Abstract
Romidepsin, a potent histone deacetylase inhibitor, has shown activity in preclinical glioma models. The primary objectives of this trial were to determine the pharmacokinetics of romidepsin in patients with recurrent glioma on enzyme-inducing antiepileptic drugs (EIAEDs) and to evaluate the antitumor efficacy of romidepsin in patients with recurrent glioblastoma who were not receiving EIAEDs. Two dose cohorts were studied in the phase I component of the trial (13.3 and 17.7 mg/m2/d). Patients in the phase II component were treated with intravenous romidepsin at a dosage of 13.3 mg/m2/day on days 1, 8, and 15 of each 28-day cycle. Eight patients were treated on the phase I component. A similar romidepsin pharmacokinetic profile was demonstrated between patients receiving EIAEDs to those not receving EIAEDs. Thirty-five patients with glioblastoma were accrued to the phase II component. There was no objective radiographic response. The median progression-free survival (PFS) was 8 weeks and only 1 patient had a PFS time ≥6 months (PFS6 = 3%). To date, 34 patients (97%) have died, with a median survival duration of 34 weeks. Despite in vitro studies showing that romidepsin is primarily metabolized by CYP3A4, no decrease in exposure to romidepsin was seen in patients receiving potent CYP3A4 inducers. Romidepsin, at its standard dose and schedule, was ineffective for patients with recurrent glioblastomas.
ClinicalTrials.gov identifier: NCT00085540.
Keywords: glioblastoma, glioma, histone deacetylase inhibitor, romidepsin
Malignant gliomas, the most common primary brain tumors in adults, have an estimated annual incidence of 15, 000 cases in the United States.1 Despite optimal treatment with maximal surgical resection, radiotherapy, and chemotherapy with the alkylating agent temozolomide, gliomas almost invariably recur. Prognosis at time of recurrence is poor, with median duration of survival of only 25 weeks for recurrent glioblastomas (grade IV glioma) and 40 weeks for recurrent anaplastic gliomas (grade III).2 Currently, there is no single accepted standard of care for patients with recurrent malignant gliomas. Re-resection with carmustine wafers,3 systemic nitrosoureas, and, more recently, bevacizumab4,5 are available options; the benefit of these therapies in either disease control or survival, however, is usually measured in a few months. Clearly, better therapeutic approaches are needed.
Several malignancies, including gliomas, present abnormal histone acetylation, histone deacetylase (HDAC) overexpression, and, consequently, aberrant DNA transcription.6 HDAC inhibitors, a relatively new class of antineoplastic drugs, promote cell-cycle arrest, cellular differentiation, and apoptosis of malignant cells.7 Inhibition of HDAC increases acetylation of histones, thereby promoting chromatin remodeling and facilitating transcription of genes involved in tumor suppression and apoptosis.6 Additionally, acetylation of nonhistone proteins involved in tumor pathogenesis, such as hypoxia-inducible factor-1, heat shock protein 90, and p53, likely plays a significant role in the antitumoral activity of HDAC inhibitors.8 More recently, HDAC inhibitors were shown to decrease formation of GBM-derived neurospheres, promote tumor stem cell differentiation, and abrogate tumorigenicity of GBM-derived neurospheres.9
Romidepsin (Istodax; Celgene; formerly known as FK228, FR901228, or depsipeptide), a peptide isolated from Chromobacterium violaceum, potently inhibits HDAC10 and has efficacy at nanomolar concentrations against several cancer models. In GBM cell lines, romidepsin decreases levels of the antiapoptotic protein Bcl-xL and increases expression of the cyclin-dependent kinase inhibitor p21.11 In addition, romidepsin decreases tumor growth and induces apoptosis in subcutaneous glioma models.11 Romidepsin has been tested clinically in several solid and hematologic malignancies and has recently received US Food and Drug Administration approval for use in cutaneous T-cell lymphomas.12,13
We performed a limited phase I pharmacokinetics (PK)–driven trial of romidepsin in patients receiving enzyme-inducing anti-epileptic drugs (EIAEDs), because romidepsin is metabolized primarily by cytochromes CYP3A4 and CYP3A514 and EIAEDs potently induce CYP3A4. To assess the clinical antitumor activity of romidepsin, we performed a phase II trial of standard dose romidepsin in patients with recurrent malignant gliomas not receiving EIAEDs.
Methods
Eligibility Criteria
Eligibility criteria included age ≥18 years, histologically confirmed intracranial malignant glioma, and unequivocal radiographic tumor progression. For the phase II component, patients could not have received treatment for >2 prior relapses; number of relapses was not an eligibility criterion for the phase I trial. Additional eligibility criteria included Karnofsky performance scale ≥60, adequate bone marrow function (white blood cell count, ≥3000 cells/µL; absolute neutrophil count, ≥1500 cells/µL; platelet count, ≥1 00 000 platelets/µL; and hemoglobin concentration, ≥10 g/dL), adequate liver function (bilirubin and aspartate aminotransferase levels <2 times the upper limit of normal), adequate renal function (creatinine level, <1.5 mg/dL), and life expectancy >8 weeks. At least 6 weeks must have elapsed from radiation therapy, 4 weeks from use of cytotoxic or investigational drugs, 6 weeks from use of nitrosoureas, 3 weeks from use of procarbazine, 2 weeks from use of vincristine, and 1 week from use of noncytotoxic agents, such as interferon, tamoxifen, thalidomide, or cis-retinoic acid. Measurable radiographic disease was not required for eligibility. A stable dose of corticosteroids for ≥5 days was needed before obtaining the baseline MRI scan.
Patients receiving EIAEDs were ineligible for the phase II trial while patients on the phase I component were required to be receiving EIAEDs. Patients receiving valproic acid, an antiepileptic drug with HDAC inhibitory activity, were not eligible for either trial component. Patients with serious intercurrent medical illness, active infection, other cancer diagnosis within 3 years of study entry, class III or IV New York Heart Association heart failure, myocardial infarction within 1 year of study entry, uncontrolled cardiac arrhythmia, history of ventricular tachycardia or ventricular fibrillation, poorly controlled angina, QTc of ≥ 500 msec, left ventricular ejection fraction ≤40% by multigated acquisition (MUGA) scan, and concomitant use of drugs that prolong QTc interval were ineligible. Pregnant or nursing women were also ineligible, and use of birth control methods was mandatory. Institutional review boards from each participating center approved the protocol, and all patients provided written informed consent before enrollment.
Treatment Plan and End Points
The National Cancer Institute's Cancer Therapy Evaluation Program (CTEP) provided romidepsin to all participating sites. Romidepsin was intravenously infused over 4 hours on days 1, 8, and 15 of a 28-day cycle with mandatory use of prophylactic antiemetic drugs. Electrocardiograms were performed prior to romidepsin infusion (hour 0), immediately following the infusion (hour 4), and 20 hours after the infusion was completed (hour 24) on days 1 and 8, and prior to infusion on day 15 of cycle 1 of both phase I and II components. Serum magnesium and potassium levels were checked prior to each infusion and replaced if levels were less than 0.85 mmol/L and 4.0 mmol/L, respectively. Medical history was determined and physical and neurological examinations were performed every cycle. Complete blood cell count, serum chemistries, and liver function tests were done weekly. A MUGA scan was performed at baseline, end of cycle 1, and every 3 cycles thereafter. A brain MRI was done prior to every other cycle. Treatment could continue for up to 12 cycles or until disease progression, unacceptable toxicity, intercurrent illness that prevented treatment continuation, or consent withdrawal. Diagnosis was confirmed by central pathological review. Patients who had a progression-free survival (PFS) duration of ≥6 months or those with radiographic responses had brain MRI scans centrally reviewed. Toxicity was evaluated using the National Cancer Institute Common Toxicity Criteria version 3.0.
Phase I
The primary end points of the phase I study were defining the maximum tolerated dose (MTD) and establishing the PK profile of romidepsin in patients receiving strong CYP3A4-inducing EIAEDs. The initial 3-patient cohort received romidepsin at dosage of 13.3 mg/m2/day on days 1, 8, and 15 of each 28-day cycle. The Data Safety Monitoring Committee (DSMC) reviewed all toxicities before deciding to escalate to the next dose level. Dose escalations were planned in groups of 3 patients; 3 additional patients were added at a certain dose level if 1 of 3 initial patients had a dose-limiting toxicity (DLT). No intra-patient dose escalation was permitted. DLT was defined as any thrombocytopenia of grade ≥3, grade ≥4 anemia and neutropenia, grade ≥3 nonhematologic toxicity attributable to romidepsin, or failure to recover from toxicities to be eligible for re-treatment within 2 weeks after the last dose of romidepsin. The MTD was based on the tolerability observed during the first 4 weeks of treatment and was predefined as the dose at which fewer than one-third of patients experienced a DLT (ie, the dose at which 0 or 1 of 6 patients experience DLT), with the next higher dose having at least 2 of 3 or 2 of 6 patients encountering DLT. Nonevaluable patients, such as those removed from study in the first cycle for reasons other than toxicity, were replaced. Dose reduction was not allowed in the first cycle of the phase I component.
Phase II
The primary efficacy end point of the phase II component of the trial was PFS at 6 months (PFS6) in patients with recurrent GBMs. Secondary end points included objective radiographic response (ORR) according to standard Macdonald criteria using the largest cross-sectional diameters of measurable lesions,15 safety profile, and overall survival (OS). Phase II patients were treated with romidepsin at dosage of 13.3 mg/m2/day on days 1, 8, and 15 of each 28-day cycle. One dose reduction to 10 mg/m2/day on days 1, 8, and 15 of each 28-day cycle was allowed for toxicity, as predefined in the protocol.
PK
Only patients on the phase I component underwent PK blood collection. Heparinized blood samples (10 mL) were obtained prior to and during romidepsin administration on days 1 and 15 of cycle 1 at the following time points: baseline, 1, 2, 3, 3.5, and 4 hours (just prior to end of the infusion). The blood samples were placed on ice to prevent decomposition and immediately centrifuged; plasma was removed and stored at −20°C or less until analyzed. Plasma concentrations of romidepsin were determined by a high-performance liquid chromatographic assay, as described elsewhere.16 Romidepsin concentrations observed during infusions were averaged and reported as steady-state concentrations (CpSS). Clearance values were determined by dividing the rate of infusion by CpSS. The area under the curve (AUC) was estimated by dividing dose by clearance.
Statistics
Phase I
PK parameters were reported as mean values. Differences in the PK variables were evaluated using the unpaired 2-tailed t test; probability values <.05 were considered statistically significant.
Phase II
PFS was calculated from study registration until radiographic tumor progression or date off treatment for clinical decline. Progression was determined according to the standard criteria of Macdonald et al.15 In addition, patients who died while receiving treatment or ≤30 days after the end of treatment without documented progression were considered to have had an event based on the date of death. All other patients without documented progression were censored for PFS at the date of their last romidepsin administration. OS was calculated from study registration until date of death or patients were censored at the last date known to be alive. Estimation of both PFS and OS was conducted using Kaplan–Meier analyses.
This single-arm phase II trial used historical control data from 8 consecutive phase II clinical trials for recurrent malignant gliomas that were deemed negative and showed a PFS6 of 15% (95% confidence interval [CI], 10%–19%) for GBM.17 The phase II trial was sized to discriminate between a PFS6 of 15% (P0) and 35% (P1). With accrual of 32 patients with GBM, this trial would be considered successful if at least 8 patients with GBM achieved the PFS6 mark. This design provided > 90% probability of rejecting the drug if the true PFS6 was 15% and 92% probability of declaring success if the true rate was 35%. Up to 35 patients with GBM could be accrued to compensate for those who might not be evaluable. An exploratory cohort of up to 20 patients with anaplastic gliomas was planned; however, slow accrual and lack of efficacy in the GBM cohort led to early termination of enrollment of the anaplastic glioma group.
Results
Patient Characteristics
Patients were accrued from February 2005 to September 2007 at 4 participating institutions of the North American Brain Tumor Consortium, a CTEP-sponsored cooperative group. Eight patients were accrued to the phase I portion of this trial, and 42 patients (37 with GBM and 5 with anaplastic gliomas) were registered to the phase II component. Two patients with GBM in the phase II component, who were registered but did not receive romidepsin, were excluded from the analysis; one was deemed ineligible and the other withdrew consent. All patients had received prior radiotherapy and a median of 1 chemotherapy regimen. Two patients had received bevacizumab prior to enrollment in this trial. Baseline patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | Phase I (n = 8) | Phase II (n = 40) |
|---|---|---|
| Sex | ||
| Male | 5 (63) | 30 (75) |
| Female | 3 (37) | 10 (25) |
| Age, median years (range) | 52 (44–57) | 55 (37–79) |
| Karnofsky performance status | ||
| 90–100 | 4 (50) | 19 (48) |
| 60–80 | 4 (50) | 21 (52) |
| Enzyme-inducing antiepileptic drug | 8 (100)a | 0 (0) |
| Histology | ||
| Anaplastic glioma (grade III) | 2 (25) | 5 (13) |
| Glioblastoma (grade IV) | 6 (75) | 35 (87) |
| Prior radiotherapy | 8 (100) | 40 (100) |
| Median number of prior chemotherapy regimens (range) | 1 (1–2) | 1 (1–3) |
Data are no. (%) of patients, unless otherwise indicated.
aSix patients receiving phenytoin, 1 receiving oxcarbazepine, and 1 receiving both phenytoin and oxcarbazepine.
Phase I
Toxicity and MTD
Three patients were treated in the 13.3 mg/m2 cohort and 5 were treated in the 17.7 mg/m2 cohort; 2 patients in the 17.7 mg/m2 cohort needed to be replaced due to tumor progression during cycle 1. No DLT occurred in either cohort; adverse events on cycle 1 were mild (grades 1 and 2 only) (Table 2). Adverse events deemed unrelated or unlikely related to romidepsin are shown in Supplementary Table 1. The DSMC decided to stop phase I dose-escalation because PK data from these 2 cohorts showed that EIAEDs did not affect romidepsin's exposure, compared with prior studies that prohibited concomitant CYP3A4 inducers. In addition, the DSMC had concerns about the potential for cardiotoxicity with doses >17.7 mg/m2 based on prior studies. Consequently, the MTD for patients receiving EIAEDs was not defined. Patients in the phase I component received a median of 1 cycle of romidepsin (range, 1–2); 7 patients discontinued treatment due to tumor progression, and 1 patient was removed from the study because of prolonged grade 2 thrombocytopenia during cycle 2.
Table 2.
Cycle 1 adverse events possibly, probably, or definitely related to romidepsin, in the phase I component (n = 8)
| Romidepsin, 13.3 mg/m2 (n = 3) |
Romidepsin, 17.7 mg/m2 (n = 5) |
|||||
|---|---|---|---|---|---|---|
| Adverse event | Grade 1 | Grade 2 | Grade 3–5 | Grade 1 | Grade 2 | Grade 3–5 |
| Hematologic | ||||||
| Anemia | 2 | 0 | 0 | 1 | 0 | 0 |
| Leucopenia | 1 | 1 | 0 | 0 | 1 | 0 |
| Neutropenia | 0 | 2 | 0 | 1 | 0 | 0 |
| Lymphopenia | 0 | 0 | 0 | 1 | 0 | 0 |
| Thrombocytopenia | 2 | 0 | 0 | 3 | 1 | 0 |
| Nonhematologic | ||||||
| Alkaline phosphatase elevation | 0 | 0 | 0 | 1 | 0 | 0 |
| Elevated ALT and/or AST level | 2 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 1 | 0 | 0 |
| Hypocalcemia | 1 | 0 | 0 | 1 | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 1 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 1 | 0 | 0 |
| Hypophosphatemia | 2 | 0 | 0 | 1 | 0 | 0 |
| Fatigue | 0 | 2 | 0 | 1 | 1 | 0 |
| Headache | 0 | 1 | 0 | 1 | 2 | 0 |
| Hot flashes | 0 | 0 | 0 | 1 | 0 | 0 |
| Gait or walking difficulties | 0 | 0 | 0 | 0 | 1 | 0 |
| Nausea | 0 | 1 | 0 | 3 | 1 | 0 |
| Rigors or chills | 0 | 0 | 0 | 1 | 0 | 0 |
| Vomiting | 0 | 1 | 0 | 2 | 1 | 0 |
ALT, alanine aminotransferse; and AST, aspartate aminotransferase.
PK
Romidepsin's PK parameters, including steady-state concentrations, AUC, and clearance, on days 1 and 15 are summarized in Table 3. There were no differences between the PK parameters on days 1 and 15. More importantly, PK data in our patients receiving EIAEDs were similar to those in prior phase I studies of patients not receiving CYP3A4 inducers treated with 4-hour intravenous infusion of romidepsin at equivalent doses.18–20
Table 3.
Romidepsin pharmacokinetics in patients receiving enzyme-inducing antiepileptic drugs, compared to findings from prior studies of patients not receiving CYP3A4 inducers
| Current Study |
Current Study |
|||||
|---|---|---|---|---|---|---|
| Parameter | Day 1 | Day 15 | Byrd et al.19 | Day 1 | Day 15 | Sandor et al.20 |
| Number of patients | 3 | 3 | 20 | 5 | 2 | 9 |
| Romidepsin dose, mg/m2 | 13.3 | 13.3 | 13 | 17.7 | 17.7 | 17.8 |
| CpSS (ng/mL) | 524 | 629 | 757 | 441 | 423 | 554 |
| AUC (µg × hr/mL) | 1.99 | 2.50 | 3.26 | 1.81 | 1.88 | 2.27 |
| Clearance (L/hr/m2) | 6.81 | 6.06 | 4.81 | 10.38 | 11.47 | 10.50 |
CpSS, steady-state concentrations; and AUC, area under the curve.
Phase II
Toxicity
Six patients (4 with GBM and 2 with anaplastic gliomas) discontinued romidepsin therapy due to toxicity (4 had prolonged thrombocytopenia, 1 had an elevated alanine aminotransferase level, and 1 had a cardiac abnormality). Nine patients (22%) required romidepsin dose reduction. Hematologic toxicity and fatigue were the most common grade ≥3 adverse events (Table 4). There were no romidepsin-related deaths. Adverse events classified as unrelated or unlikely related to romidepsin are shown in Supplementary Table 2.
Table 4.
Adverse events possibly, probably, or definitely related to romidepsin use, in the phase II component (n = 40)
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 8 | 0 | 0 | 0 |
| Leucopenia | 1 | 6 | 1 | 0 |
| Neutropenia | 1 | 0 | 6 | 0 |
| Lymphopenia | 1 | 4 | 4 | 0 |
| Thrombocytopenia | 13 | 2 | 0 | 0 |
| Nonhematologic | ||||
| Ventricular arrhythmia | 1 | 0 | 0 | 0 |
| Cardiac general | 0 | 0 | 1 | 0 |
| Hypotension | 0 | 0 | 1 | 0 |
| PT prolongation | 1 | 0 | 0 | 0 |
| Fatigue | 7 | 6 | 3 | 1 |
| Fever without neutropenia | 1 | 0 | 0 | 0 |
| Weight loss | 1 | 0 | 0 | 0 |
| Alopecia | 1 | 0 | 0 | 0 |
| Dry skin | 1 | 0 | 0 | 0 |
| Injection site reaction | 0 | 0 | 1 | 0 |
| Pruritis | 0 | 1 | 0 | 0 |
| Skin rash | 2 | 0 | 1 | 0 |
| Anorexia | 4 | 0 | 0 | 0 |
| Constipation | 6 | 0 | 1 | 0 |
| Diarrhea | 3 | 1 | 0 | 0 |
| Dry mouth | 1 | 0 | 0 | 0 |
| Flatulence | 0 | 1 | 0 | 0 |
| Heartburn | 1 | 1 | 0 | 0 |
| Mucositis | 0 | 1 | 0 | 0 |
| Nausea | 11 | 2 | 0 | 0 |
| Taste alteration | 1 | 0 | 0 | 0 |
| Vomiting | 1 | 1 | 1 | 0 |
| Petechiae | 1 | 0 | 0 | 0 |
| Edema–limb | 1 | 1 | 0 | 0 |
| ALT elevation | 7 | 0 | 1 | 0 |
| AST elevation | 0 | 1 | 0 | 0 |
| Alkaline phosphatase elevation | 1 | 0 | 0 | 0 |
| Bilirubin elevation | 0 | 1 | 0 | 0 |
| Cholesterol elevation | 0 | 1 | 0 | 0 |
| Creatinine elevation | 1 | 0 | 0 | 0 |
| Hyperglycemia | 1 | 3 | 0 | 0 |
| Hypermagnesemia | 1 | 0 | 0 | 0 |
| Hypoalbuminemia | 1 | 0 | 0 | 0 |
| Hypocalcemia | 2 | 0 | 0 | 0 |
| Hypokalemia | 3 | 0 | 0 | 0 |
| Hypomagnesemia | 1 | 0 | 0 | 0 |
| Hyponatremia | 1 | 0 | 0 | 0 |
| Hypophosphatemia | 2 | 2 | 1 | 0 |
| Muscle weakness (lower extremity) | 0 | 2 | 0 | 0 |
| Muscle weakness (whole body) | 0 | 1 | 0 | 0 |
| Extremity-upper (function) | 0 | 1 | 0 | 0 |
| Joint–function | 1 | 0 | 0 | 0 |
| Confusion | 1 | 0 | 0 | 0 |
| Dizziness | 1 | 2 | 0 | 0 |
| Blurred vision | 1 | 0 | 0 | 0 |
| Abdominal pain | 3 | 0 | 0 | 0 |
| Chest/thorax pain | 1 | 0 | 0 | 0 |
| Limb pain | 1 | 0 | 0 | 0 |
| Headache | 0 | 1 | 0 | 0 |
| Joint pain | 2 | 1 | 0 | 0 |
| Thrombosis or embolism of vascular access | 0 | 0 | 0 | 1 |
ALT, alanine aminotransferse; AST, aspartate aminotransferase; and PT, prothrombin time.
Outcomes
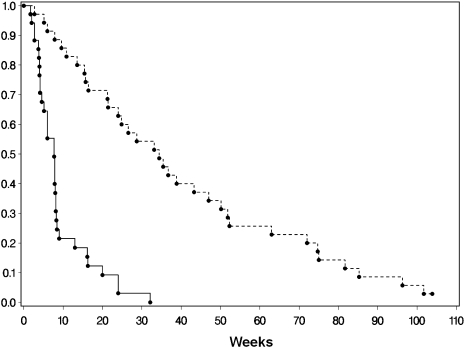
Thirty-five patients with GBM enrolled in the phase II component were included in the PFS and OS analyses (Fig. 1). The median number of cycles of romidepsin was 2 (range, 1–8 cycles). Among the 35 patients with GBM, only 1 had PFS ≥6 months (PFS6 3%) and this patient developed tumor progression at 32 weeks. Twenty-nine patients with GBM (83%) stopped romidepsin therapy because of tumor progression, 4 (11%) because of toxicity, and 2 (6%) because of intercurrent illnesses unrelated to romidepsin. The 2 patients removed from the study because of intercurrent illnesses and 1 who discontinued due to toxicity died ≤1 month after receipt of the last dose of treatment. Thus, these cases were considered events for PFS calculation. Two patients with GBM were censored for progression, and the median PFS of all 35 patients with GBM was 8 weeks (95% CI, 5–8 wks). Only 1 patient with GBM, who was observed for 104 weeks, was censored for survival; all others had died by the time of analysis. The median OS for patients with GBM was 34 weeks (95% CI, 21–47 wks). Thirty-two of 35 patients with GBM were evaluable for ORR. No radiographic responses were seen, and the best ORR was progressive disease in 23 patients (72%) and stable disease in 9 patients (28%).
Fig. 1.
Progression-free (solid line) and overall survival (dashed line) curves of 35 patients with recurrent glioblastoma in the phase II trial of romidepsin.
Five patients with anaplastic glioma received a median of 2 cycles (range, 1–8 cycles) of romidepsin. Two patients discontinued treatment because of toxicity at 1 and 5 weeks; the other 3 patients had tumor progression at 4, 8, and 35 weeks. All patients with anaplastic glioma died, with a median OS of 36 weeks (95% CI, 13–79 wks). No patients with anaplastic glioma had radiographic responses; among 3 evaluable patients, the best response was stable disease in 1 patient and progressive disease in 2 patients.
Discussion
The promising preclinical data for HDAC inhibition in glioma and the proven activity in patients with T cell lymphoma prompted us to evaluate the activity of romidepsin in patients with recurrent high-grade gliomas. Despite in vitro studies showing that romidepsin is primarily metabolized by CYP3A4 and, to a lesser degree, by CYP3A5, our results did not show any decrease in the exposure of romidepsin in patients receiving potent CYP3A4 inducer EIAEDs. Thus, to avoid the expected dose-limiting toxicity of the romidepsin, we chose to terminate further dose escalation after the first 2 dose levels, because the PK parameters of romidepsin in our patients receiving EIAEDs were very similar to those reported in adults18–20 or children aged ≥2 years21 who were not receiving CYP3A4-inducing drugs. We do not know for certain why we did not observe altered metabolism of romidepsin in patients receiving EIAEDs; however, prediction or extrapolation of in vitro drug interaction data to clinical situations is often difficult. One possible explanation for our findings is that clearance of drugs with baseline rapid first pass liver metabolism, especially if given intravenously, is not significantly affected by CYP induction, because their high hepatic clearance is limited by the rate of hepatic blood flow.22 In fact, a phase II trial of 98 patients with T cell lymphoma that evaluated the effects of germ line polymorphisms in CYP3A4*1B and CYP3A5*3 on romidepsin metabolism did not show any correlation between genetic variants known to increase clearance of CYP3A4/5-metabolized drugs.23 Regardless of the reason for the lack of altered metabolism, our data indicate that no romidepsin dose adjustment is required in patients receiving strong CYP3A4 inducers who are being treated with the dose regimen that was used in our study and that is currently approved for the treatment of cutaneous T cell lymphomas (ie, 14 mg/m2/d on days 1, 8, and 15 of a 28-d cycle).
Romidepsin was reasonably well tolerated in our study, although dose reductions were required in 22% of patients in the phase II component of the trial. Fatigue and nausea were the most common nonhematologic toxicities and were similar to that previously reported in other romidepsin trials.12,13 Hematologic toxicity was usually mild, but prolonged thrombocytopenia caused discontinuation of romidepsin in 5 patients. In spite of frequent monitoring, cardiac abnormalities were seen less often than previously reported,24 likely in association with proactive correction of hypokalemia and hypomagnesemia prior to romidepsin infusions.
Despite preclinical and mechanistic rationale for HDAC inhibition as a potential anti-glioma therapy, our trial showed that romidepsin had no significant clinical activity as a single agent in unselected patients with recurrent GBM. The PFS6 of 3% and lack of radiographic responses are comparable to the findings of prior negative phase II trials from our consortium.25 A phase II trial of vorinostat, a class I and II HDAC inhibitor, demonstrated a slightly higher PFS6 of 15.2% in a similar population of patients with recurrent GBM but was likewise deemed to have too low a level of activity to justify its use as a single agent.
There are several possible reasons for the disappointing results of romidepsin in recurrent GBM. One possibility is that romidepsin, a peptide with relative high molecular weight and strong serum protein binding, did not achieve therapeutic intratumoral levels. A primate study showed that the level of cerebrospinal fluid exposure of romidepsin was only 2% after intravenous administration;26 however, because GBM lacks an intact blood-brain barrier, intratumoral drug levels are usually higher than those seen in primates with normal blood-brain barrier. In addition, even if only 2% of romidepsin's steady-state plasma levels (∼8 ng/mL) distributed into brain tumors, it would be greater than the in vitro concentration reported to induce apoptosis (1 ng/mL) of glioma cell lines.11 This simple estimation does not account, however, for romidepsin's short half-life (<3 h), for its serum protein binding, and for the fact that romidepsin is a substrate of the ATP-binding cassette efflux-transporter, ABCB1 (P-glycoprotein, MDR1),23 which is often overexpressed in gliomas and can further decrease intratumoral drug levels.27
The minimal single-agent activity of HDAC inhibitors in gliomas is consistent with that seen in clinical trials of several other solid and hematologic malignancies, with the single exception of cutaneous T-cell lymphomas.12,13 HDAC inhibitors have widespread effects on gene transcription and posttranslational nonhistone acetylation, and the mechanisms underpinning their therapeutic activity are not defined. For example, a study demonstrated that ∼10% of 3600 acetylation sites on 1750 different proteins showed increased acetylation levels after exposure to HDAC inhibitors.28 It has also been shown that HDAC inhibitors can increase transcription of both pro-apoptotic and anti-apoptotic genes and that their efficacy depends, in fact, on the overall balance of genes that are transcribed.29 Thus, the complexity of the effects of HDAC inhibition on cellular signaling networks, intertumoral genetic heterogeneity, and the poorly predictive preclinical model systems we have for studying clinically relevant glioma biology likely conspire to explain the disconnect between the preclinical and clinical observations. Consequently, additional laboratory and clinical studies are needed to characterize in more detail the molecular pathways involved in the antitumor effects following inhibition of HDAC and to identify biomarkers predictive of patients most likely to benefit from such treatment, either as a single agent or when used in combination with other cytotoxic or biological agents.
Despite the lack of activity of single-agent romidepsin in recurrent malignant gliomas, HDAC inhibition continues to be a potentially viable therapeutic target for these tumors, especially in combination with other agents or radiotherapy. Ongoing studies of vorinostat and valproic acid in combination with radiation and temozolomide for patients with GBM will provide further information on the efficacy of this approach.
Supplementary Material
Funding
National Institutes of Health (CA 062399; “The North American Brain Tumor Consortium”).
Supplementary Material
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Wu W, Lamborn KR, Buckner JC, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12:164–172. doi: 10.1093/neuonc/nop019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. doi:10.1016/S0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 4.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. doi:10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 6.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. doi:10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010;107:14639–14644. doi: 10.1073/pnas.1008522107. doi:10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X, Guo ZS, Marcu MG, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 9.Sun P, Xia S, Lal B, et al. DNER, an epigenetically modulated gene, regulates glioblastoma-derived neurosphere cell differentiation and tumor propagation. Stem Cells. 2009;27:1473–1486. doi: 10.1002/stem.89. doi:10.1002/stem.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furumai R, Matsuyama A, Kobashi N, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 11.Sawa H, Murakami H, Kumagai M, et al. Histone deacetylase inhibitor, FK228, induces apoptosis and suppresses cell proliferation of human glioblastoma cells in vitro and in vivo. Acta Neuropathol. 2004;107:523–531. doi: 10.1007/s00401-004-0841-3. doi:10.1007/s00401-004-0841-3. [DOI] [PubMed] [Google Scholar]
- 12.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. doi:10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2010.28.9066. doi:10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 14.Shiraga T, Tozuka Z, Ishimura R, Kawamura A, Kagayama A. Identification of cytochrome P450 enzymes involved in the metabolism of FK228, a potent histone deacetylase inhibitor, in human liver microsomes. Biol Pharm Bull. 2005;28:124–129. doi: 10.1248/bpb.28.124. doi:10.1248/bpb.28.124. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.Chassaing C, Marshall JL, Wainer IW. Determination of the antitumor agent depsipeptide in plasma by liquid chromatography on serial octadecyl stationary phases. J Chromatogr B Biomed Sci Appl. 1998;719:169–176. doi: 10.1016/s0378-4347(98)00387-9. doi:10.1016/S0378-4347(98)00387-9. [DOI] [PubMed] [Google Scholar]
- 17.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 18.Klimek VM, Fircanis S, Maslak P, et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin Cancer Res. 2008;14:826–832. doi: 10.1158/1078-0432.CCR-07-0318. doi:10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 19.Byrd JC, Marcucci G, Parthun MR, et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105:959–967. doi: 10.1182/blood-2004-05-1693. doi:10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 20.Sandor V, Bakke S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 21.Fouladi M, Furman WL, Chin T, et al. Phase I study of depsipeptide in pediatric patients with refractory solid tumors: a Children's Oncology Group report. J Clin Oncol. 2006;24:3678–3685. doi: 10.1200/JCO.2006.06.4964. doi:10.1200/JCO.2006.06.4964. [DOI] [PubMed] [Google Scholar]
- 22.Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35:361–390. doi: 10.2165/00003088-199835050-00003. doi:10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Woo S, Gardner ER, Chen X, et al. Population pharmacokinetics of romidepsin in patients with cutaneous T-cell lymphoma and relapsed peripheral T-cell lymphoma. Clin Cancer Res. 2009;15:1496–1503. doi: 10.1158/1078-0432.CCR-08-1215. doi:10.1158/1078-0432.CCR-08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah MH, Binkley P, Chan K, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12:3997–4003. doi: 10.1158/1078-0432.CCR-05-2689. doi:10.1158/1078-0432.CCR-05-2689. [DOI] [PubMed] [Google Scholar]
- 25.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. doi:10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg SL, Stone J, Xiao JJ, et al. Plasma and cerebrospinal fluid pharmacokinetics of depsipeptide (FR901228) in nonhuman primates. Cancer Chemother Pharmacol. 2004;54:85–88. doi: 10.1007/s00280-004-0766-5. doi:10.1007/s00280-004-0766-5. [DOI] [PubMed] [Google Scholar]
- 27.Calatozzolo C, Gelati M, Ciusani E, et al. Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human glioma. J Neurooncol. 2005;74:113–121. doi: 10.1007/s11060-004-6152-7. doi:10.1007/s11060-004-6152-7. [DOI] [PubMed] [Google Scholar]
- 28.Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. doi:10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 29.Peart MJ, Smyth GK, van Laar RK, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. doi:10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.