Abstract
Recurrence of primary central nervous system lymphoma (PCNSL) after initial diagnosis and treatment occurs within 2 years in most patients, and relapse after 5 years is rare. We evaluated late relapse in our PCNSL population. We identified 10 patients from our database of 378 patients (268 achieved a complete response and 230 had relapse) with PCNSL who had relapse ≥5 years after initial diagnosis. At initial diagnosis, their median age was 47 years; all patients had brain involvement and achieved a complete response to initial therapy (9 received high-dose methotrexate). Median time to first relapse was 7.4 years (range, 5.2–14.6 y). Eight patients had relapse in the brain, 1 had ocular relapse, and 1 had a systemic relapse. The histologic specimens at initial diagnosis and relapse were examined for clonal rearrangement in 3 patients; 1 had the identical clone at initial diagnosis and relapse 13.8 years later, and the other 2 were uninformative. All patients received salvage therapy (9 received systemic therapy and 1 received intraocular chemotherapy. Nine patients achieved a complete response to salvage therapy and 1 achieved a partial response. Four patients had relapse a second time. The median progression-free survival after first relapse was 31 months (range, 7.9–82.4). Late relapses accounted for 4% of all recurrences (10 of 230 patients) in our PCNSL population. Long-term persistence of the PCNSL clone was observed in one patient. Patients with late relapses have a good response to salvage therapy and prolonged survival.
Keywords: high-dose methotrexate, late relapse, primary central nervous system lymphoma, salvage therapy
Primary central nervous system lymphoma (PCNSL) is an aggressive extra-nodal non-Hodgkin lymphoma that arises from the brain, spinal cord, leptomeninges, or eye. Although uncommon, its incidence has been increasing steadily over the past few decades and currently accounts for ∼3% of all primary brain tumors.1 The overall survival (OS) and progression-free survival (PFS) have increased with the introduction of high-dose methotrexate–based regimens, with or without cranial irradiation. Despite this, relapse is common and the 5-year survival rate is low (22%–40%). Approximately 35%–60% of patients have relapse, and most relapses occur within the first two years of diagnosis.2–4 Late relapses are uncommon, and to our knowledge, there have been only 2 patients with PCNSL described in the literature with relapse after 5 years in remission.5,6 We examined the Memorial Sloan-Kettering Cancer Center (MSKCC) PCNSL population to determine the frequency of late relapse.
Methods
This retrospective study was approved by the MSKCC Institutional Review Board.
We reviewed our database from 1983–2004 of 378 patients with PCNSL to identify those whose first relapse occurred ≥5 years after initial diagnosis. Only patients who achieved a complete response (CR) to initial therapy were evaluated for late relapse. We excluded immunocompromised patients and those with a prior history of systemic lymphoma. PCNSL was confirmed at MSKCC in all patients. All patients underwent cranial MRI and body PET/CT at relapse, and 8 underwent cerebrospinal fluid (CSF) and ocular assessment. Response to salvage treatment was evaluated by MRI. CR was defined as resolution of enhancing tumor with clinical improvement or stability after discontinuation of corticosteroids. Partial response was defined as a ≥50% reduction in the size of the lesion when the patient's corticosteroid dose was stable or decreasing. Progressive disease was defined as an unequivocal increase in tumor size or appearance of new lesions; stable disease was all other situations.
Clonality was assessed in the initial as well as relapsed biopsy samples if both were available. The samples were analyzed in duplicate using the Biomed-2 protocols and the multiplex primer sets IGH (VH–JH), TCRB (Vb–Jb and Db–Jb), and TCRG from InVivoScribe Technologies, in accordance with the manufacturer's protocol. Each sample was analyzed by GeneScan analysis on the ABI 3730 DNA analyzer (Life Technologies).
Statistics
Survival was measured from the date of initial diagnosis to death or last follow-up visit. Median time to relapse was calculated from initial diagnosis to the date of first relapse. PFS from the first relapse to the second relapse or death was also calculated. Survival was assessed using the Kaplan–Meier estimation methods.
Results
Patient Characteristics
There were 378 patients with PCNSL seen at MSKCC, 268 of whom achieved a CR. Of these patients, 230 had relapse; 220 had relapsed <5 years after diagnosis. Ten patients had relapsed ≥5 years after initial diagnosis. At initial diagnosis, the median age of these 10 patients was 47 years (range, 35–71 y); 6 patients were <50 years of age. There were 7 men and 3 women. Karnofsky performance status (KPS) was evaluated in 7 patients at diagnosis and was ≥70 in 5. One patient had a remote history of colon cancer; no patient had a history of systemic lymphoma or immunosuppression. All patients had brain involvement at initial diagnosis; 6 had a single brain lesion, and 4 had multiple lesions. Two patients had evidence of leptomeningeal enhancement on brain MRI. CSF specimens were examined in 9 patients, and test results were positive for malignant cells in 1. Seven patients underwent a slit-lamp examination and were found to have no evidence of ocular lymphoma. The histology was diffuse large B-cell lymphoma in all patients.
Treatment
Initial treatment included a high-dose methotrexate (HD-MTX)–based regimen in 9 patients, 5 of whom underwent whole brain radiation (WBRT) as well (Table 1). One patient was treated with WBRT alone. All patients achieved a CR to initial therapy within 1.6–7.8 months (median, 4.3 mo) after starting treatment.
Table 1.
Initial and salvage treatment
| Patient | Initial treatment | Relapse location | Interval to relapse (y) | Salvage treatment | Response | PFS after relapse (mo) | Second relapse | Overall survival (y) |
|---|---|---|---|---|---|---|---|---|
| 1 | HD-MTX, IO MTX, WBRT, Ara-C | Brain (original site), CSF | 7.8 | HD-MTX | PR | ≥10.1 | − | ≥8.7 |
| 2 | HD-MTX, IO MTX, WBRT, Ara-C | Brain (unknown if original or other site), CSF, eyes | 7.3 | HD-MTX, ocular RT | CR | 20.4 | + | ≥13.3 |
| 3 | MPV, Ara-C | Kidney | 7.3 | R-CHOP | CR | ≥82.4 | − | ≥14.1 |
| 4 | HD-MTX, Ara-C | Brain (other site) | 7.5 | R-temo | CR | ≥57.7 | − | ≥12.2 |
| 5 | HD-MTX, Ara-C, BEAM, ASCT | Brain (other site) | 5.5 | R-MPV, BuCyTT, ASCT | CR | ≥58.2 | − | ≥10.3 |
| 6 | WBRT | Brain (other site), CSF | 5.2 | HD-MTX, IO MTX, WBRT, Ara-C | CR | ≥41.6 | − | 8.6 |
| 7 | MPV,WBRT, Ara-C | Brain (other site) | 13.8 | MPV | CR | 15 | + | ≥15.9 |
| 8 | HD-MTX, WBRT, PCV | Eyes | 6.9 | Intra-ocular MTX | CR | 17.8 | + | 8.4 |
| 9 | MPV,WBRT, Ara-C | Brain (other site) | 14.6 | MV, Ara-C | CR | 10.9 | + | ≥15.8 |
| 10 | MPV, Ara-C | Brain (original and other sites) | 8 | R-MP, Ara-C | CR | ≥7.9 | − | ≥8.6 |
Ara-C, cytarabine; ASCT, autologous stem-cell transplant; BEAM, carmustine, etoposide, cytarabine, melphalan; BuCyTT, busulfan, cyclophosphamide, thiotepa; CR, complete response; HD-MTX, high-dose methotrexate; IO MTX, intra-Ommaya methotrexate; MPV, high-dose methotrexate, procarbazine, vincristine; PCV, procarbazine, lomustine, vincristine; PR, partial response; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-temo, rituximab, temozolomide; WBRT, whole-brain radiation therapy.
Relapse
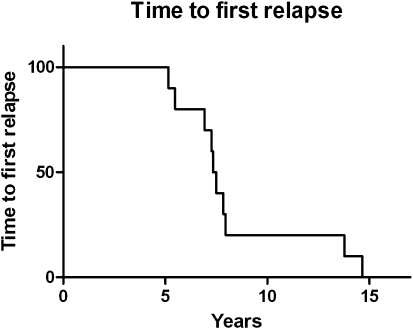
Median time to first relapse was 7.4 years (range, 5.2–14.6 y) (Fig. 1). Eight patients had relapse in the brain, 1 patient had ocular relapse only, and 1 had an isolated systemic (renal) relapse. Of the 8 patients who had relapse in the brain, 6 developed lesions in sites remote from the original disease; 2 of these 8 patients had relapse in the brain and leptomeninges, and 1 had relapse in the brain, leptomeninges, and eye. Nine patients had histologic confirmation of relapse. Pathologic specimens from initial diagnosis and relapse were available in 3 patients with central nervous system (CNS) recurrence and examined for clonal rearrangement. The identical clone was present at both initial diagnosis and relapse 13.8 years later in 1 patient (Fig. 2). Tissue examination was uninformative in the other 2 patients.
Fig. 1.
Time to first relapse.
Fig. 2.
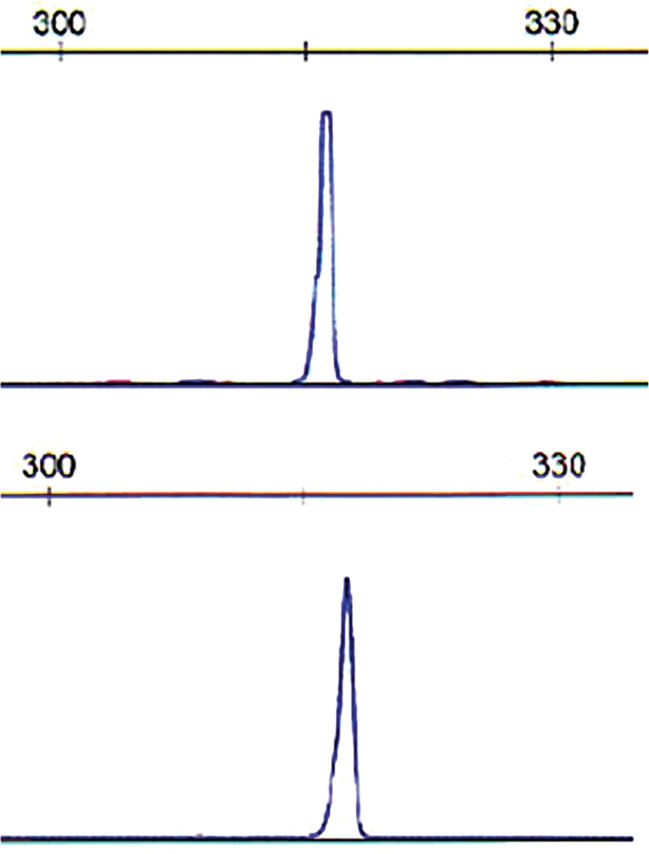
Clonality analysis of lymphoma samples at diagnosis and relapse. Polymerase chain reaction (PCR) was performed using established Biomed-2 protocols and analyzed by capillary electrophoresis. An identical immunoglobulin heavy chain (IgH) gene rearrangement is identified in the lymphoma at initial diagnosis (upper) and at relapse (lower). PCR for framework region 1 (FR1) is shown. The scale indicates the size of the PCR product in base pairs.
Salvage Therapy
All patients received salvage therapy; 9 underwent systemic chemotherapy, and 1 was treated with intra-ocular chemotherapy alone (Table 1). The patient with relapse limited to the kidney received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). The remaining 8 patients received a HD-MTX–based regimen (7 patients) or rituximab and temozolomide (1 patient).
Outcome
Nine patients achieved a CR to salvage therapy, and one patient had a partial response. Four patients with CR relapsed again, a median of 16.4 months (range, 10.9–20.4 months) after commencement of salvage therapy. One died of PCNSL, which was confirmed at autopsy. One patient had an isolated ocular relapse again and refused treatment other than steroid eye drops and is doing well, with no evidence of parenchymal disease for the past 4 years. The other 2 patients had relapse in the brain: one achieved a third CR with re-initiation of salvage chemotherapy, and the other is currently receiving chemotherapy.
Two patients died: one died of PCNSL, and the other died of kidney carcinoma and stroke, which may have been a consequence of prior WBRT. Eight patients are alive, 6 of whom continue to be seen at our institution; 2 were lost to follow-up. Three of the patients examined at MSKCC had a KPS of 90 at the most recent visit; none of these patients ever received radiation. Two had a KPS of 60 as a consequence of treatment-related leukoencephalopathy; both had received WBRT. One is currently under treatment.
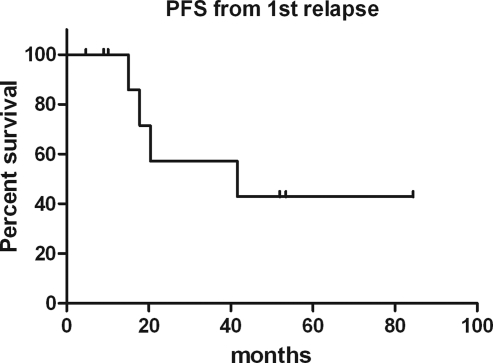
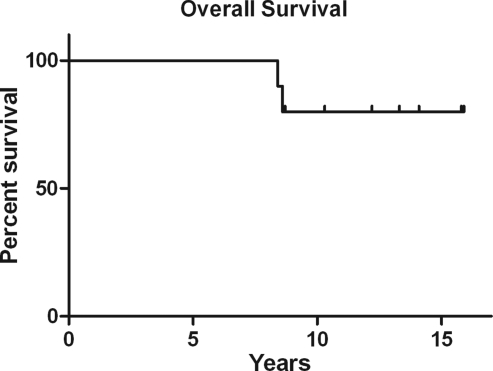
The median PFS after first relapse was 31 months (range, 7.9–82.4 mo) (Fig. 3). The median OS has not been reached (Fig. 4). The median duration of follow-up for surviving patients was 12.8 years (range, 8.6–15.9 y).
Fig. 3.
Progression-free survival (PFS) after first relapse.
Fig. 4.
Overall survival (OS) after initial diagnosis.
Discussion
PCNSL often responds rapidly to initial treatment, but when relapse occurs, it is seen within the first few years after diagnosis in the overwhelming majority of patients. The longest reported median OS in PCNSL is 60 months with the combination of chemotherapy and radiation.2 However, a significant subset of patients experience very long survival and may even be considered cured if no relapse is seen after ≥5 years. Because we began to observe late recurrences in our long-term survivors, we sought to study those who had relapse ≥5 years after initial diagnosis. These relapses accounted for 4% of the recurrences (10 of 230 patients) seen in our PCNSL population.
Prognostic factors strongly influence outcome in PCNSL patients of which the most consistent are age and performance status, as assessed in a recursive partitioning analysis.7 Six (60%) of our 10 patients were aged <50 years, and 5 (71%) of 7 patients had a KPS ≥70 prior to starting initial treatment, so most were in the better prognostic groups at diagnosis. It is unsurprising to find patients with a preponderance of better prognostic factors among our long-term survivors; only these patients would be vulnerable to delayed recurrences of their disease.
The CNS pattern of late relapse was similar to that seen in patients whose cases recur earlier—predominantly in the brain, often at distant sites. Despite this typical distribution, pathologic confirmation of recurrent PCNSL is advised in those who experience late relapse. Such patients may be vulnerable to other malignancies, particularly if WBRT was administered as a component of initial treatment or the relapse is systemic.
All of our patients responded well to salvage therapy, and only 1 died of PCNSL. In general, patients who are refractory to primary therapy or relapse after an initial response have a poor prognosis without treatment, with a median survival of only 2 months.8 In a study of patients with relapsed PCNSL, the median duration of disease-free survival after initial diagnosis was 10.25 months, and the median OS after relapse was 4.5 months (range, 0.5–40.5 mo) despite treatment.4 In contrast, our patients did exceptionally well after salvage therapy, with a median PFS of 31 months, perhaps reflecting a less aggressive biology of the disease that was responsible for the prolonged first remission. Most importantly, 3 of the 6 patients who are currently being followed at our institution are functioning independently and at a high level. Two have cognitive decline from treatment-related leukoencephalopathy as a consequence of their cumulative therapy.
Remarkably, we could demonstrate persistence of the original clone >13 years after initial diagnosis in one of our patients. Unfortunately, the tissue examinations were uninformative in the other 2 patients. Most patients with PCNSL do not undergo an additional biopsy at relapse, so there are few biologic data on this entity. In 1 patient, PCNSL tissue from both diagnosis and recurrence 14 months later showed a common ancestor B cell, but the specimen obtained at relapse had evolved to contain additional somatic mutations not found in the original specimen.9 We did not perform subclone analysis in our patient, but clonality was identical, demonstrating that these malignant lymphocytes can persist in the CNS for many years before becoming apparent. Furthermore, despite the long interval from diagnosis to relapse in our patients, only 1 patient developed systemic lymphoma, which occurred in the absence of CNS relapse and could have represented a separate primary. Unfortunately, we did not have tumor samples from both events for analysis.
There are no guidelines for treatment of relapsed PCNSL. In 27 patients with relapsed or refractory PCNSL, salvage therapy with cytarabine and etoposide followed by thiotepa-busulfan-cyclophosphamide and autologous stem-cell rescue achieved a median OS of 58.6 months.10 Our patient who received high-dose chemotherapy followed by stem cell rescue at initial diagnosis and again at relapse has been free of disease for the past 58.2 months. Patients may respond to re-introduction of HD-MTX as salvage therapy, as noted in 22 patients with a 91% response rate and a median survival of 61.9 months.11 However, other studies report lower response rates and survival durations.12–14 Nevertheless, response to salvage therapy and long post-relapse survival durations seen in our patients suggest chemoresistance has not occurred when the disease course is protracted.9 Seven of our 8 patients were re-treated with HD-MTX and responded; one responded twice. Treatments for PCNSL are becoming more successful and the disease-free survival rates are increasing. Therefore, it is imperative to maintain close follow-up of these patients and monitor for late relapses.
Acknowledgment
We thank Judith Lampron for her expert editorial assistance.
Conflict of interest statement. None declared.
References
- 1.Central Brain Tumor Registry of the United States. 2010 CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. 2010. Accessed at http://wwwcbtrusorg/2010-NPCR-SEER/CBTRUS-WEBREPORT-Final-3-2-10pdf .
- 2.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis LM, Iwamoto FM. An update on therapy of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2006;20:1267–85. doi: 10.1016/j.hoc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80:159–165. doi: 10.1007/s11060-006-9165-6. doi:10.1007/s11060-006-9165-6. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki T, Shima N, Yamabe H, Nagaoka S, Moritake K, Kikuchi H. Primary malignant lymphoma of the central nervous system—report of four long-term survivors. Neurol Med Chir (Tokyo) 1995;35:655–662. doi: 10.2176/nmc.35.655. doi:10.2176/nmc.35.655. [DOI] [PubMed] [Google Scholar]
- 6.Herrlinger U, Hebart H, Kanz L, Dichgans J, Weller M. Relapse of primary CNS lymphoma after more than 10 years in complete remission. J Neurol. 2005;252:1409–1410. doi: 10.1007/s00415-005-0854-4. doi:10.1007/s00415-005-0854-4. [DOI] [PubMed] [Google Scholar]
- 7.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. doi:10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 8.Reni M, Ferreri AJ, Villa E. Second-line treatment for primary central nervous system lymphoma. Br J Cancer. 1999;79:530–534. doi: 10.1038/sj.bjc.6690083. doi:10.1038/sj.bjc.6690083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pels H, Montesinos-Rongen M, Schaller C, et al. Clonal evolution as pathogenetic mechanism in relapse of primary CNS lymphoma. Neurology. 2004;63:167–169. doi: 10.1212/01.wnl.0000132649.24618.8a. [DOI] [PubMed] [Google Scholar]
- 10.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. doi:10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 11.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10:5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. doi:10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 12.Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901–903. doi: 10.1212/01.wnl.0000137050.43114.42. [DOI] [PubMed] [Google Scholar]
- 13.Voloschin AD, Betensky R, Wen PY, Hochberg F, Batchelor T. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J Neurooncol. 2008;86:211–215. doi: 10.1007/s11060-007-9464-6. doi:10.1007/s11060-007-9464-6. [DOI] [PubMed] [Google Scholar]
- 14.Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, Nomdedeu B, Montserrat E, Graus F. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–224. doi: 10.1034/j.1600-0609.2003.00045.x. doi:10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]