BRCA2, Ku70, Chk2, and Rad51 protein levels in 2 glioma cell lines (T98G [TP53mut] and U87 [TP53wt]) and normal human astrocytes (NHAs) measured on western blot. These data demonstrate higher levels of Rad51 in glioma cells compared with NHA, as well as higher expression of BRCA2 and Chk2. U87, but not T98G, cells also demonstrated slightly higher Ku70 levels compared to NHA. We then assessed Rad51 levels in a larger series of glioma cell lines and included different conditions to establish whether TMZ or Bg treatment affected Rad51 expression. Figure 1B shows Rad51 levels in four glioma cell lines (A7, T98G, U373, U87) compared with NHA in untreated conditions (c), on day 3 post XR (D3), with Bg (D3Bg) or Bg and TMZ (D3Bg TMZ). Figure 1C shows the corresponding flow cytometry data, confirming a TMZ-induced G2 arrest in glioma cell lines at 72 h post exposure as expected. The lack of G2 arrest in NHA is likely to be due to the very long doubling time of these cells. These data suggest that high Rad51 levels are a feature of HGG cell lines and that this is not altered by treatment with TMZ or the resulting G2 cell cycle arrest, with or without Bg treatment.
Fig. 1.
(A) Western blot analysis of BRCA2, KU70, Chk2, and Rad51 protein levels in 2 high-grade glioma cell lines (T98G, U373) and normal human astrocytes (NHA). (B) Rad51 levels in 4 glioma cell lines (A7, T98G, U373, U87) and NHA, with and without benzyl guanine (Bg) and TMZ treatment. Asynchronous cells were exposed to Bg + TMZ and harvested for western blot on day 0 (c, control) and 3 days post treatment (D3 = day 3 control, D3Bg = day 3 with Bg, D3BgTMZ = day 3 with Bg and TMZ). Actin levels are shown as a loading control. On day 3, flow cytometry data demonstrate a marked TMZ-induced G2 phase arrest in the glioma cells compared to untreated controls (C).
Rad 51 foci post XR and TMZ
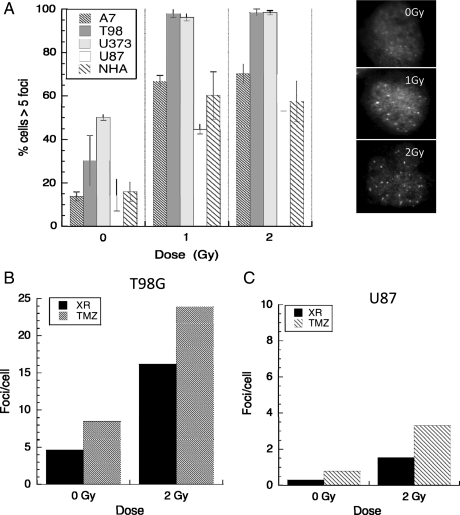
Rad51 forms repair associated foci following DNA damage and at sites of replication fork collapse. Damage-induced foci are most prominent at relatively late time points after irradiation, between 4 and 24 h, suggesting that they may locate at complex or poorly repaired lesions. We investigated Rad51 foci formation following radiation, TMZ, and combined treatment in 4 glioma cell lines. Data are presented in Fig. 2A as the proportion of cells with ≥5 foci at 4 h post-XR treatment in each cell line and show an increase in foci numbers after XR. A panel showing the typical appearance of T98G cells stained for Rad51 foci after 0, 1, and 2 Gy are also shown (right panel). We have previously demonstrated that this high proportion of cells with significant foci numbers is not simply due to high S-phase fractions, as they also occur in non-S phase cells.28 Although these numbers reach a plateau after 1 Gy XR, a further increase in mean foci numbers/cells occurs when TMZ is added to 2 Gy XR in T98G (TP53mut) cells, the cell line that shows the most marked XR effect (Fig. 2B), and in TP53wt U87 cells, which show a smaller XR effect and lower foci numbers (Fig. 2C). These data suggest that both clinically relevant XR doses and TMZ induce Rad51-dependent repair in these glioma cells and that combination treatment may be associated with higher activation levels than XR alone.
Fig. 2.
Rad51 foci numbers induced by radiation, temozolomide, and combination treatment in 4 high-grade glioma cell lines (A7, T98G, U373, U87) and normal human astrocytes (NHAs). Asynchronously growing cells were irradiated (0, 1, or 2 Gy) and fixed for fluorescent immunocytochemistry 4 h post XR or treated with TMZ then irradiated on day 3 and fixed 4 h later. Data are expressed as proportion of cells with ≥5 foci (A) and mean foci/cell in T98G (B) and U87 cells (C) post 0 Gy, 2 Gy ± TMZ. Error bars are standard errors. Typical appearances of Rad51 foci in T98G cells after 0, 1, and 2 Gy are shown in the right panel.
Rad 51 knockdown and radiosensitivity
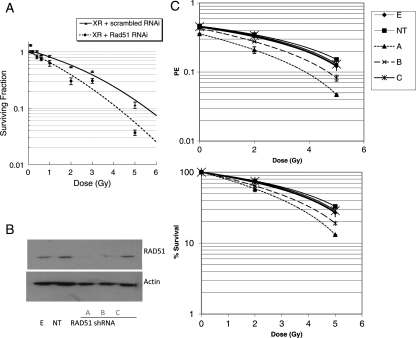
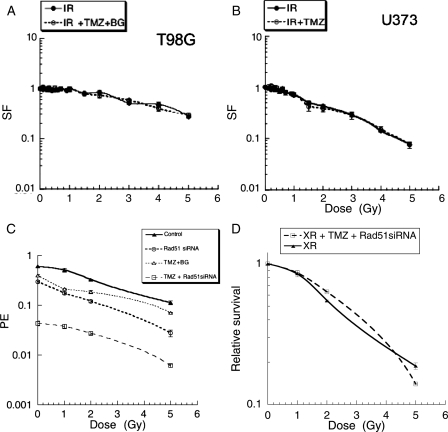
Since the data presented above suggest that Rad51-directed repair may be relevant to XR-induced DNA DSB, we also assessed the effect of Rad51 knockdown on radiosensitivity using siRNA. Rad51 siRNA treatment alone reduced plating efficiency in these cell lines by a small amount (T98G cells PE control = 0.47, PE Rad51 siRNA = 0.4). The results of Rad51 knockdown on radiosensitivity in the TP53mut cell line T98G are shown in Figure 3A expressed as surviving fraction. This demonstrates significant reduction in clonogenic survival associated with Rad51 knockdown compared with nonspecific sequence across the XR dose range 0.1 to 5 Gy. To further confirm the specificity of this response, we used shRNA technology to expose cells to graded levels of Rad51 protein. Figure 3B shows the effect of different shRNA constructs designated a–c on Rad 51 protein levels. Construct a is associated with lower protein levels than b and c, respectively. The corresponding clonogenic survival data for cell lines stably transfected with these constructs are shown in Figure 3C, as plating efficiency (PE; upper panel) and surviving fraction (lower panel). As in the siRNA data, there is a small reduction in PE associated with all 3 constructs, but the reduction in surviving fraction following radiation is more marked with the construct (a), which leads to the lowest protein levels. These data support a correlation between Rad51 protein levels and cell survival following radiation.
Fig. 3.
The effect of Rad51 knockdown using short interfering RNA on clonogenic survival of T98G glioma cells following XR (A). Asynchronous T98G cells were transfected with Rad51 siRNA on day 0 (dashed line) or scrambled control (solid line) then irradiated. Data points represent means of surviving (SF) from 3 different flasks. Error bars are standard errors. Stable transfection using shRNA constructs producing different levels of knockdown were also established and used for single dose irradiation experiments. Differential Rad51 expression levels were confirmed in 3 different cell lines on western blot (B). Survival data are summarized in 3 (C) as plating efficiency (upper panel) and surviving fraction (lower panel). Data points represent means from 2 different experiments, 3 flasks per data point in each experiment.
Rad51 knockdown and chemosensitivity
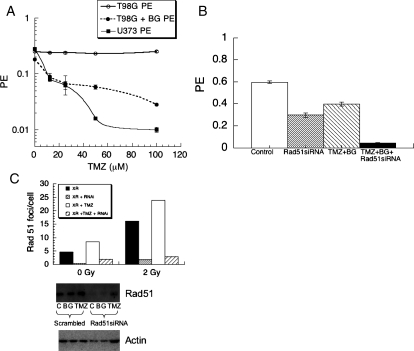
HGGs are frequently treated with TMZ as well as XR. Therefore, we also assessed the affect of Rad51 knockdown on sensitivity to TMZ in the same cells. Figure 4A shows that TMZ alone has no effect on PE of T98G (solid line, open symbols), as has been shown previously, due to high MGMT levels.16 Cytotoxicity could be promoted when Bg was coadministered (Fig. 4A dashed line), which produced toxicity levels approaching those in an MGMT deficient cell line, U373 (Fig. 4A solid line filled symbols). Figure 4B shows the effect of Rad51 siRNA compared with nonspecific sequence with and without TMZ and Bg treatment on PE in T98G cells, demonstrating an increase in cytotoxicity when siRNA is used prior to TMZ. PE with TMZ + Bg (hatched bar) = 0.397 with Rad51 knockdown (grey bar) = 0.29, with TMZ + Rad51 knockdown (black bar) = 0.04. Figure 4C demonstrates that knockdown reduced protein levels assessed on western blot (lower panel) and abolished Rad51 foci formation measured as mean foci numbers per cell on immunocytochemistry (bar chart, upper panel) after XR or combined treatment. These data suggest that Rad51 knockdown enhances TMZ toxicity at a dose that induces DNA damage, consistent with previously published studies showing that repair of TMZ-induced damage is Rad51 dependent.
Fig. 4.
Cytotoxicity of TMZ ± BG on T98G (high MGMT, circles) and U373 (low MGMT, squares) glioma cells (A). Asynchronous cells were exposed to TMZ ± BG for 3 h then replated in fresh medium and assessed for colony formation 10–14 days later. The effect of Rad51 knockdown on cytotoxicity of TMZ was assessed using siRNA (B), showing significantly enhanced toxicity with combined treatment in T98G cells. Knockdown was associated with abolition of Rad51 foci assessed by immunocytochemistry in T98G cells (C, upper panel) and reduced protein levels on western blot (C, lower panel).
Rad51 knockdown and combination treatment
Newly diagnosed HGGs are treated with combination XR and concomitant TMZ. We assessed cytotoxicity of combination treatment in 2 of the cell lines discussed above; T98G (high MGMT) and U373 (low MGMT). As described above, TMZ alone had no effect on plating efficiency of T98G, as expected, but cytotoxicity could be promoted when Bg was coadministered, U373 has low MGMT levels and is sensitive to TMZ alone. There was no effect of Bg alone in either cell line (data not shown). When cytotoxic doses of TMZ were administered prior to XR, no radiosensitizing effect of TMZ ± Bg was observed in either cell line in clonogenic survival experiments. Figures 5A and 5B show equivalent surviving fraction data when the reduced PE due to TMZ is taken into account in both cell lines. Since Rad51 knockdown produced increased cell killing when these agents were used alone, we postulated that reducing Rad51 levels would significantly improve cytotoxicity after combined treatment. Figure 5C shows the results of Rad51 knockdown on cytotoxicity in clonogenic survival experiments after combined treatment in T98G cells expressed as changes in PE, confirming a significant effect on cytotoxicity when siRNA directed against Rad51 is added to combined treatment with XR + TMZ. It is noteworthy that the effect of Rad51 knockdown in these circumstances is mainly due to reduced PE associated with TMZ treatment. There does not appear to be any additional radiosensitizing effect in these experiments. This is illustrated in Fig. 5D, which shows these data as relative survival after TMZ and XR accounting for the effect of Rad51 knockdown in unirradiated cells.
Fig. 5.
The effect of TMZ ± BG on clonogenic survival after XR. Asynchronous cells were exposed to TMZ 100 µM then irradiated with single doses in the range 0.1–5 Gy on day 3. Data points represent means from 3 flasks at each data point and are shown as surviving fractions (SF), normalized for the effect of TMZ on unirradiated cells. T98G (high MGMT cells with BG) upper panel (A), U373 (low MGMT cells) lower panel (B). Solid line, XR only, dashed line XR + TMZ. (C) The effect of Rad51 knockdown using short interfering RNA on clonogenic survival of T98G glioma cells following XR, or XR + TMZ. Solid triangle symbols, solid line = XR alone, open triangle symbols dashed line = XR + Rad51siRNA, open circular symbols = XR + TMZ, open square symbols = XR + TMZ + Rad51siRNA expressed as plating efficiency (PE). Relative survival of combined treatment with TMZ + Rad51siRNA (square symbols dashed line) compared to XR alone (triangular symbols solid line) is shown separately (D).
Repair of DSB after XR, TMZ, and combined treatment
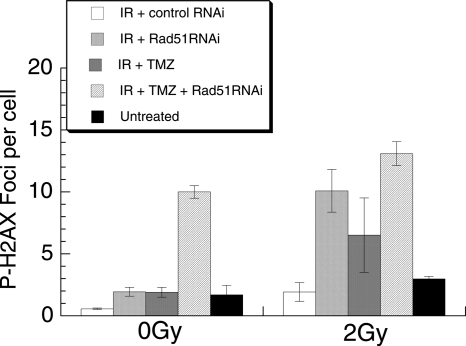
In addition to promoting sensitivity to both XR and TMZ, inhibiting Rad51-dependent repair may be expected to change the kinetics of DSB repair, since it is responsible for a predominantly slow repair component. We have measured this using induction and resolution of phosphorylated H2AX foci in cells treated with each agent alone and with combination treatment with and without Rad51 siRNA. H2AX foci form rapidly at sites of chromatin structural change associated with DNA DSB, and enumeration of these foci has been shown to correlate closely with induction and repair of radiation–induced DSB.35 It has been demonstrated recently that persistence of H2AX foci at 24 h is closely correlated with loss of clonogenicity following exposure to a variety of DNA-damaging agents, including radiation and TMZ.36 Data assessing H2AX foci after XR, TMZ, and combination treatments are shown in Figure 6, which demonstrates increased residual H2AX foci at 24 h following combined treatment with XR, TMZ, and Rad51 knockdown. Following 2 Gy or TMZ alone, foci numbers return to baseline at 24 h; however, Rad51 siRNA significantly increases foci numbers at 24 h after TMZ (P = .0002) and after 2 Gy (P = .012). The addition of siRNA also causes a significant increase in foci at 24 h following combination treatment compared with the effect of nonspecific sequences (P = .0008), which is larger than the effect of siRNA with TMZ only (P = .05).
Fig. 6.
γ-H2AX foci at 24 h post treatment with XR, TMZ, Rad51 siRNA, and combined treatment of T98G cells. Cells were treated with XR alone + control siRNA (white bar), XR + Rad51siRNA (close hatched bar), XR + TMZ + control siRNA (grey bar) or XR + TMZ + Rad51siRNA or untreated (black bar). Cells were fixed for immunocytochemistry 24 h later and fluorescent foci were counted manually. γ-H2AX data points represent means from 3 different samples, error bars are SEM.
Rad51 knockdown in newly established GBM cells
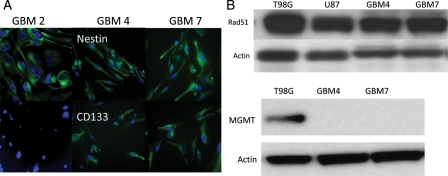
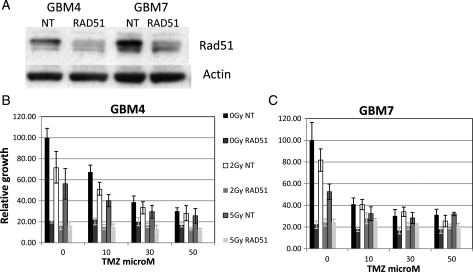
Since established glioma cell lines may have undergone specific adaption and mutation that have allowed for their prolonged passage in culture and may therefore display different repair responses compared with GBM cells newly established from tumors, we also investigated the role of Rad51 in newly established GBM cell lines. We first confirmed that these cell lines express the stem cell marker proteins CD133 and nestin, as shown in representative immunoflourescent staining images in Figure 7A. We then investigated Rad51 protein levels in 2 of these cell lines. Western blots from cell lines designated GBM 4 and 7 are shown in Figure 7B alongside T98G and U87 cells and demonstrate that the newly established cells have similar Rad51 levels to the established cell lines. These experiments also confirmed no significant MGMT protein in these cell lines, predicting sensitivity to TMZ. In preliminary experiments, we defined TMZ cytotoxicity in 2 of these cell lines, GBM 4 and GBM 7, and confirmed an additive effect of TMZ + XR, as in the established glioma cells. Figure 8 shows toxicity data measured using the SRB assay in GBM4 and GBM7 cells exposed to graded TMZ concentrations and 0, 2, and 5 Gy XR. We then targeted Rad51 using siRNA in these cells and measured the effect of Rad51 knockdown on cytotoxicity of XR and TMZ using the SRB assay. The results of these experiments are shown in Figure 9, in which the effect of Rad51 knockdown following 0, 2, or 5 Gy XR is shown across a range of TMZ concentrations between 10 and 50 µM. We repeated each experiment twice in each cell line with very similar results. These data suggest that compared with the established cell lines, GBM4 and GBM7 are extremely sensitive to Rad51 inhibition, which causes a dramatic reduction in viability compared with nontargeting control sequences. This effect is so marked that an additional effect of Rad51 targeting on the cytotoxicity of XR and/or TMZ cannot be quantified in these experiments. This is dissimilar to the effect in established glioma cells, in which PE was much less affected by Rad51 knockdown and suggests that even in the absence of exogenous damage Rad51 is important for viability in these newly established cells.
Fig. 7.
Immunohistochemistry using fluorescent antibodies to detect Cd133 and nestin protein in 3 newly established glioma cell lines, GBM 2, 4, and 7 (A). (B) Western blot of repair proteins Rad51 and MGMT in GBM 4 and 7 cells compared to T98G and U87.
Fig. 8.
Cytotoxicity of temozolomide in the 2 newly established glioma cell lines GBM 4 (A) and 7 (B). Cells were exposed to graded doses of TMZ for 3 h on day 0 then irradiated with XR doses 0, 2, or 5 Gy on day 8 then assessed for viability on day 5 post XR using the SRB assay. Data points represent means from 6 experimental points at each dose combination in a single experiment.
Fig. 9.
(A) Western blot showing Rad51 knockdown in GBM4 and GBM 7 cells. (B) and (C) Survival data in GBM4 and GBM 7 cells following a 3 h exposure to TMZ (0, 10, 30, 50 µM) on day 1 followed by XR (0, 2, or 5 Gy) on day 6, assessed by SRB assay on day 3 post XR. Data points represent means from 6 experimental points within one experiment. Error bars are standard deviations.
Rad51 in fixed tumor specimens
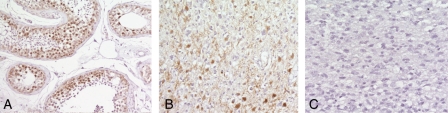
Since these in vitro data suggest that Rad51 may be relevant to toxicity of XR and TMZ in glioma cell lines and to maintaining viability in newly established GBM cells, we then investigated whether high Rad51 levels in glioma specimens could be associated with prognosis, as suggested by other authors.37 We stained a series of 19 different formalin fixed glioma specimens for Rad51 protein levels. Table 1 lists the histological diagnosis and proportion of cells staining for Rad51 in these samples. Figure 10 shows a representative section of testis (used as positive control), a grade III astrocytoma, and a grade IV astrocytoma (i.e., GBM). These data suggest that the proportion of cells staining for Rad51 in tissue sections of glioma is variable and does not correlate with tumor grade.
Table 1.
Glioma specimens examined for tissue levels of Rad51 protein using immunohistochemistry of paraffin embedded material. Nineteen specimens of varying grade (column 2) were examined and scored as % cells positive (column 3).
| Tumor type | Grade | % Cells Rad51 +ve |
|---|---|---|
| Pilocytic astrocytoma | I | >50 |
| Pilocytic astrocytoma | I | 0 |
| Gemistocytic astrocytoma | II | 10–50 |
| Oligodendroglioma | II | >50 |
| Oligodendroglioma | II | 0 |
| Diffuse astrocytoma | II | 0 |
| Fibrillary astrocytoma | II | 0 |
| Diffuse astrocytoma | II | >50 |
| Fibrillary astrocytoma | II | 0 |
| Fibrillary astrocytoma | II | 0 |
| Anaplastic astocytoma | III | 10–50 |
| GBM | IV | 0 |
| GBM | IV | 0 |
| GBM | IV | 0 |
| GBM | IV | 0 |
| GBM | IV | 10–50 |
| GBM | IV | >50 |
| GBM | IV | 0 |
Fig. 10.
Glioma specimens stained for Rad51 protein compared to a positive control (testis). Panel (A) testis, showing nuclear Rad51 staining in the seminferous tubules; (B) a grade III astrocytoma, showing nuclear staining; and (C) a grade IV tumor with no Rad51 staining.
Discussion
In the studies discussed here we have confirmed that radioresistant HGG cells exhibit high Rad51 protein levels in vitro. This is also true of recently established GBM cell lines and is consistent with published data in several tumor types in which it has been correlated to treatment outcome—for example, in lung, head and neck, and breast carcinomas, high Rad51 protein levels have been related to poorer outcome,38–40 and high expression levels may be related to an aggressive phenotype in prostate cancer.41 Recent data have also related low Rad51 foci numbers at 24 h post treatment to improved response to neoadjuvant chemotherapy in breast cancer.42 In glioblastoma specimens, however, there are data suggesting that high Rad51 levels may be associated with a better prognosis.37 Despite convincing in vitro evidence of high Rad51 levels in HGG cell lines, in our series of clinical glioma specimens it is clear that Rad51 expression measured using immunohistochemistry is variable and does not correlate with grade. This may reflect differences among subpopulations of cells within these tumors, which represent those that can be established as cell lines but which cannot be identified separately on immunohistochemistry. It is also possible that within tumors of the same grade—for example, GBM—this relationship may be different. Nevertheless, considering the large number of clinical and pathological variables that are known to impact on prognosis even within glioma subtypes, it will be difficult to establish the relevance of Rad51 expression outside very large clinical series.
In addition to high Rad51 expression, all the established glioma cell lines we studied also showed an increase in Rad51 foci positive cells after low radiation doses, which could be increased further with the addition of TMZ in both TP53wt and TP53mut cells, consistent with a model in which these protein complexes are formed at sites of ongoing DSB repair. The relationship between induction and resolution of Rad51 foci with radiosensitivity and chemosensitivity is not clear. One previous study found no correlation between numbers of foci at 4 h and SF2, but suggested that persistence of foci beyond 24 h was associated with radiosensitivity, and this has been confirmed by recent studies using a variety of DNA damaging agents in cells that express GFP-tagged Rad51.43 In the radioresistant established glioma cell lines that we assessed, Rad51 foci induced by XR alone disappeared by 24 h (data not shown), consistent with the notion that foci resolution is associated with efficient repair and radioresistance. Other authors have also documented a plateau effect in Rad51 foci numbers above a certain XR dose level, but the mechanism behind this remains unclear.44
High Rad51 levels and dose-dependent foci formation suggest that Rad51-dependent repair makes a significant contribution to DSB repair after clinically relevant radiation doses in glioma cells. The data we have presented here using Rad51 knockdown confirm that this is the case; asynchronous cells are significantly radiosensitized when Rad51 protein levels are reduced and the sensitization is proportional to the protein levels. This is consistent with previous data showing marked G2 delay after low XR doses and large component of slow repair in these cells28 and suggests that in these cells HR contributes to a larger proportion of repair following XR than in other cell lines, including NHA.
Other studies have suggested a significant influence of Rad51 in chemoresistance of some tumor cell lines such as sarcomas, implying that inhibition may potentially sensitize tumor cells.26 The relevance of Rad51 levels to TMZ resistance is predictable, as this drug causes a prominent G2 cell cycle delay, which is thought to promote efficient repair of cytotoxic DSBs. Since a large proportion of treated cells are delayed in G2 phase, Rad51-dependent repair would be predicted to make a significant contribution to recovery. This has been confirmed by recently published data, which suggest that resolution of DSBs produced by alkylator induced O6-methlylation of guanine is critically dependent on homologous recombination.11 Our data confirm that TMZ cytotoxicity is significantly enhanced by Rad51 siRNA and that this is associated with reduced repair foci formation and excess unrepaired DSB at 24 h assessed by γ-H2AX labeling.
We also postulated that in the presence of high Rad51 levels, HGG cells may be efficient at repairing DSB caused by both XR and TMZ and therefore no radiosensitizing effect of TMZ would be apparent, but that inhibition of relevant repair pathways may promote more marked cytotoxicity of combined treatment. The data shown here suggest that combination treatment including XR, TMZ, and Rad51 siRNA produces a marked reduction in survival of T98G cells and that this triple combination treatment is associated with a large fraction of DSBs that remain unrepaired at 24 h post treatment. Nevertheless, our survival data do not suggest a synergistic effect, in fact an additional effect of Rad51 knockdown on XR survival is not apparent when all 3 agents are used. This may in part be due to the difficulty of defining changes in survival in the context of the low PEs apparent when Rad51 knockdown is used with TMZ. It is also possible that the effect of Rad51 knockdown in the presence of TMZ selectively kills cells in S/G2 phase, which would also be the population that is sensitized to XR, therefore no additional toxicity is apparent with the combined treatment. Despite this, the DSB repair data suggest that a further exposure after a 24-h gap, as in clinical radiotherapy regimens, may promote further cytotoxicity due to residual unrepaired DNA DSB.
Our data using newly established GBM cell lines also confirm high Rad51 levels; however, unlike the established glioma cells, these cell lines show a marked sensitivity to Rad51 knockdown even in untreated cells. This effect was so marked that we could not assess whether there was an additional effect on XR or TMZ sensitivity in the dose ranges we studied. This suggests a dependence on Rad51 repair in the absence of exogenous damage in newly established glioma cells that is not apparent in established cell lines. The reason for this difference in sensitivity is unclear; however, a role for Rad51 in replication in the absence of exogenous damage has recently been suggested, in which it may protect single-strand intermediates from nuclease digestion. This may suggest that high levels of replication stress could increase Rad51 dependence.45 Whether this is the explanation in glioma cell lines used here requires further investigation.
Overall, the data presented here suggest an important influence of Rad51-dependent repair on radioresistance and chemoresistance in glioma cell lines at clinically relevant doses and on the viability of newly established glioma cell lines. Preclinical studies are beginning to confirm that Rad51 is a potentially effective target in gliomas treated with radiation. Most notably gene therapy delivering siRNA targeted against HR has recently been demonstrated to be an effective radiosensitizer in small animal models.46 Clinical studies have also suggested that agents that inhibit homologous recombination, including Gleevec, can be effective in treating gliomas when combined with DNA damaging agents such as hydroxyurea, which promotes HR intermediates at stalled replication forks.47 Targeting HR is a particularly appealing strategy because of the potential selectivity for tumor cells and new approaches to reducing Rad51 activity are being developed—for example, by utilizing high affinity peptides derived from BRCA2 binding site proteins to reduce functional Rad51 levels or to target the gene promoter in suicide gene therapy.48,49 This approach would produce a favorable therapeutic ratio for locally delivered therapy, since noncycling surrounding brain tissue is not expected to exhibit significant dependence on Rad51-dependent repair. For systemically delivered treatment, there may be a risk of sensitizing nontumor, dividing tissue, although the very high level of Rad51 in glioma cells is likely to provide a therapeutic window, which would need to be defined in preclinical in vivo models.
In summary, these data confirm the importance of Rad51-dependent repair in glioma cells and suggest that approaches that inhibit recombination repair may prove to be an effective adjunct in standard clinical regimens using XR and TMZ.
Funding
Cancer Research UK (C7817).
Acknowledgments
This work was supported by UCLH/UCL Comprehensive Biomedical Research Centre.
Conflict of interest statement. None declared.
References
- 1.Douglas JG, Stelzer KJ, Mankoff DA, et al. [F-18]-fluorodeoxyglucose positron emission tomography for targeting radiation dose escalation for patients with glioblastoma multiforme: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2006;64:886–891. doi: 10.1016/j.ijrobp.2005.08.013. doi:10.1016/j.ijrobp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Tisdale MJ. Antitumor imidazotetrazines–XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem Pharmacol. 1987;36:457–462. doi: 10.1016/0006-2952(87)90351-0. doi:10.1016/0006-2952(87)90351-0. [DOI] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. doi:10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 5.Ceccotti S, Aquilina G, Macpherson P, et al. Processing of O6-methylguanine by mismatch correction in human cell extracts. Curr Biol. 1996;6:1528–1531. doi: 10.1016/s0960-9822(96)00758-0. doi:10.1016/S0960-9822(96)00758-0. [DOI] [PubMed] [Google Scholar]
- 6.Caporali S, Falcinelli S, Starace G, et al. DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol. 2004;66:478–491. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 7.Quiros S, Roos W, Kaina B. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas aopotosis is also induced in subsequent cell cycles. Cell Cycle. 2010;9:168–178. doi: 10.4161/cc.9.1.10363. doi:10.4161/cc.9.1.10363. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-methylguanine adducts. Mol Cell. 2006;22:501–510. doi: 10.1016/j.molcel.2006.04.023. doi:10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13:2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. doi:10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermisson M, Klumpp A, Wick W, et al. O6-methylguanine DNA methyltransferase and TP53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. doi:10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 11.Roos WP, Nikolova T, Quiros S, et al. Brca2/Xrcc2 dependent HR, but not NHEJ, is required for protection against O(6)-methylguanine triggered apoptosis, DSBs and chromosomal aberrations by a process leading to SCEs. DNA Repair (Amst) 2009;8:72–86. doi: 10.1016/j.dnarep.2008.09.003. doi:10.1016/j.dnarep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12:4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. doi:10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 13.Wedge SR, Porteous JK, Glaser MG, et al. In vitro evaluation of temozolomide combined with X-irradiation. Anticancer Drugs. 1997;8:92–97. doi: 10.1097/00001813-199701000-00013. doi:10.1097/00001813-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 14.van Rijn J, Heimans JJ, van den Berg J, et al. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–784. doi: 10.1016/s0360-3016(99)00539-8. doi:10.1016/S0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 15.van Nifterik KA, van den Berg J, Stalpers LJ, et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69:1246–1253. doi: 10.1016/j.ijrobp.2007.07.2366. doi:10.1016/j.ijrobp.2007.07.2366. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers AJ, Ruff EM, Martindale C, et al. Cytotoxic effects of temozolomide and radiation are additive- and schedule-dependent. Int J Radiat Oncol Biol Phys. 2009;75:1511–1519. doi: 10.1016/j.ijrobp.2009.07.1703. doi:10.1016/j.ijrobp.2009.07.1703. [DOI] [PubMed] [Google Scholar]
- 17.Lees-Miller SP, Godbout R, Chan DW, et al. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. doi:10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 18.He F, Li L, Kim D, et al. Adenovirus-mediated expression of a dominant negative Ku70 fragment radiosensitizes human tumor cells under aerobic and hypoxic conditions. Cancer Res. 2007;67:634–642. doi: 10.1158/0008-5472.CAN-06-1860. doi:10.1158/0008-5472.CAN-06-1860. [DOI] [PubMed] [Google Scholar]
- 19.Daido S, Yamamoto A, Fujiwara K, et al. Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy. Cancer Res. 2005;65:4368–4375. doi: 10.1158/0008-5472.CAN-04-4202. doi:10.1158/0008-5472.CAN-04-4202. [DOI] [PubMed] [Google Scholar]
- 20.Short SC, Bourne S, Martindale C, et al. DNA damage responses at low radiation doses. Radiat Res. 2005;164:292–302. doi: 10.1667/rr3421.1. doi:10.1667/RR3421.1. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarti A, Zhai GG, Zhang M, et al. Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene. 2004;23:7494–7506. doi: 10.1038/sj.onc.1208049. doi:10.1038/sj.onc.1208049. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto A, Taki T, Yagi H, et al. Cell cycle-dependent expression of the mouse Rad51 gene in proliferating cells. Mol Gen Genet. 1996;251:1–12. doi: 10.1007/BF02174338. [DOI] [PubMed] [Google Scholar]
- 23.Esashi F, Christ N, Gannon J, et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 2005;434:598–604. doi: 10.1038/nature03404. doi:10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- 24.Bahassi EM, Ovesen JL, Riesenberg AL, et al. The checkpoint kinases Chk1 and Chk2 regulate the functional associations between hBRCA2 and Rad51 in response to DNA damage. Oncogene. 2008;27:3977–3985. doi: 10.1038/onc.2008.17. [DOI] [PubMed] [Google Scholar]
- 25.Koehn H, Magan N, Isaacs RJ, Stowell KM. Differential regulation of DNA repair protein Rad51 in human tumor cell lines exposed to doxorubicin. Anticancer Drugs. 2007;18:419–425. doi: 10.1097/CAD.0b013e328012a9a0. doi:10.1097/CAD.0b013e328012a9a0. [DOI] [PubMed] [Google Scholar]
- 26.Hannay JA, Liu J, Zhu QS, et al. Rad51 overexpression contributes to chemoresistance in human soft tissue sarcoma cells: a role for TP53/activator protein 2 transcriptional regulation. Mol Cancer Ther. 2007;6:1650–1660. doi: 10.1158/1535-7163.MCT-06-0636. doi:10.1158/1535-7163.MCT-06-0636. [DOI] [PubMed] [Google Scholar]
- 27.Beucher A, Birraux J, Tchouandong L, et al. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009;28:3413–3427. doi: 10.1038/emboj.2009.276. doi:10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Short SC, Martindale C, Bourne S, et al. DNA repair after irradiation in glioma cells and normal human astrocytes. Neuro Oncol. 2007;9:404–411. doi: 10.1215/15228517-2007-030. doi:10.1215/15228517-2007-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golding SE, Rosenberg E, Khalil A, et al. Double strand break repair by homologous recombination is regulated by cell cycle-independent signaling via ATM in human glioma cells. J Biol Chem. 2004;279:15402–15410. doi: 10.1074/jbc.M314191200. doi:10.1074/jbc.M314191200. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi T, Taki T, Hiraga S, et al. In vitro and in vivo potentiation of radiosensitivity of malignant gliomas by antisense inhibition of the RAD51 gene. Biochem Biophys Res Commun. 1998;245:319–324. doi: 10.1006/bbrc.1998.8440. doi:10.1006/bbrc.1998.8440. [DOI] [PubMed] [Google Scholar]
- 31.Russell JS, Brady K, Burgan WE, et al. Gleevec-mediated inhibition of Rad51 expression and enhancement of tumor cell radiosensitivity. Cancer Res. 2003;63:7377–7383. [PubMed] [Google Scholar]
- 32.Short SC, Mitchell SA, Boulton P, et al. The response of human glioma cell lines to low-dose radiation exposure. Int J Radiat Biol. 1999;75:1341–1348. doi: 10.1080/095530099139214. doi:10.1080/095530099139214. [DOI] [PubMed] [Google Scholar]
- 33.Pollard S, Clarke ID, Smith A, et al. Brain cancer stem cells: A level playing field. Cell Stem Cell. 2009;5:468–469. doi:10.1016/j.stem.2009.10.016. [Google Scholar]
- 34.Durand RE. Use of a cell sorter for assays of cell clonogenicity. Cancer Res. 1986;46:2775–2778. [PubMed] [Google Scholar]
- 35.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. doi:10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banath JP, Klokov D, Macphail SH, et al. Residual gammaH2AX foci as an indication of lethal DNA lesions. BMC Cancer. 2010;10:4. doi: 10.1186/1471-2407-10-4. doi:10.1186/1471-2407-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsh JW, Ellsworth RK, Kumar R, et al. Rad51 protein expression and survival in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;74:1251–1255. doi: 10.1016/j.ijrobp.2009.03.018. doi:10.1016/j.ijrobp.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Connell PP, Jayathilaka K, Haraf DJ, et al. Pilot study examining tumor expression of RAD51 and clinical outcomes in human head cancers. Int J Oncol. 2006;28:1113–1119. [PubMed] [Google Scholar]
- 39.Qiao GB, Wu YL, Yang XN, et al. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer. 2005;93:137–143. doi: 10.1038/sj.bjc.6602665. doi:10.1038/sj.bjc.6602665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Scodan RC-CG, Fourme E, et al. DNA repair gene expression and risk of locoregional relapse in breast cancer patients. Int J Radiat Oncol Biol Phys. 2010;78:328–336. doi: 10.1016/j.ijrobp.2009.07.1735. [DOI] [PubMed] [Google Scholar]
- 41.Mitra A, Jameson C, Barbachano Y, et al. Overexpression of RAD51 occurs in aggressive prostatic cancer. Histopathology. 2009;55:696–704. doi: 10.1111/j.1365-2559.2009.03448.x. doi:10.1111/j.1365-2559.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graeser MK, McCarthy A, Lord CJ, et al. A marker of homologous recombination predicts pathological complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16:6159–6168. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sak A, Stueben G, Groneberg M, et al. Targeting of Rad51-dependent homologous recombination: implications for the radiation sensitivity of human lung cancer cell lines. Br J Cancer. 2005;92:1089–1097. doi: 10.1038/sj.bjc.6602457. doi:10.1038/sj.bjc.6602457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staudt C, Iliakis G. Rad51 foci plateau with increasing radiation dose in mammalian cells; Proc 11th International Wolfsberg Meeting on Molecular Radiation/Oncology, Ermatingen, Switzerland 2009. [Google Scholar]
- 45.Hashimoto Y, Chaudhuri AR, Lopes M, et al. Rad51 protects nascent DNA from Mre11-dependent degradation and promotes continuous DNA synthesis. Nature Struct Mol Biol. 2010;17:1305–1311. doi: 10.1038/nsmb.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saydam O, Saydam N, Glauser DL, et al. HSV-1 amplicon-mediated post-transcriptional inhibition of Rad51 sensitizes human glioma cells to ionizing radiation. Gene Ther. 2007;14:1143–1151. doi: 10.1038/sj.gt.3302967. doi:10.1038/sj.gt.3302967. [DOI] [PubMed] [Google Scholar]
- 47.Desjardins A, Quinn JA, Vredenburgh JJ, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83:53–60. doi: 10.1007/s11060-006-9302-2. doi:10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 48.Nomme J, Takizawa Y, Martinez SF, et al. Inhibition of filament formation of human Rad51 protein by a small peptide derived from the BRC-motif of the BRCA2 protein. Genes Cells. 2008;13:471–481. doi: 10.1111/j.1365-2443.2008.01180.x. doi:10.1111/j.1365-2443.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- 49.Hine CM, Seluanov A, Gorbunova V. Use of the Rad51 promoter for targeted anti-cancer therapy. Proc Natl Acad Sci U S A. 2008;105:20810–20815. doi: 10.1073/pnas.0807990106. doi:10.1073/pnas.0807990106. [DOI] [PMC free article] [PubMed] [Google Scholar]