Abstract
Background:
We modelled the efficiency of a personalised approach to screening for prostate and breast cancer based on age and polygenic risk-profile compared with the standard approach based on age alone.
Methods:
We compared the number of cases potentially detectable by screening in a population undergoing personalised screening with a population undergoing screening based on age alone. Polygenic disease risk was assumed to have a log-normal relative risk distribution predicted for the currently known prostate or breast cancer susceptibility variants (N=31 and N=18, respectively).
Results:
Compared with screening men based on age alone (aged 55–79: 10-year absolute risk ⩾2%), personalised screening of men age 45–79 at the same risk threshold would result in 16% fewer men being eligible for screening at a cost of 3% fewer screen-detectable cases, but with added benefit of detecting additional cases in younger men at high risk. Similarly, compared with screening women based on age alone (aged 47–79: 10-year absolute risk ⩾2.5%), personalised screening of women age 35–79 at the same risk threshold would result in 24% fewer women being eligible for screening at a cost of 14% fewer screen-detectable cases.
Conclusion:
Personalised screening approach could improve the efficiency of screening programmes. This has potential implications on informing public health policy on cancer screening.
Keywords: polygenic risk, personalised screening, breast cancer, prostate cancer
The benefits of any cancer screening programme may be offset by adverse consequences, such as false-positive findings (positive screening findings that do not result in a diagnosis of cancer), overdiagnosis (diagnosis of a cancer as a result of screening that would not have been diagnosed in a person's lifetime had screening not taken place) (Paci et al, 2004), and overtreatment. A screening programme becomes viable if it does more good than harm at reasonable cost (Gray et al, 2008).
Prostate and breast cancers are the two most commonly diagnosed cancers in men and women, respectively, in the Western countries (Parkin et al, 2005). The value of screening for prostate cancer using serum prostate-specific antigen (PSA) remains controversial even after the publication of the two major randomised controlled trials of screening (Andriole et al, 2009; Schroder et al, 2009). Early detection of prostate cancer by screening can prevent death for a subset of men, but overdiagnosis and overtreatment may be substantial. The European Study of Screening for Prostate Cancer showed that to prevent one death from prostate cancer, 1410 men would need to be screened and 48 would need treatment (Schroder et al, 2009), although more mature data may demonstrate greater effectiveness. In all, 8 out of 1000 men undertaking PSA testing are likely to be overdiagnosed (Pashayan et al, 2009). In breast cancer, the benefit of mammographic screening in preventing death is greater than the harm in terms of overdiagnosis. On the basis of the UK Breast Screening Programme, 2.3 out of 1000 women screened for 20 years are likely to be overdiagnosed (Duffy et al, 2010).
Genome-wide association studies (GWAS) have identified genetic variants that are common in the population and confer susceptibility to different types of cancers. Most susceptibility variants identified by GWAS in different cancers have low effect size (per-allele relative risks of 1.1–1.3) (Chung et al, 2010) and so the clinical utility of the individual variants in predicting future risk is limited. However, the combination of multiple risk alleles, each with a weak effect may result in a distribution of risk in the population that is sufficiently wide to be clinically useful (Pharoah et al, 2002).
Several studies have shown that risk-profiles based on the known common susceptibility alleles have limited discrimination for breast cancer (Gail, 2008; Pharoah et al, 2008; Wacholder et al, 2010) and for prostate cancer (Salinas et al, 2009), leading some investigators to conclude that the clinical utility of risk prediction based on polygenic profiling is still limited. However, discrimination is not the only measure of clinical utility of a risk prediction model and it has been suggested that polygenic risk profiling may provide sufficient information to enable screening for breast cancer to be targeted to those women at highest risk (Pharoah et al, 2008; Devilee and Rookus, 2010; Wacholder et al, 2010). The aim of this study was to model the efficiency of a personalised screening strategy based on a combination of age and polygenic risk-profile compared with a strategy based on age alone in prostate and breast cancer.
Materials and methods
We compared the number of individuals eligible for screening and the number of cases potentially detectable by screening in the population undergoing screening based on age alone compared with a population undergoing personalised screening based on age and polygenic risk-profile, in which eligibility for screening depends on 10-year absolute risk of being diagnosed with prostate or breast cancer.
Absolute risk calculation
The number of prostate and breast cancer registrations, deaths from prostate and breast cancer, deaths from all causes, and mid-year population estimates in 1-year age bands for England from 2002 to 2006 were obtained from the Office for National Statistics. These data were used to estimate prostate and breast cancer incidence and mortality rates for prostate cancer, breast cancer, and other causes. We then used the DevCan 6.4.1 software (National Cancer Institute and Information Management Services, 2009) to derive the age-conditional absolute risk (risk between ages x and y, given alive and cancer-free at age x) of being diagnosed with prostate or breast cancer among the general population. DevCan is based on competing risk methods developed by Fay et al (2003). We also estimated age-conditional absolute risk for individuals at different levels of polygenic risk by underlying cancer-specific incidence with the polygenic relative risk.
Polygenic risk distribution
In all, 31 prostate cancer and 18 breast cancer susceptibility loci with common risk alleles have been published (Table 1).
Table 1. Common susceptibility variants for prostate and breast cancer identified through GWAS.
| dbSNP No. | Locus/gene | Risk-allele frequency | Odds ratio per allele | Variance | Reference |
|---|---|---|---|---|---|
| Prostate | |||||
| rs12621278 | 2q31/ITGA6 | 0.94 | 1.30 | 0.008 | Eeles et al (2009) |
| rs721048 | 2p15 | 0.19 | 1.15 | 0.002 | Gudmundsson et al (2008) |
| rs1465618 | 2p21/THADA | 0.23 | 1.08 | 0.002 | Eeles et al (2009) |
| rs2660753 | 3p12 | 0.11 | 1.18 | 0.002 | Eeles et al (2008) |
| rs10934853 | 3q21.3 | 0.28 | 1.12 | 0.002 | Gudmundsson et al (2009) |
| rs7679673 | 4q24 /TET2 | 0.55 | 1.09 | 0.004 | Eeles et al (2009) |
| rs17021918 | 4q22/PDLIM5 | 0.66 | 1.10 | 0.003 | Eeles et al (2009) |
| rs12500426 | 4q22/PDLIM6 | 0.46 | 1.08 | 0.007 | Eeles et al (2009) |
| rs9364554 | 6q25 | 0.29 | 1.17 | 0.013 | Eeles et al (2008) |
| rs6465657 | 7q21 | 0.46 | 1.12 | 0.007 | Eeles et al (2008) |
| rs10486567 | 7p15 /JAZF1 | 0.77 | 1.12 | 0.009 | Thomas et al (2008) |
| rs2928679 | 8p21 | 0.42 | 1.05 | 0.010 | Eeles et al (2009) |
| rs1512268 | NKX3.1 | 0.45 | 1.18 | 0.014 | Eeles et al (2009) |
| rs620861 | 8q24 | 0.61 | 1.28 | 0.024 | Al Olama et al (2009) |
| rs10086908 | 8q24 | 0.70 | 1.25 | 0.007 | Al Olama et al (2009) |
| rs445114 | 8q24 | 0.64 | 1.14 | 0.041 | Gudmundsson et al (2009) |
| rs16902094 | 8q24 | 0.15 | 1.21 | 0.015 | Gudmundsson et al (2009) |
| rs6983267 | 8q24 | 0.50 | 1.26 | 0.010 | Yeager et al (2007) |
| rs1447295 | 8q24 | 0.10 | 1.62 | 0.004 | Amundadottir et al (2006) |
| rs16901979 | 8q24 | 0.03 | 2.10 | 0.002 | Gudmundsson et al (2007a) |
| rs4962416 | 10q26 /CTBP2 | 0.27 | 1.17 | 0.013 | Thomas et al (2008) |
| rs10993994 | 10q11/MSMB | 0.24 | 1.25 | 0.014 | Eeles et al (2008), Thomas et al (2008) |
| rs7127900 | 11p15 | 0.20 | 1.22 | 0.011 | Eeles et al (2009) |
| rs7931342 | 11q13 | 0.51 | 1.16 | 0.012 | Eeles et al (2008), Thomas et al (2008) |
| rs4430796 | 17q12 /HNF1B | 0.49 | 1.24 | 0.015 | Gudmundsson et al (2007b) |
| rs11649743 | HNF1B | 0.80 | 1.28 | 0.015 | Sun et al (2008) |
| rs1859962 | 17q24.3 | 0.46 | 1.24 | 0.017 | Gudmundsson et al (2007b) |
| rs2735839 | 19q13/KLK2,KLK3 | 0.85 | 1.20 | 0.001 | Eeles et al (2008) |
| rs8102476 | 19q13.2 | 0.54 | 1.12 | 0.011 | Gudmundsson et al (2009) |
| rs5759167 | 22q13 | 0.53 | 1.16 | 0.015 | Eeles et al (2009) |
| rs5945619 | Xp11 | 0.28 | 1.12 | 0.002 | Eeles et al (2008), Gudmundsson et al (2008) |
| Breast | |||||
| rs11249433 | 1p11.2 | 0.39 | 1.16 | 0.010 | Thomas et al (2009) |
| rs1045485 | 2q33 /CASP8 | 0.85 | 1.14 | 0.004 | Cox et al (2007) |
| rs13387042 | 2q35 | 0.49 | 1.12 | 0.006 | Milne et al (2009) |
| rs4973768 | 3p24 /NEK10, SLC4A7 | 0.46 | 1.11 | 0.005 | Ahmed et al (2009) |
| rs889312 | 5q11/MAP3K1 | 0.28 | 1.13 | 0.006 | Easton et al (2007) |
| rs4415084 | 5p12/MRPS30 | 0.40 | 1.19 | 0.015 | Stacey et al (2008) |
| rs2046210 | 6p12/ESR1 | 0.36 | 1.29 | 0.030 | Zheng et al (2009) |
| rs13281615 | 8q24 | 0.40 | 1.08 | 0.003 | Easton et al (2007) |
| rs1011970 | 9 | 0.17 | 1.09 | 0.002 | Turnbull et al (2010) |
| rs2981582 | 10q26/FGFR2 | 0.38 | 1.26 | 0.025 | Udler et al (2009) |
| rs2380205 | 10p15 | 0.43 | 0.94 | 0.002 | Turnbull et al (2010) |
| rs10995190 | 10q21/ZNF365 | 0.85 | 1.16 | 0.006 | Turnbull et al (2010) |
| rs704010 | 10q22 | 0.39 | 1.07 | 0.002 | Turnbull et al (2010) |
| rs614367 | 11q13 | 0.15 | 1.15 | 0.005 | Turnbull et al (2010) |
| rs3817198 | 11p15/LSP1 | 0.30 | 1.07 | 0.002 | Easton et al (2007) |
| rs999737 | 14q24/RAD51L1 | 0.76 | 1.06 | 0.001 | Thomas et al (2009) |
| rs1244362 | 16q12/TOX3 | 0.25 | 1.20 | 0.014 | Easton et al (2007), Stacey et al (2007) |
| rs6504950 | 17q/COX11 | 0.73 | 1.05 | 0.001 | Ahmed et al (2009) |
Abbreviations: dbSNP=Single Nucleotide Polymorphism database; GWAS=genome-wide association study. Reported risk allele frequency in Europeans.
We estimated the variance of the distribution of polygenic risk in the population from the published risk allele frequencies and per-allele relative risk, assuming a log-additive model of interaction between risk alleles both within and between loci. Under this model, the distribution of risk on a relative risk scale in the population at birth is log-normal with mean, μ, and variance, σ2. We set μ=−σ2/2, so that the mean relative risk in the population at birth is equal to unity. The distribution of relative risk among cases at young ages is also log-normal with the same variance, but shifted (on the log scale) to the right by σ2 (Pharoah et al, 2002). The 31 prostate cancer susceptibility variants result in a polygenic variance of 0.377, accounting for approximately 24% of the familial risk of prostate cancer. The 18 breast cancer susceptibility variants confer a polygenic variance of 0.121 and account for approximately 8.4% of the familial risk of breast cancer.
The percentile rank associated with a given polygenic relative risk (or age-conditional absolute risk) in the population or in cases can be calculated given the mean and variance of the log-normal relative risk distribution. We thus estimated the proportion of the population that has a polygenic risk greater than a given absolute risk threshold, and the proportion of cases that will occur within this high-risk subgroup.
We compared two approaches with screening for prostate cancer in men aged 45–79 – screening based on age alone in which men are only eligible for screening from age 55 (10-year absolute risk of 2% or greater), and personalised screening in which men are eligible for screening at a 2% absolute risk that is age and polygenic risk dependent. We then compared the number of individuals eligible for screening under the two approaches and the number of cases occurring in the eligible population that are therefore potentially screen detectable. Similarly, we compared breast cancer screening based on age alone in women aged 47–79 (10-year absolute risk with screening ⩾2.5%) with screening women aged 35–79 with a 2.5% 10-year risk based on age and polygenic profile.
Results
Prostate cancer
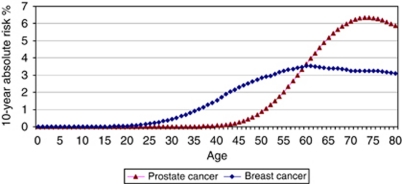
On average, there were 22 836 new cases of prostate cancer per year in men 45–79 years in England during the period 2002 to 2006 (total population 8 655 126). The age-conditional absolute risk of being diagnosed with prostate over 10 years in the general population of men in England is shown in Figure 1. Under the age-based screening programme, 63% of men would be eligible for screening (aged 55 and over) and 96% of cases would occur in this subset of the population (Table 2). These are the cases that are potentially screen detectable. Under the personalised strategy, 53% of men would be eligible for screening with 93 of cases being screen detectable. Thus, the number of men eligible for screening would be 17% fewer at a cost of detecting 3% fewer cases. For the population of men aged 45–79 in England, there would be an additional three screen-detectable cases per 100 000 population in men younger than 55 years of age with polygenic risk ⩾2%, and 12 cases per 100 000 population would be missed in men older than 55 years with polygenic risk <2%.
Figure 1.
Ten-year absolute risk of being diagnosed with prostate or breast cancer, England, 2002–2006.
Table 2. Reclassification of population of 100 000 men 45–79 years eligible for screening and in whom prostate cancer could be detectable, under age-based or personalised screening strategies.
| Personalised screening |
Age-based screening
|
||
|---|---|---|---|
| Polygenic risk threshold | <51 years | ⩾51 years | Total |
| Population | |||
| <1% | 20 355 | 9377 | 29 733 |
| ⩾1% | 2079 | 68 188 | 70 267 |
| Total | 22 434 | 77 566 | 100 000 |
| Cases | |||
| <1% | 2 | 3 | 5 |
| ⩾1% | 1 | 258 | 259 |
| Total | 3 | 261 | 264 |
| Polygenic risk threshold | <55 years | ⩾55 years | Total |
| Population | |||
| <2% | 33 802 | 13 328 | 47 130 |
| ⩾2% | 2841 | 50 029 | 52 871 |
| Total | 36 643 | 63 357 | 100 000 |
| Cases | |||
| <2% | 6 | 12 | 18 |
| ⩾2% | 3 | 243 | 246 |
| Total | 9 | 255 | 264 |
| Polygenic risk threshold | <58 years | ⩾58 years | Total |
| Population | |||
| <3% | 46 499 | 16 152 | 60 408 |
| ⩾3% | 4993 | 35 960 | 39 592 |
| Total | 51 492 | 52 113 | 100 000 |
| Cases | |||
| <3% | 15 | 26 | 41 |
| ⩾3% | 7 | 216 | 223 |
| Total | 22 | 242 | 264 |
Eligibility based on age or polygenic risk equivalent to 10-year absolute for that age considering three scenarios: age 51 vs risk 1%, age 55 vs risk 2%, age 58 vs risk 3% England 2002–2006.
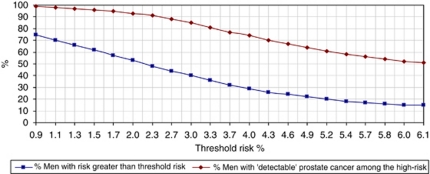
The proportion of men 45–79 years that would be eligible for screening and the proportion of cases potentially detectable within the eligible population at different risk thresholds are given in Figure 2.
Figure 2.
Men eligible for screening and cases detectable by screening at different risk thresholds. Percentage of men 45–79 years of age with polygenic risk for prostate cancer greater than a given threshold risk and percentage of men with detectable prostate cancer within this high-risk population, England, 2002–2006.
The eligible population for the personalised approach based on a 1.4% 10-year risk threshold would be the same size as the age 55 and over population. The number of screen-detectable cases would then be 0.4% (one case per 100 000 population) greater under the personalised approach. Alternatively, a 1.5% threshold for personalised screening would be 2.6% (1637 men eligible for screening per 100 000 population) smaller than the age 55 and over population and have the same number of screen-detectable cases. At a higher age threshold, such as a 2.2% threshold for personalised screening, the number eligible for screening would be 4% (1983 per 100 000 population) smaller than the age 58 and over population and have the same number of potentially screen-detectable cases.
Table 2 shows the eligible population and screen-detectable cases for screening from age 51 or an absolute risk threshold of 1%, and screening from age 58 or an absolute risk threshold of 3%.
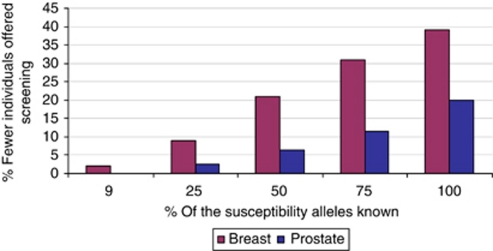
If all possible susceptibility variants for prostate cancer were known (predicted polygenic variance 1.58), 35% of men aged 45–79 would be at 2% 10-year risk with 90% of cases being potentially screen detectable. Compared with screening from age 55, 44% fewer men would be offered screening at a cost of 7% fewer cases being potentially screen detectable. To detect the same number of cases as screening from age 55, 20% (12 768 men eligible for screening per 100 000 population) fewer men would be eligible for screening (Figure 3).
Figure 3.
Change in proportion of individuals eligible for screening with increase in the known susceptibility variants. The likely percentage fewer individuals that would be eligible for screening under the personalised screening strategy as compared with the standard screening while detecting the same number of cases with increase in the percentage of the known susceptibility alleles. Prostate: compared with screening men 55–79; currently ∼24% of the variants known. Breast: compared with screening women 47–79; currently ∼9% of the variants known.
Breast cancer
On average, there were 30 936 new cases of breast cancer per year in women 35–79 years in England during the period 2002–2006 (total population 13 126 890). The age-conditional absolute risk of being diagnosed with breast cancer over 10 years in the general population of women in England is given in Figure 1. Under the age-based programme, 65% of women aged 35–79 would be eligible for screening with 85% of cases being potentially screen detectable (Table 3). Under the personalised strategy, 50% of women would be eligible for screening with 73 of cases being potentially screen detectable. Thus, the number of women eligible for screening would be 24% fewer at a cost of 14% fewer screen-detectable cases. There would be nine screen-detectable cases per 100 000 population under personalised screening in women not eligible under age-based screening and 38 potentially screen-detectable cases per 100 000 population under age-based screening in women not eligible for screening based on polygenic risk (Table 3).
Table 3. Reclassification of population of 100 00 women 35–79 years eligible for screening and in whom breast cancer could be detectable, under age-based or personalised screening strategies.
| Personalised screening |
Age-based screening
|
||
|---|---|---|---|
| Polygenic risk threshold | <47 years | ⩾47 years | Total |
| Population | |||
| <2.5% | 30 276 | 19 926 | 50 202 |
| ⩾2.5% | 4429 | 45 368 | 49 798 |
| Total | 34 705 | 65 295 | 100 000 |
| Cases | |||
| <2.5% | 26 | 38 | 64 |
| ⩾2.5% | 9 | 162 | 172 |
| Total | 35 | 200 | 236 |
Eligibility based on age 47 or polygenic risk equivalent to 10-year absolute risk for age 47 (2.5% 10-year absolute risk); England 2002–2006.
The eligible population for the personalised approach based on a 2.02% 10-year risk threshold would be the same size as the age 47 and over population. The number of screen-detectable cases would then be 1% (two cases per 100 000 population) greater under the personalised approach. Alternatively, a 2% threshold for personalised screening would entail screening 2% fewer women (1477 women eligible for screening per 100 000 population) than the age 47 and over population and yield the same number of potentially screen-detectable cases.
In a best-case scenario analysis, assuming all possible susceptibility variants for breast cancer were known, 28% of women 35–79 years would be at 2.5% risk and 76% of the cases would occur in this group. Compared with screening from age 47, 57% fewer women would be offered screening at a cost of detecting 10% fewer cases. To detect the same number of cases as screening from age 47, 39% (25 678 women eligible for screening per 100 000 population) fewer women would need to be screened (Figure 3).
Discussion
These data show that personalised screening with eligibility for screening based on an absolute risk that is dependent on age and polygenic risk and equivalent to the risk threshold for eligibility based on age alone could reduce the number of people eligible for screening while detecting the majority of the cancers identified through a programme based on age alone. Alternatively, screening the same number of individuals in a personalised screening programme could potentially detect a greater number of cases than a screening programme based on age alone.
However, we have estimated the proportion of the population to be offered screening and the proportion of cancer cases that might be screen detectable in this subgroup of the population, from the distribution of genetic risk in the population. The estimate of potentially detectable cases is based on cancer incidence derived from cancer registration and is independent of the detection rate by screening. Given the normal distribution of polygenic risk among the cases, and the number of cases in single age group, we estimated the proportion and the expected number of cases that will occur above a certain absolute risk threshold. We have not estimated the expected number of cases to be detected following a screening programme, as this would depend on screening programme sensitivity. Screening programme sensitivity is the probability of detecting cancer by screening in a population subjected to screening. The programme sensitivity increases with decrease in the inter-screening interval, with increase in test sensitivity and with increase in the duration of the pre-clinical screen-detectable phase (Launoy et al, 1998). In subjects of a given age at high genetic risk, the test sensitivity is likely to be the same or better than in those of the same age at low genetic risk. However, both the PSA test (Hoffman et al, 2002) and mammogram are less sensitive in younger subjects (at lower risk). It is not known how test sensitivity will compare between younger and older subjects at the same absolute risk. The duration of the pre-clinical, screen-detectable phase may also vary by underlying genetic risk. Thus, the comparative sensitivity of the screening programme under the two approaches is not known, and empirical data will be needed in order to estimate this. Assuming equivalent or improved screening programme sensitivity, personalised screening has the potential for cost saving as the cost of the genetic test for risk profiling may be offset by savings on repeat screening and diagnostic work-up of false positives.
Reducing the number of screening tests may also reduce some of the harms associated with screening. Fewer screen tests will, at the population level, reduce the anxiety and inconvenience associated with having the test. Assuming that the probability of a false positive is independent of polygenic risk-profile, reducing the number of screen tests will also reduce the number of false-positive screens, with a reduction in the harms associated with a false positive and the benefit of saving further resources on diagnostic tests. Personalised screening also has the potential to reduce the harms associated with overdiagnosis and overtreatment, but this depends on the nature of the relationship between polygenic risk and disease aggressiveness. To date, there is equivocal evidence on the association of combination of prostate cancer susceptibility variants with disease aggressiveness (Xu et al, 2008; Bao et al, 2010).
Personalised screening may potentially confer additional benefits. It can detect cancer in younger subjects at high risk. Prostate and breast cancer detected in younger subjects may tend to behave more aggressively (Fredholm et al, 2009; Lin et al, 2009). If polygenic high risk is associated with disease aggressiveness, then potentially additional life years would be gained by early detection of cancer in younger subjects. The majority of breast cancer susceptibility variants identified to date confer risk for oestrogen receptor-positive breast cancers (Turnbull et al, 2010), which are responsive to hormonal treatment and have a favourable prognosis (Dunnwald et al, 2007). However, the nature of the complex interaction between disease risk, tumour subtype, natural history of disease, and benefit from screening are not understood and the true benefits of screening according to genetic risk cannot be estimated.
In addition to polygenic risk, there is scope for individualised screening based on phenotypic risk markers. Already, there is considerable screening activity below the age range of the UK national programme for women with a significant family history of breast cancer (Maurice et al, 2006). There is also interest in tailoring screening to risk based on mammographic breast density. This might be used to prescribe screening frequency or indeed modality, since in addition to risk, density affects the sensitivity and the potential lead time of mammographic screening (Chiu et al, 2010). Further studies are needed using empirical data to test the implications of adding information on PSA test level and family history to polygenic risk profiling for personalised screening in prostate cancer (Zheng et al, 2008).
The threshold risk for personalised screening will be population specific. We have used data from England to estimate the proportion of men 45–79 years that would be eligible for screening and the proportion of cases potentially detectable within the eligible population at different risk thresholds. The optimum threshold risk for population of England will be different from that of another population with different incidence of cancer, such as of Asian population with low incidence of prostate cancer.
Other issues need to be considered. Screening based on a personalised risk-profile would add complexity to a screening programme. Perhaps of greater importance is the fact that eligibility for screening based on age is generally acceptable to both professionals and the public, but whether eligibility based on age and other risk factors would also be acceptable is not known. Furthermore, there are ethical and legal issues associated with genetic testing and risk prediction that would need to be addressed before personalised programmes could be implemented.
Personalised screening strategy based on age and genetic risk would potentially improve the efficiency of screening programmes and reduce their adverse consequences. Questions remain whether higher genetic risk affects cancer detection and cancer behaviour and so affecting test sensitivity, overdiagnosis and outcome. Further evidence from empirical data is needed. Nevertheless, this approach has the potential to inform public health policy decision making in the context of population screening.
Acknowledgments
NP is a Cancer Research UK Training Fellow in Cancer Public Health and Epidemiology. SC is funded through a FP7 grant of the European Union. DFE is a Principal Research Fellow of Cancer Research UK. RE is NIHR Senior Investigator. This work was supported by funding from the European Community Seventh Framework Programme under grant agreement 223175 (HEALTH-F2-2009-223175). The funding source had no role in the study design, collection, analysis, interpretation of the data, writing of the report, or in the decision to submit the paper for publication. All the authors had the responsibility for the decision to submit for publication.
Authors’ contributions
PP developed the concept. NP and PP contributed to designing the methodology, analysis, interpretation of the findings, and drafting the paper. SWD contributed to designing the analysis. SC collated the data and contributed to the analysis. SWD, SC, TD, HB, DEN, DFE, and RE contributed to the interpretation of the results and revising of the paper. All authors have read and approved the final paper. NP and PP had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The authors declare no conflict of interest.
References
- Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA, Luben R, Eccles D, Evans DG, Fletcher O, Johnson N, dos SSI, Peto J, Stratton MR, Rahman N, Jacobs K, Prentice R, Anderson GL, Rajkovic A, Curb JD, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver WR, Bojesen S, Nordestgaard BG, Flyger H, Dork T, Schurmann P, Hillemanns P, Karstens JH, Bogdanova NV, Antonenkova NN, Zalutsky IV, Bermisheva M, Fedorova S, Khusnutdinova E, Kang D, Yoo KY, Noh DY, Ahn SH, Devilee P, van Asperen CJ, Tollenaar RA, Seynaeve C, Garcia-Closas M, Lissowska J, Brinton L, Peplonska B, Nevanlinna H, Heikkinen T, Aittomaki K, Blomqvist C, Hopper JL, Southey MC, Smith L, Spurdle AB, Schmidt MK, Broeks A, van Hien RR, Cornelissen S, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Schmutzler RK, Burwinkel B, Bartram CR, Meindl A, Brauch H, Justenhoven C, Hamann U, Chang-Claude J, Hein R, Wang-Gohrke S, Lindblom A, Margolin S, Mannermaa A, Kosma VM, Kataja V, Olson JE, Wang X, Fredericksen Z, Giles GG, Severi G, Baglietto L, English DR, Hankinson SE, Cox DG, Kraft P, Vatten LJ, Hveem K, Kumle M, Sigurdson A, Doody M, Bhatti P, Alexander BH, Hooning MJ, van den Ouweland AM, Oldenburg RA, Schutte M, Hall P, Czene K, Liu J, Li Y, Cox A, Elliott G, Brock I, Reed MW, Shen CY, Yu JC, Hsu GC, Chen ST, nton-Culver H, Ziogas A, Andrulis IL, Knight JA, Beesley J, Goode EL, Couch F, Chenevix-Trench G, Hoover RN, Ponder BA, Hunter DJ, Pharoah PD, Dunning AM, Chanock SJ, Easton DF (2009) Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41: 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Olama AA, Kote-Jarai Z, Giles GG, Guy M, Morrison J, Severi G, Leongamornlert DA, Tymrakiewicz M, Jhavar S, Saunders E, Hopper JL, Southey MC, Muir KR, English DR, Dearnaley DP, rdern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Sawyer E, Lophatananon A, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper C, Donovan JL, Hamdy FC, Neal DE, Eeles RA, Easton DF (2009) Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet 41: 1058–1060 [DOI] [PubMed] [Google Scholar]
- Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Cazier JB, Sainz J, Jakobsdottir M, Kostic J, Magnusdottir DN, Ghosh S, Agnarsson K, Birgisdottir B, Le RL, Olafsdottir A, Blondal T, Andresdottir M, Gretarsdottir OS, Bergthorsson JT, Gudbjartsson D, Gylfason A, Thorleifsson G, Manolescu A, Kristjansson K, Geirsson G, Isaksson H, Douglas J, Johansson JE, Balter K, Wiklund F, Montie JE, Yu X, Suarez BK, Ober C, Cooney KA, Gronberg H, Catalona WJ, Einarsson GV, Barkardottir RB, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K (2006) A common variant associated with prostate cancer in European and African populations. Nat Genet 38: 652–658 [DOI] [PubMed] [Google Scholar]
- Andriole GL, Grubb III RL, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, Crawford ED, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD (2009) Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao BY, Pao JB, Lin VC, Huang CN, Chang TY, Lan YH, Lu TL, Lee HZ, Chen LM, Ting WC, Hsieh CJ, Huang SP (2010) Individual and cumulative association of prostate cancer susceptibility variants with clinicopathologic characteristics of the disease. Clin Chim Acta 411: 1232–1237 [DOI] [PubMed] [Google Scholar]
- Chiu SY, Duffy S, Yen AM, Tabar L, Smith RA, Chen HH (2010) Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Biomarkers Prev 19: 1219–1228 [DOI] [PubMed] [Google Scholar]
- Chung CC, Magalhaes WC, Gonzalez-Bosquet J, Chanock SJ (2010) Genome-wide association studies in cancer--current and future directions. Carcinogenesis 31: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Dunning AM, Garcia-Closas M, Balasubramanian S, Reed MW, Pooley KA, Scollen S, Baynes C, Ponder BA, Chanock S, Lissowska J, Brinton L, Peplonska B, Southey MC, Hopper JL, McCredie MR, Giles GG, Fletcher O, Johnson N, dos SSI, Gibson L, Bojesen SE, Nordestgaard BG, Axelsson CK, Torres D, Hamann U, Justenhoven C, Brauch H, Chang-Claude J, Kropp S, Risch A, Wang-Gohrke S, Schurmann P, Bogdanova N, Dork T, Fagerholm R, Aaltonen K, Blomqvist C, Nevanlinna H, Seal S, Renwick A, Stratton MR, Rahman N, Sangrajrang S, Hughes D, Odefrey F, Brennan P, Spurdle AB, Chenevix-Trench G, Beesley J, Mannermaa A, Hartikainen J, Kataja V, Kosma VM, Couch FJ, Olson JE, Goode EL, Broeks A, Schmidt MK, Hogervorst FB, Van’t Veer LJ, Kang D, Yoo KY, Noh DY, Ahn SH, Wedren S, Hall P, Low YL, Liu J, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Sigurdson AJ, Stredrick DL, Alexander BH, Struewing JP, Pharoah PD, Easton DF (2007) A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet 39: 352–358 [DOI] [PubMed] [Google Scholar]
- Devilee P, Rookus MA (2010) A tiny step closer to personalized risk prediction for breast cancer. N Engl J Med 362: 1043–1045 [DOI] [PubMed] [Google Scholar]
- Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen TH, Yen AM, Smith RA (2010) Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen 17: 25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, Wareham N, Ahmed S, Healey CS, Bowman R, Meyer KB, Haiman CA, Kolonel LK, Henderson BE, Le ML, Brennan P, Sangrajrang S, Gaborieau V, Odefrey F, Shen CY, Wu PE, Wang HC, Eccles D, Evans DG, Peto J, Fletcher O, Johnson N, Seal S, Stratton MR, Rahman N, Chenevix-Trench G, Bojesen SE, Nordestgaard BG, Axelsson CK, Garcia-Closas M, Brinton L, Chanock S, Lissowska J, Peplonska B, Nevanlinna H, Fagerholm R, Eerola H, Kang D, Yoo KY, Noh DY, Ahn SH, Hunter DJ, Hankinson SE, Cox DG, Hall P, Wedren S, Liu J, Low YL, Bogdanova N, Schurmann P, Dork T, Tollenaar RA, Jacobi CE, Devilee P, Klijn JG, Sigurdson AJ, Doody MM, Alexander BH, Zhang J, Cox A, Brock IW, MacPherson G, Reed MW, Couch FJ, Goode EL, Olson JE, Meijers-Heijboer H, van den OA, Uitterlinden A, Rivadeneira F, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Hopper JL, McCredie M, Southey M, Giles GG, Schroen C, Justenhoven C, Brauch H, Hamann U, Ko YD, Spurdle AB, Beesley J, Chen X, Mannermaa A, Kosma VM, Kataja V, Hartikainen J, Day NE, Cox DR, Ponder BA (2007) Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447: 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, Muir K, Hopper JL, Henderson BE, Haiman CA, Schleutker J, Hamdy FC, Neal DE, Donovan JL, Stanford JL, Ostrander EA, Ingles SA, John EM, Thibodeau SN, Schaid D, Park JY, Spurdle A, Clements J, Dickinson JL, Maier C, Vogel W, Dork T, Rebbeck TR, Cooney KA, Cannon-Albright L, Chappuis PO, Hutter P, Zeegers M, Kaneva R, Zhang HW, Lu YJ, Foulkes WD, English DR, Leongamornlert DA, Tymrakiewicz M, Morrison J, rdern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Saunders EJ, Page EC, Sawyer EJ, Edwards SM, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van AN, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Southey MC, Lophatananon A, Liu JF, Kolonel LN, Le ML, Wahlfors T, Tammela TL, Auvinen A, Lewis SJ, Cox A, FitzGerald LM, Koopmeiners JS, Karyadi DM, Kwon EM, Stern MC, Corral R, Joshi AD, Shahabi A, McDonnell SK, Sellers TA, Pow-Sang J, Chambers S, Aitken J, Gardiner RA, Batra J, Kedda MA, Lose F, Polanowski A, Patterson B, Serth J, Meyer A, Luedeke M, Stefflova K, Ray AM, Lange EM, Farnham J, Khan H, Slavov C, Mitkova A, Cao G, Easton DF (2009) Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet 41: 1116–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, Donovan JL, Hamdy FC, Dearnaley DP, Muir KR, Smith C, Bagnato M, rdern-Jones AT, Hall AL, O’Brien LT, Gehr-Swain BN, Wilkinson RA, Cox A, Lewis S, Brown PM, Jhavar SG, Tymrakiewicz M, Lophatananon A, Bryant SL, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Fisher C, Jamieson C, Cooper CS, English DR, Hopper JL, Neal DE, Easton DF (2008) Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 40: 316–321 [DOI] [PubMed] [Google Scholar]
- Fay MP, Pfeiffer R, Cronin KA, Le C, Feuer EJ (2003) Age-conditional probabilities of developing cancer. Stat Med 22: 1837–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H (2009) Breast cancer in young women: poor survival despite intensive treatment. PLoS One 4: e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail MH (2008) Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst 100: 1037–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Patnick J, Blanks RG (2008) Maximising benefit and minimising harm of screening. BMJ 336: 480–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, Agnarsson BA, Benediktsdottir KR, Magnusdottir DN, Orlygsdottir G, Jakobsdottir M, Stacey SN, Sigurdsson A, Wahlfors T, Tammela T, Breyer JP, McReynolds KM, Bradley KM, Saez B, Godino J, Navarrete S, Fuertes F, Murillo L, Polo E, Aben KK, van OI, Suarez BK, Helfand BT, Kan D, Zanon C, Frigge ML, Kristjansson K, Gulcher JR, Einarsson GV, Jonsson E, Catalona WJ, Mayordomo JI, Kiemeney LA, Smith JR, Schleutker J, Barkardottir RB, Kong A, Thorsteinsdottir U, Rafnar T, Stefansson K (2009) Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet 41: 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Xu J, Blondal T, Kostic J, Sun J, Ghosh S, Stacey SN, Mouy M, Saemundsdottir J, Backman VM, Kristjansson K, Tres A, Partin AW, bers-Akkers MT, Godino-Ivan MJ, Walsh PC, Swinkels DW, Navarrete S, Isaacs SD, Aben KK, Graif T, Cashy J, Ruiz-Echarri M, Wiley KE, Suarez BK, Witjes JA, Frigge M, Ober C, Jonsson E, Einarsson GV, Mayordomo JI, Kiemeney LA, Isaacs WB, Catalona WJ, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K (2007a) Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 39: 631–637 [DOI] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Rafnar T, Bergthorsson JT, Manolescu A, Gudbjartsson D, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Blondal T, Jakobsdottir M, Stacey SN, Kostic J, Kristinsson KT, Birgisdottir B, Ghosh S, Magnusdottir DN, Thorlacius S, Thorleifsson G, Zheng SL, Sun J, Chang BL, Elmore JB, Breyer JP, McReynolds KM, Bradley KM, Yaspan BL, Wiklund F, Stattin P, Lindstrom S, Adami HO, McDonnell SK, Schaid DJ, Cunningham JM, Wang L, Cerhan JR, St Sauver JL, Isaacs SD, Wiley KE, Partin AW, Walsh PC, Polo S, Ruiz-Echarri M, Navarrete S, Fuertes F, Saez B, Godino J, Weijerman PC, Swinkels DW, Aben KK, Witjes JA, Suarez BK, Helfand BT, Frigge ML, Kristjansson K, Ober C, Jonsson E, Einarsson GV, Xu J, Gronberg H, Smith JR, Thibodeau SN, Isaacs WB, Catalona WJ, Mayordomo JI, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K (2008) Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet 40: 281–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van BE, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen TO, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K (2007b) Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39: 977–983 [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Gilliland FD, ms-Cameron M, Hunt WC, Key CR (2002) Prostate-specific antigen testing accuracy in community practice. BMC Fam Pract 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launoy G, Duffy SW, Prevost TC, Bouvier V (1998) Detection of cancer, sensitivity of the test and sensitivity of the screening program. Rev Epidemiol Sante Publique 46: 420–426 [PubMed] [Google Scholar]
- Lin DW, Porter M, Montgomery B (2009) Treatment and survival outcomes in young men diagnosed with prostate cancer: a Population-Based Cohort Study. Cancer 115: 2863–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice A, Evans DG, Shenton A, Ashcroft L, Baildam A, Barr L, Byrne G, Bundred N, Boggis C, Wilson M, Duffy SW, Howell A (2006) Screening younger women with a family history of breast cancer--does early detection improve outcome? Eur J Cancer 42: 1385–1390 [DOI] [PubMed] [Google Scholar]
- Milne RL, Benitez J, Nevanlinna H, Heikkinen T, Aittomaki K, Blomqvist C, Arias JI, Zamora MP, Burwinkel B, Bartram CR, Meindl A, Schmutzler RK, Cox A, Brock I, Elliott G, Reed MW, Southey MC, Smith L, Spurdle AB, Hopper JL, Couch FJ, Olson JE, Wang X, Fredericksen Z, Schurmann P, Bremer M, Hillemanns P, Dork T, Devilee P, van Asperen CJ, Tollenaar RA, Seynaeve C, Hall P, Czene K, Liu J, Li Y, Ahmed S, Dunning AM, Maranian M, Pharoah PD, Chenevix-Trench G, Beesley J, Bogdanova NV, Antonenkova NN, Zalutsky IV, nton-Culver H, Ziogas A, Brauch H, Justenhoven C, Ko YD, Haas S, Fasching PA, Strick R, Ekici AB, Beckmann MW, Giles GG, Severi G, Baglietto L, English DR, Fletcher O, Johnson N, dos SSI, Peto J, Turnbull C, Hines S, Renwick A, Rahman N, Nordestgaard BG, Bojesen SE, Flyger H, Kang D, Yoo KY, Noh DY, Mannermaa A, Kataja V, Kosma VM, Garcia-Closas M, Chanock S, Lissowska J, Brinton LA, Chang-Claude J, Wang-Gohrke S, Shen CY, Wang HC, Yu JC, Chen ST, Bermisheva M, Nikolaeva T, Khusnutdinova E, Humphreys MK, Morrison J, Platte R, Easton DF (2009) Risk of estrogen receptor-positive and -negative breast cancer and single-nucleotide polymorphism 2q35-rs13387042. J Natl Cancer Inst 101: 1012–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute and Information Management Services (2009) DevCan: Probability of Developing or Dying of Cancer Software. [6.4.1]. National Cancer Institute: Bethesda, MD. http://srab.cancer.gov/devcan [Google Scholar]
- Paci E, Warwick J, Falini P, Duffy SW (2004) Overdiagnosis in screening: is the increase in breast cancer incidence rates a cause for concern? J Med Screen 11: 23–27 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108 [DOI] [PubMed] [Google Scholar]
- Pashayan N, Duffy SW, Pharoah P, Greenberg D, Donovan J, Martin RM, Hamdy F, Neal DE (2009) Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer 100: 1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharoah PD, Antoniou A, Bobrow M, Zimmern RL, Easton DF, Ponder BA (2002) Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet 31: 33–36 [DOI] [PubMed] [Google Scholar]
- Pharoah PD, Antoniou AC, Easton DF, Ponder BA (2008) Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med 358: 2796–2803 [DOI] [PubMed] [Google Scholar]
- Salinas CA, Koopmeiners JS, Kwon EM, FitzGerald L, Lin DW, Ostrander EA, Feng Z, Stanford JL (2009) Clinical utility of five genetic variants for predicting prostate cancer risk and mortality. Prostate 69: 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der KT, Blijenberg BG, Moss SM, De Koning HJ, Auvinen A (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360: 1320–1328 [DOI] [PubMed] [Google Scholar]
- Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, Masson G, Jakobsdottir M, Thorlacius S, Helgason A, Aben KK, Strobbe LJ, bers-Akkers MT, Swinkels DW, Henderson BE, Kolonel LN, Le ML, Millastre E, Andres R, Godino J, Garcia-Prats MD, Polo E, Tres A, Mouy M, Saemundsdottir J, Backman VM, Gudmundsson L, Kristjansson K, Bergthorsson JT, Kostic J, Frigge ML, Geller F, Gudbjartsson D, Sigurdsson H, Jonsdottir T, Hrafnkelsson J, Johannsson J, Sveinsson T, Myrdal G, Grimsson HN, Jonsson T, von HS, Werelius B, Margolin S, Lindblom A, Mayordomo JI, Haiman CA, Kiemeney LA, Johannsson OT, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K (2007) Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 39: 865–869 [DOI] [PubMed] [Google Scholar]
- Stacey SN, Manolescu A, Sulem P, Thorlacius S, Gudjonsson SA, Jonsson GF, Jakobsdottir M, Bergthorsson JT, Gudmundsson J, Aben KK, Strobbe LJ, Swinkels DW, van Engelenburg KC, Henderson BE, Kolonel LN, Le ML, Millastre E, Andres R, Saez B, Lambea J, Godino J, Polo E, Tres A, Picelli S, Rantala J, Margolin S, Jonsson T, Sigurdsson H, Jonsdottir T, Hrafnkelsson J, Johannsson J, Sveinsson T, Myrdal G, Grimsson HN, Sveinsdottir SG, Alexiusdottir K, Saemundsdottir J, Sigurdsson A, Kostic J, Gudmundsson L, Kristjansson K, Masson G, Fackenthal JD, Adebamowo C, Ogundiran T, Olopade OI, Haiman CA, Lindblom A, Mayordomo JI, Kiemeney LA, Gulcher JR, Rafnar T, Thorsteinsdottir U, Johannsson OT, Kong A, Stefansson K (2008) Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 40: 703–706 [DOI] [PubMed] [Google Scholar]
- Sun J, Zheng SL, Wiklund F, Isaacs SD, Purcell LD, Gao Z, Hsu FC, Kim ST, Liu W, Zhu Y, Stattin P, Adami HO, Wiley KE, Dimitrov L, Sun J, Li T, Turner AR, Adams TS, Adolfsson J, Johansson JE, Lowey J, Trock BJ, Partin AW, Walsh PC, Trent JM, Duggan D, Carpten J, Chang BL, Gronberg H, Isaacs WB, Xu J (2008) Evidence for two independent prostate cancer risk-associated loci in the HNF1B gene at 17q12. Nat Genet 40: 1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Jacobs KB, Kraft P, Yeager M, Wacholder S, Cox DG, Hankinson SE, Hutchinson A, Wang Z, Yu K, Chatterjee N, Garcia-Closas M, Gonzalez-Bosquet J, Prokunina-Olsson L, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver R, Prentice R, Jackson R, Kooperberg C, Chlebowski R, Lissowska J, Peplonska B, Brinton LA, Sigurdson A, Doody M, Bhatti P, Alexander BH, Buring J, Lee IM, Vatten LJ, Hveem K, Kumle M, Hayes RB, Tucker M, Gerhard DS, Fraumeni Jr JF, Hoover RN, Chanock SJ, Hunter DJ (2009) A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat Genet 41: 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni Jr JF, Hoover R, Hayes RB, Hunter DJ, Chanock SJ (2008) Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet 40: 310–315 [DOI] [PubMed] [Google Scholar]
- Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, Seal S, Ghoussaini M, Hines S, Healey CS, Hughes D, Warren-Perry M, Tapper W, Eccles D, Evans DG, Hooning M, Schutte M, van den OA, Houlston R, Ross G, Langford C, Pharoah PD, Stratton MR, Dunning AM, Rahman N, Easton DF (2010) Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42: 504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udler MS, Meyer KB, Pooley KA, Karlins E, Struewing JP, Zhang J, Doody DR, MacArthur S, Tyrer J, Pharoah PD, Luben R, Bernstein L, Kolonel LN, Henderson BE, Le ML, Ursin G, Press MF, Brennan P, Sangrajrang S, Gaborieau V, Odefrey F, Shen CY, Wu PE, Wang HC, Kang D, Yoo KY, Noh DY, Ahn SH, Ponder BA, Haiman CA, Malone KE, Dunning AM, Ostrander EA, Easton DF (2009) FGFR2 variants and breast cancer risk: fine-scale mapping using African American studies and analysis of chromatin conformation. Hum Mol Genet 18: 1692–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson HS, Diver WR, Thun MJ, Cox DG, Hankinson SE, Kraft P, Rosner B, Berg CD, Brinton LA, Lissowska J, Sherman ME, Chlebowski R, Kooperberg C, Jackson RD, Buckman DW, Hui P, Pfeiffer R, Jacobs KB, Thomas GD, Hoover RN, Gail MH, Chanock SJ, Hunter DJ (2010) Performance of common genetic variants in breast-cancer risk models. N Engl J Med 362: 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Isaacs SD, Sun J, Li G, Wiley KE, Zhu Y, Hsu FC, Wiklund F, Turner AR, Adams TS, Liu W, Trock BJ, Partin AW, Chang B, Walsh PC, Gronberg H, Isaacs W, Zheng S (2008) Association of prostate cancer risk variants with clinicopathologic characteristics of the disease. Clin Cancer Res 14: 5819–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni Jr JF, Hoover R, Hunter DJ, Chanock SJ, Thomas G (2007) Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 39: 645–649 [DOI] [PubMed] [Google Scholar]
- Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Balter K, Kader AK, Turner AR, Liu W, Bleecker ER, Meyers DA, Duggan D, Carpten JD, Chang BL, Isaacs WB, Xu J, Gronberg H (2008) Cumulative association of five genetic variants with prostate cancer 1. N Engl J Med 358: 910–919 [DOI] [PubMed] [Google Scholar]
- Zheng W, Long J, Gao YT, Li C, Zheng Y, Xiang YB, Wen W, Levy S, Deming SL, Haines JL, Gu K, Fair AM, Cai Q, Lu W, Shu XO (2009) Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet 41: 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]