Abstract
The objective of this phase II study was to evaluate the efficacy and safety of subcutaneous octreotide therapy for the treatment of recurrent meningioma and meningeal hemangiopericytoma. Octreotide is an agonist of somatostatin receptors, which are frequently expressed in meningioma, and reports have suggested that treatment with somatostatin agonists may lead to objective response in meningioma. Patients with recurrent/progressive meningioma or meningeal hemangiopericytoma were eligible for enrollment; those with atypical/anaplastic meningioma or hemangiopericytoma must have experienced disease progression despite radiotherapy or have had a contraindication to radiation. Patients received subcutaneous octreotide with a goal dose of 500 μg 3 times per day, as tolerated. Imaging was performed every 3 months during therapy. The primary outcome measure was radiographic response rate. Eleven patients with meningioma and 1 with meningeal hemangiopericytoma were enrolled during the period 1992–1998. Side effects included diarrhea (grade 1 in 4 patients and grade 2 in 2), nausea or anorexia (grade 1 in 4 patients), and transaminitis (grade 1 in 1 patient). One patient developed extra hepatic cholangiocarcinoma, which was likely unrelated to octreotide therapy. No radiographic responses were observed. Eleven of the 12 patients experienced progression, with a median time to progression of 17 weeks. Two patients experienced long progression-free intervals (30 months and ≥18 years). Eleven patients have died. Median duration of survival was 2.7 years. Immunohistochemical staining of somatostatin receptor Sstr2a expression in a subset of patients did not reveal a correlation between level of expression and length of progression-free survival. Octreotide was well-tolerated but failed to produce objective tumor response, although 2 patients experienced prolonged stability of previously progressive tumors.
Keywords: hemangiopericytoma, meningioma, octreotide, somatostatin, trial
Meningiomas are tumors of the arachnoidal cap cells, and they represent the most common benign intracranial tumor in adults.1 Meningeal hemangiopericytoma, formerly called “hemangiopericytic meningioma,” is a pathologically distinct meningeal mesenchymal tumor that shares some clinical similarities with meningioma and is usually associated with high rates of local and distant recurrences.2–4 The first line of treatment for meningioma and hemangiopericytoma is surgical resection, with a goal of surgical cure via total resection. Complete excision is often achievable in the cerebral convexities, but meningiomas located in regions such as the skull base and the sagittal sinus often cannot be entirely resected. Recurrent benign meningiomas (World Health Organization [WHO] grade I) and meningiomas with atypical (WHO grade II) or anaplastic (WHO grade III) pathology, which are associated with higher risks of recurrence, are often treated with radiation after surgery.2,5–7 Both fractionated radiation and stereotactic radiosurgery have been used in this setting. If meningiomas or hemangiopericytomas continue to recur or progress after resection and radiation, the remaining therapeutic options are limited. No chemotherapeutic approaches are proven to be of significant clinical benefit in this setting.8–13 The indolent nature of meningioma growth suggests that prolonged maintenance therapies may be beneficial, but extended treatment with standard cytotoxic chemotherapy can be poorly tolerated and have long-term toxicity.
Somatostatin is a neuropeptide produced in the hypothalamus and released into systemic circulation, where its primary sites of action are the pituitary, pancreas, and gastrointestinal tract.14 In these sites, somatostatin inhibits endocrine and exocrine secretions, as well as gastrointestinal motility. Somatostatin also plays a role in several pathways relevant to cancer by inhibiting angiogenesis and invasion, as well as inducing apoptosis.15 Somatostatin itself is impractical for clinical use because of a short half-life, but a number of somatostatin analogues with longer half-lives have been developed. The prototype somatostatin receptor agonist is octreotide (Sandostatin; Novartis Pharmaceuticals). Somatostatin analogue therapy has proven to be an effective treatment for pituitary adenomas16,17 and gastroenteropancreatic endocrine tumors.18,19 A significant majority of meningiomas express somatostatin receptors detectable by scintillography or biochemical analysis.20–23 There are 5 somatostatin receptor subtypes (sstr1–sstr5). Meningiomas most frequently express the sstr2a receptor, 1 of the subtypes preferentially bound by octreotide.20 Inhibition of meningioma cell growth by somatostatin has been shown in vivo,24 and the somatostatin analogue octreotide was used in a small series of recurrent meningiomas with minimal toxicity.25 There is a single report of a patient treated with a somatostatin analogue for a suprasellar hemangiopericytoma, initially thought to be meningioma, with a partial response.26,27 More recently, a prospective pilot study of 16 patients treated with a sustained-release somatostatin analogue Sandostatin LAR, which has the same somatostatin recepter affinity profile as octreotide, showed several partial responses and acceptable side effects.28
We conducted a single-arm, open-label phase 2 trial of subcutaneous octreotide in adults with recurrent or progressive unresectable meningioma or hemangiopericytoma.
Material and Methods
Eligibility Criteria
Patients aged ≥18 years who had recurrent or progressive biopsy-proven unresectable meningioma or meningeal hemangiopericytoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 were recruited from the outpatient clinic population at the Mayo Clinic (Rochester, MN) during the period 1992–1998. Radiographically evaluable tumor with evidence of tumor progression on CT or MRI scan was required at the time of study enrollment. For patients with atypical meningioma (WHO grade II), anaplastic meningioma (WHO grade III), or meningeal hemangiopericytoma (WHO grade II or III), the tumor must have progressed despite prior radiotherapy or the patient was not a candidate for radiotherapy or gamma knife radiotherapy. At least 6 weeks must have elapsed since radiation or previous chemotherapy. Adequate hematologic, renal, and hepatic function were required before treatment. All patients were evaluated by a radiation oncologist and a neurosurgeon and were not considered candidates for additional radiation or surgery. Pregnant or nursing women were excluded from study enrollment, as were patients with active infection or coexistent malignant disease. All patients provided informed consent. This study was approved by the Mayo Clinic Institutional Review Board.
Treatment Regimen
The somatostatin analogue octreotide was delivered via self-administered subcutaneous injection. The dose was 150 μg twice per day on day 1, 250 μg twice per day on day 2, and 500 μg 3 times per day thereafter. If toxicity occurred with either dose escalation, the dose was reduced to the preceding level. If a radiographic response was observed, treatment was continued indefinitely until disease progression or unacceptable toxicity occurred. Patients with radiographically stable disease were treated for at least 6 months or until unacceptable toxicity was noted. Patients with stable disease could be treated for >6 months at the discretion of the treating physician.
Subject Evaluation
Patients were evaluated clinically within 3 days after starting therapy, at 1 month, and then every 3 months thereafter. Patient compliance with the treatment regimen was assessed via self-report at each study visit. Treatment-related toxicities were graded at each evaluation according to the National Cancer Institute Common Toxicity Criteria, version 1.0. Blood cell counts and chemistries were performed every 3 months. MRI and/or CT scan was obtained ≤10 days before treatment and then every 3 months thereafter. The primary end point of the study was radiographic response rate. In patients with bidimensionally measurable disease, response was graded as complete response if total disappearance of all tumor was observed, partial response if the product of the perpendicular diameters of contrast enhancement decreased by ≥50%, progression when a ≥25% increase in the product of the perpendicular diameters of the contrast-enhancing tumor was seen, and stable in all other cases. In subjects with radiographically evaluable but not bidimensionally measurable disease, total disappearance of all tumor was considered complete response, unequivocal reduction in disease burden was termed regression, unequivocal increase in disease burden was considered progression, and all other situations constituted stable disease. Supplementary response end points included progression-free survival and overall survival.
Statistical Analysis
This trial had a 1-stage phase 2 design with target accrual of 14 patients to provide 85% power to detect a 30% response rate using a .05-level binomial test of the null hypothesis that the true response rate was 5%. However, because of slow accrual, the study was stopped after only 12 patients had been enrolled. Kaplan-Meier curves were generated for progression-free survival and overall survival.
Pathology
Standard hematoxylin and eoisin–stained meningioma and meningeal hemangiopericytoma tumor specimens were reviewed and graded by experienced Mayo Clinic neuropathologists to confirm the diagnosis before study enrollment. Pathology slides of patients who underwent tumor resection outside of the Mayo Clinic were returned to the performing institution after review.
Tumor expression of somatostatin receptor sstr2a was evaluated by immunohistochemistry in all patients who underwent meningioma resection at Mayo Clinic. Five-micron sections were cut from the formalin-fixed paraffin-embedded tumor blocks. Pancreas sections were used as controls, given the presence of sstr2 in pancreatic islet β cells. Slides were dried for 30 min at 60°C and placed into 3 changes of xylene for 5 min each to remove paraffin. Slides were immersed in 3 changes of 100% ethanol and 1 change of 95% ethanol and room temperature tap water to rinse for 5 min. Slides were immersed in a steam bath of Diva Decloaker 10× antibody retrieval solution (Biocare Medical) at 90°C for 20 min, then removed and allowed to rise to room temperature, followed by a tap water rinse. Slides were placed into Wash Buffer (DAKO) for 5 min then immersed into 3% hydrogen peroxide for 5 min, followed by an additional Wash Buffer rinse. A DAKO IHC instrument was used, and slides were treated sequentially with Non-Serum Blocking Solution (DAKO) for 5 min, somatostatin receptor 2a-specific antibody (Novus Biologicals) at 1:2500 for 60 min room temperature, labeled polymer-HRP anti-rabbit (DAKO #K4011) for 30 min, Chromogen–DAB+ (DAKO K4007 and K4011) for 5 min, and hematoxylin-3 (Lerner #1931433) for 30 s. Water rinses were performed between steps. Slides were then dehydrated in 1-change 95% ethanol, 3changes 100% ethanol, and 3changes xylene before mounting. Immunostained sections were examined with light microscopy by a study pathologist (C.G.) blinded to the patient clinical history and graded as described by Barresi et al.29 In brief, the immunostaining intensity was graded as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The area of staining positivity, which was recorded as a percentage of positive cells, was graded as follows: 0 (<5%), 1 (5%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (76%–100%). Slides were stained and analyzed as a single batch to minimize technical variation.
Results
Twelve patients were enrolled in the study during the period from May 1992 through March 1998. All patients were eligible and could be evaluated for response and toxicity. No patients left the study for reasons other than disease progression or loss to follow-up. Table 1 lists the clinical characteristics of the 12 patients. All patients had assessable disease. The histologic diagnosis was meningioma (WHO grade I) in 3 patients, clear cell meningioma (WHO grade II) in 1, atypical meningioma (WHO grade II) in 2, anaplastic meningioma (WHO grade III) in 5, and meningeal hemangiopericytoma (WHO grade III) in 1. All patients except 2 had undergone prior radiation therapy; 1 of these patients had radiation-induced WHO grade II meningioma and could not receive more therapy to the region, and the other had WHO grade I meningioma. Three patients had undergone prior chemotherapy or hormonal therapy. Six had multiple tumors, including 1 patient with histologic confirmation of lung metastases from an anaplastic meningioma.
Table 1.
Clinical characteristics and response of patients treated with octreotide.
| Patient | Age, years | Sex | Histological finding | Prior therapy | Best response | Time to progression, days | sstr2A IS | sstr2A ASP |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | Meningioma | Surgery | Stable | 194 | 3 | 4 |
| 2 | 38 | M | Atypical meningioma | Surgery | Stable | ≥6700 | 3 | 4 |
| 3 | 43 | M | Meningioma | Surgery, XRT | Stable | 125 | 2–3 | 4 |
| 4 | 64 | M | Atypical meningoma | Surgery, XRT | Stable | 118 | ||
| 5 | 38 | M | Hemangiopericytoma | Surgery, XRT | Stable | 196 | ||
| 6 | 36 | F | Meningoma | Surgery, XRT | Stable | 93 | 1–2 | 4 |
| 7 | 48 | M | Anaplastic meningioma | Surgery, XRT | Progression | 33 | … | … |
| 8 | 59 | M | Anaplastic meningoma | Surgery, XRT, BCNU | Progression | 31 | … | … |
| 9 | 52 | F | Anaplastic meningioma | Surgery, XRT | Progression | 22 | 2–3 | 4 |
| 10 | 65 | M | Anaplastic meningoma | Surgery, XRT, tamoxifen | Stable | 939 | … | … |
| 11 | 56 | M | Anaplastic meningioma | Surgery, XRT | Stable | 45 | … | … |
| 12 | 35 | M | Clear cell meningioma | Surgery, XRT, hydroxyurea | Stable | 134 | 2 | 4 |
ASP indicates area of staining positivity; BCNU indicates 1,3-bis(2-choroethyl)-1-nitrosourea; IS indicates intensity of staining; XRT indicates radiotherapy.
Best response data are listed in Table 1. No radiographic or clinical responses were observed; thus, the binomial exact 95% confidence interval for the response rate was 0%–26.5%. Nine patients had stable disease, ranging from 45 days to >18 years in duration; the median stable disease duration was 19 weeks. Only 2 patients had stable disease for >1 year. The 1 remaining patient with stable disease has an atypical meningioma. No patient had improvement in any clinical symptoms or signs.
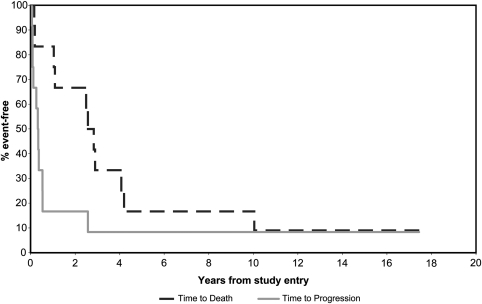
To date, disease has progressed in 11 of the 12 patients. Three patients had progressive disease at first evaluation. All 3 had anaplastic meningioma. The median time to progression for all patients was 17 weeks. The duration of survival from the start of therapy ranged from 22 days to 9.4 years, with a median survival duration of 2.7 years. At the time of this report, 1 of 12 patients is alive at >18 years, whereas 11 have died. Kaplan-Meier plots of time to progression and survival are displayed in Figure 1. Of the 11 patients who died, 3 had progressive disease and 8 had stable disease as a best response. The single patient who remains progression-free has WHO grade II meningioma, which is believed to have been induced by radiation after treatment for a pituitary adenoma. The meningioma was initially treated with gross total resection, with recurrent disease occurring <2 years later, prompting trial enrollment.
Fig. 1.
Kaplan-Meier plot of time to progression and overall survival.
In general, therapy with octreotide was well tolerated. Ten of 12 patients achieved the target dose of 500 μg 3 times per day. Two patients were treated at 150 μg twice per day because of diarrhea. The most common toxicity was diarrhea, which was mild (grade 1) in 4 patients and moderate (grade 2) in 4. Mild anorexia or nausea developed in 4 patients. A mild elevation of liver function test values occurred in 1 patient. One patient developed extra-hepatic cholangiocarcinoma, which was likely unrelated to octreotide therapy. No neurologic toxicity was observed. No patient had to discontinue therapy because of toxicity.
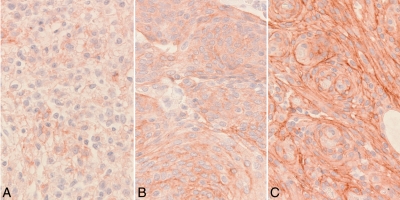
As an exploratory analysis, somatostatin receptor 2a (sstr2a) staining was performed on the 6 patients who received an operation for meningioma at Mayo Clinic. One patient had WHO grade I meningioma, 4 had atypical meningioma (WHO grade II), and 1 patient had an anaplastic meningioma (WHO grade III). All 6 patients showed sstr2a expression in >75% of tumor cells in the sample examined. Two of the 6 patients had strong sstr2a expression throughout the tumor tissue; 2 patients had heterogenous staining, with a mixture of strong and moderate expression; and 2 patients had a mixture of areas of moderate and weak expression. Examples of weak, moderate, and strong staining intensity are displayed in Figure 2. No correlation between strength of sstr2a staining and duration of progression-free survival was seen.
Fig. 2.
Somatostatin receptor immunostaining at 400× magnification. (A) Weak. (B) Moderate. (C) Strong.
Discussion
Inoperable recurrent or progressive meningioma is a significant clinical problem. No chemotherapy options offer proven benefit when surgical and radiation options have been exhausted. This prospective trial showed no evidence of meningioma regression in response to subcutaneous octreotide therapy, in contrast to case reports and series25–27 and the only other published trial of a somatostatin analogue for recurrent meningioma, which reported a 31% response rate.28 In our trial, 2 patients experienced prolonged stability of disease, including ongoing response more than 18 years after study entry in a patient with recurrent atypical meningioma refractory to other treatments. Although radiation-induced meningiomas are often biologically aggressive, it is possible that this tumor would also have behaved favorably in the absence of octreotide therapy. Although the median survival time in this trial was >2 years, many previous trials have reported median survival times of well under 1 year.10–12 This difference is likely due to differences in case mixture rather than the efficacy of octreotide.
Strengths of this clinical trial include prospective design, frequent clinical and radiographic evaluation, and uniform use of response criteria. This trial also has several limitations. First, our patient population consisted primarily of patients with aggressive tumors and, therefore, may not reflect the general population of patients with recurrent unresectable meningiomas. Second, our patient population size is small, limiting the statistical power of the study. In addition, no formal phase 1 dose-escalation study of octreotide has been performed in patients with meningioma. Instead, a dose known to be biologically active against other tumor types (eg, carcinoid tumors) was chosen.18,19 The total dose of 1500 μg/day used in this trial is higher than the usual effective dose in these other tumors, and this dose was reported to be effective against meningioma in previous case reports and case series.25–27 However, we cannot exclude the possibility that use of a higher dose would have led to prolonged survival or clinical response.
At the time this study was designed, octreotide scintillography scanning had been described elsewhere,21,23,30 but the technique was not yet in widespread clinical practice. Thus, patients were enrolled without pretreatment confirmation of the presence of somatostatin receptors in their tumors. It is possible that, by chance, our population contained a large proportion of patients without somatostatin receptor expression in their tumors and who were thus unable to benefit from treatment. To retrospectively determine the level and pattern of sstr2a expression in our patient population, immunostaining with a commercially available sstr2a stain was performed on all available tumor specimens. In each of the 6 cases, widespread somatostatin expression was seen, and in 4 of the 6 patients, the intensity of expression was entirely strong (2 patients) or a mixture of strong and moderate (2 patients). Thus, it is unlikely that our negative result was simply due to lack of receptor expression.
Our study did not demonstrate a significant benefit of somatostatin analogue treatment for patients with recurrent meningiomas and meningeal hemangiopericytomas. Although 9 patients had some period of stable disease, the median time to progression was short, and only 2 patients had stable disease for >6 months during treatment. Although these results do not exclude the possibility of a beneficial effect of octreotide on recurrent meningioma, given the inconvenience of 3-times-daily octreotide injection and the high radiographic tumor response rate reported in response to the long-acting somatostatin analogue Sandostatin LAR,27 there is little rationale for further study of octreotide in this setting. Two ongoing clinical trials are continuing to examine the role of somatostatin-analogue therapy in recurrent meningioma. One study (ClinicalTrials.gov identifier: NCT00813592), which is no longer enrolling patients, utilizes twice-daily pasireotide (SOM230), a somatostatin analogue engineered to bind to somatostatin receptors 1, 2, 3, and 5. The second study (ClinicalTrials.gov identifier: NCT00859040), which is currently enrolling patients, uses monthly injections of pasireotide LAR (SOM230c), a long-acting analogue with the same somatostatin receptor specificity as standard pasireotide. These studies, when complete, will provide further insight into the utility of somatostatin analogue therapy in meningioma.
Conflict of interest statement. None declared.
Funding
Supported in part by National Institutes of Health grant P30 CA 15083 (Diasio R).
References
- 1.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. doi:10.1227/01.NEU.0000188281.91351.B9. [DOI] [PubMed] [Google Scholar]
- 2.Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82:1915–1920. [PubMed] [Google Scholar]
- 3.Goellner JR, Laws ER, Jr, Soule EH, Okazaki H. Hemangiopericytoma of the meninges: Mayo Clinic experience. Am J Clin Pathol. 1978;70:375–380. doi: 10.1093/ajcp/70.3.375. [DOI] [PubMed] [Google Scholar]
- 4.Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100:1491–1497. doi: 10.1002/cncr.20109. doi:10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 5.Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102(Suppl):283–286. doi: 10.3171/jns.2005.102.s_supplement.0283. [DOI] [PubMed] [Google Scholar]
- 6.Mattozo CA, De Salles AA, Klement IA, et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. J Neurosurg. 2007;106:846–854. doi: 10.3171/jns.2007.106.5.846. [DOI] [PubMed] [Google Scholar]
- 7.Wara WM, Sheline GE, Newman H, Townsend JJ, Boldrey EB. Radiation therapy of meningiomas. Am J Roentgenol Radium Ther Nucl Med. 1975;123:453–458. doi: 10.2214/ajr.123.3.453. [DOI] [PubMed] [Google Scholar]
- 8.Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96:211–217. doi: 10.1007/s11060-009-9948-7. doi:10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrell UM, Rittig MG, Anders M, et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840–844. doi: 10.3171/jns.1997.86.5.0840. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62:1210–1212. doi: 10.1212/01.wnl.0000118300.82017.f4. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78:271–276. doi: 10.1007/s11060-005-9093-x. doi:10.1007/s11060-005-9093-x. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain MC, Glantz MJ. Interferon-alpha for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer. 2008;113:2146–2151. doi: 10.1002/cncr.23803. doi:10.1002/cncr.23803. [DOI] [PubMed] [Google Scholar]
- 13.Wen PY, Yung WK, Lamborn KR, et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01–08) Neuro Oncol. 2009;11:853–860. doi: 10.1215/15228517-2009-010. doi:10.1215/15228517-2009-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 15.Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286:230–237. doi: 10.1016/j.mce.2008.02.002. doi:10.1016/j.mce.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Ezzat S, Snyder PJ, Young WF, et al. Octreotide treatment of acromegaly: a randomized, multicenter study. Ann Intern Med. 1992;117:711–718. doi: 10.7326/0003-4819-117-9-711. [DOI] [PubMed] [Google Scholar]
- 17.Petersenn S, Schopohl J, Barkan A, et al. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010;95:2781–2789. doi: 10.1210/jc.2009-2272. doi:10.1210/jc.2009-2272. [DOI] [PubMed] [Google Scholar]
- 18.Arnold R, Benning R, Neuhaus C, Rolwage M, Trautmann ME. Gastroenteropancreatic endocrine tumours: effect of Sandostatin on tumour growth. The German Sandostatin Study Group. Digestion. 1993;54(Suppl 1):72–75. doi: 10.1159/000201081. doi:10.1159/000201081. [DOI] [PubMed] [Google Scholar]
- 19.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. doi:10.1002/1097-0142(19930701)72:1<244::AID-CNCR2820720143>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Arena S, Barbieri F, Thellung S, et al. Expression of somatostatin receptor mRNA in human meningiomas and their implication in in vitro antiproliferative activity. J Neurooncol. 2004;66:155–166. doi: 10.1023/b:neon.0000013498.19981.55. doi:10.1023/B:NEON.0000013498.19981.55. [DOI] [PubMed] [Google Scholar]
- 21.Bohuslavizki KH, Brenner W, Braunsdorf WE, et al. Somatostatin receptor scintigraphy in the differential diagnosis of meningioma. Nucl Med Commun. 1996;17:302–310. doi: 10.1097/00006231-199604000-00157. doi:10.1097/00006231-199604000-00157. [DOI] [PubMed] [Google Scholar]
- 22.Reubi JC, Maurer R, Klijn JG, et al. High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab. 1986;63:433–438. doi: 10.1210/jcem-63-2-433. doi:10.1210/jcem-63-2-433. [DOI] [PubMed] [Google Scholar]
- 23.Scheidhauer K, Hildebrandt G, Luyken C, Schomacker K, Klug N, Schicha H. Somatostatin receptor scintigraphy in brain tumors and pituitary tumors: first experiences. Horm Metab Res Suppl. 1993;27:59–62. [PubMed] [Google Scholar]
- 24.Kunert-Radek J, Stepien H, Radek A, Pawlikowski M. Somatostatin suppression of meningioma cell proliferation in vitro. Acta Neurol Scand. 1987;75:434–436. doi: 10.1111/j.1600-0404.1987.tb05474.x. doi:10.1111/j.1600-0404.1987.tb05474.x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Luna PP, Relimpio F, Pumar A, et al. Clinical use of octreotide in unresectable meningiomas. A report of three cases. J Neurosurg Sci. 1993;37:237–241. [PubMed] [Google Scholar]
- 26.Runzi MW, Jaspers C, Windeck R, et al. Successful treatment of meningioma with octreotide. Lancet. 1989;1:1074. doi: 10.1016/s0140-6736(89)92465-3. doi:10.1016/S0140-6736(89)92465-3. [DOI] [PubMed] [Google Scholar]
- 27.Runzi MW, Jaspers C, Windeck R, et al. Treatment of meningioma with octreotide. Lancet. 1989;2:217–218. doi: 10.1016/s0140-6736(89)90400-5. doi:10.1016/S0140-6736(89)90400-5. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. 2007;69:969–973. doi: 10.1212/01.wnl.0000271382.62776.b7. doi:10.1212/01.wnl.0000271382.62776.b7. [DOI] [PubMed] [Google Scholar]
- 29.Barresi V, Alafaci C, Salpietro F, Tuccari G. Sstr2A immunohistochemical expression in human meningiomas: is there a correlation with the histological grade, proliferation or microvessel density? Oncol Rep. 2008;20:485–492. [PubMed] [Google Scholar]
- 30.Sciuto R, Ferraironi A, Semprebene A, et al. Clinical relevance of 111In-octreotide scans in CNS tumors. Q J Nucl Med. 1995;39(4 Suppl 1):101–103. [PubMed] [Google Scholar]