Abstract
Introduction
We sought to examine the association between livelihood security and adherence to antiretroviral therapy (ARVs) in low- and middle-income countries (LIMC).
Methods
Performing a systematic review, we searched, independently and in duplicate, 7 electronic databases and 2 conference websites for quantitative surveys that examined the association between indicators of livelihood security and adherence to ARVs in LIMC between 2000–2010. Criteria for relevance were applied to complete papers (quantitative study with estimates of associations) and quality assessment was conducted on those deemed relevant. We performed three regressions to measure the association between each type of livelihood and adherence.
Results
Twenty original studies and 6 conference abstracts were included, the majority from Africa (n = 16). Seventeen studies and 3 conference abstracts were cross-sectional and 3 studies and 3 abstracts were prospective clinical cohort studies, with considerable variation in quality for studies of each design type. Among the diverse populations represented, we observed considerable variation in associations between measurements of livelihood indicators and increasingly accepted adherence measures, irrespective of study design or quality. A financial capital indicator, financial constraints/payment for ARV medication, was more commonly associated with non-adherence (3/5 studies). A human capital indicator, educational level, was most commonly associated with adherence (11/20 studies).
Discussion
Additional better quality research examining livelihood security is required to inform provision of optimal supports for adherence and mitigation of the impacts of HIV/AIDS.
Introduction
The HIV/AIDS epidemic has taken a particular toll on low- and middle-income countries (LIMC), with sub-Saharan Africa heavily affected by both disease and poverty. Among the many challenges faced by clinicians and AIDS organizations are maintaining health in the face of poverty that may preclude access to food and medication adherence [1]. Although several development initiatives have been established by different AIDS organizations, such as micro-finance and support groups, little is understood about the impact of livelihood security and its eventual impact on long-term patient status, including mortality.
Livelihood is closely linked to socio-economic status (SES), a term often used to reflect an individual's access to resources such as food, potable water, health facilities, educational opportunities, and housing [1], [2]. Assets include the types of capital that can be used directly or indirectly to generate livelihoods and reflect natural (e.g., land, water), physical (e.g., infrastructure, roads), financial (e.g., money, savings, income), human (e.g., knowledge, education, ability to work), and social (e.g., networks, kin, membership in a group) forms [3]. A livelihood approach, as a framework, explores how individuals, households, or communities behave under specific conditions, analyzing their ability to cope and adapt in response to external shocks such as drought or civil strife [4], [5].
In the context of HIV/AIDS, there has been growing recognition that the various aspects of livelihoods that increase risk of illness and death need to be identified [1]. Limited livelihood security can lead to engaging in risky behaviours that increase HIV incidence [5]. Among those receiving ARVs, limited livelihoods can reduce adherence, create adverse gastrointestinal and other adverse events due to poor diets, and lead to disrupted medication supplies [1], [5]–[7].
Highly active antiretroviral therapy (HAART) provides the hope that people living with HIV/AIDS (PLWHA) can now live longer [8], [9] and more productive lives. Nevertheless, as of 2008, only 42% of clinically eligible individuals in LIMC were receiving treatment [10], despite the fact that treatment has been recognized as an essential tool for mitigating the impacts of HIV on affected communities [11]. Treatment efficacy with ARVs relies on sustained adherence, critical for viral suppression and the prevention of resistance, disease progression, and death [12], [13]. Unfortunately, adherence remains a challenge for many [14]–[16], given obstacles such as dosing schedules, dietary requirements, and adverse effects [15], [17].
Since the rapid scale-up of ARVs in resource-limited settings, numerous studies have focused on treatment adherence [14], [15], [18]. In 2006, we previously reviewed facilitators and barriers to adherence in developed and developing nations, some of which were livelihood-related (e.g., cost, available social support). However, our review was limited in its ability to directly measure the associations between identified factors and adherence levels [15]. There remain important gaps in our understanding of the relationship between livelihood security and adherence to ARVs, specifically in the context of treatment sustenance. The objective of our review is to evaluate the adherence literature specifically focused on the role of livelihood security on adherence to ARVs in LMIC.
Methods
Inclusion Criteria
We aimed to include all observational studies that examined the association between financial, human, and social capital, as important indicators of livelihood security, and adherence to ARVs in LMIC settings.
Ethics
Ethical approval was not sought for this systematic review as only published data was included. Furthermore, no personal identifiers from patients described in included studies were included. Therefore, written consent from such patients was neither sought nor needed.
Search Strategy
We searched the following databases: AMED (inception to January 2010), Campbell Collaboration (inception to January 2010), CinAhl (inception to January 2010), CAB Abstracts (inception to January 2010), Cochrane Library (inception to January 2010), Embase (inception to January 2010), and PubMed via Medline (inception to January 2010). Conference abstracts from the International AIDS Society conferences (inception to 2009) and Conferences on Retroviruses and Opportunistic Infections (inception to 2009) were also sought.
Our search strategies combined terms that represented livelihood security and HIV. An initial scan of the literature noted that the majority of potentially relevant studies focused on financial, human, and/or social forms of capital. While the role of natural and physical capital was referred to in the qualitative literature, their association with adherence were infrequently estimated. Therefore, in the present study, we focused solely on financial, human, and social types of capital. Using the UK Department of International Development (DFID) Sustainable Livelihood Framework as a guideline [19], financial capital in the present study denotes access to financial resources; human capital encompasses skills, knowledge, the ability to work, and nutritional factors; and social capital refers to formal and informal social relationships.
As we were interested in the interaction between adherence and antiretroviral therapy, HIV and livelihoods, our search strategy combined terms representing “HIV OR AIDS” AND “adherence to antiretroviral therapy” AND “financial capital OR human capital OR social capital”. We supplemented this search by reviewing the bibliographies of key papers. As the PRISMA Guidelines for Meta-Analyses and MOOSE guidelines for Systematic Reviews of Observational Studies [20] suggest that observational studies are often not indexed well, we did not limit our search by study design.
Study selection
BR and DCC independently reviewed the abstracts. Initially, eligible studies met the following criteria: (1) reported an original research study; (2) measured adherence to antiretroviral therapy; (3) contained content addressing the association between social, human, or financial capital and adherence to antiretroviral therapy; and (4) was set in a low-income or middle-income country as defined by the World Bank Country Classification [21]. The relevant qualitative studies, though useful with discussions on the contribution of livelihood factors, could not contribute information on the estimate of the association between the livelihood measures of interest and adherence, and, as a result, were excluded.
Quality Assessment
We extracted data on the quality of included studies using criteria consolidated from existing critical appraisal sources [14], [22], [23]. As many studies were clinical case series, with populations of patients being asked additional questions during regular visits, some criteria relevant for more traditional population surveys were not helpful for our assessment (e.g., representativeness of population, use of random selection). For longitudinal studies we added criteria with respect to follow-up: 1) the proportion followed at each stage of the study was described-(e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed) and 2) participants reasons for non-participation at each follow-up were presented [23]–[25]. Conference abstracts usually did not contain sufficient information upon which to conduct quality assessments. Given the limited number of available studies and our interest in exploring the association of livelihood and adherence in a range of LMIC, we chose not to exclude any study based on quality.
Data Abstraction
When the full-text of an abstract was not available or when information was not available in the full-text paper, we contacted the study authors for additional information. BR initially appraised quality and content and abstracted relevant data. DCC acted as a secondary reviewer. When disagreement occurred we reached consensus through discussion. The reviewers discussed the studies including characterization of different livelihood measures reported, the strengths of different adherence measures, and the patterns of findings encountered. In addition to descriptive material, we abstracted data on prevalence e.g. of other livelihood factors associated with adherence, and the types and magnitudes of associations e.g. of education with adherence, reported in each study.
Quantitative Data Synthesis
Studies were organized and sorted by year of publication, by study design, by sample size, by response rate, and by the livelihood measures examined. The prevalence of various livelihood measures were determined and the proportion of participants reporting each livelihood factor was also captured from individual studies. Patterns across studies were then examined with respect to the estimates of the given associations as well as the precision around these estimates. While few studies consistently measured the same independent (i.e. livelihood) factors and dependent variable (i.e. adherence) we chose to run three separate meta-regressions for each type of livelihood measure (financial, human, social) as a predictor to determine if there were individual effects on adherence levels. Analyses were performed in STATA. A p-value of less than 0.05 was considered statistically significant.
Results
Study Selection and Characteristics
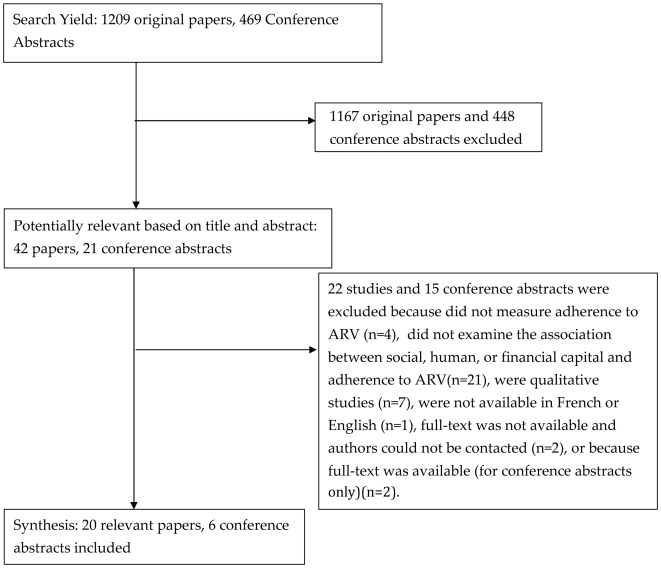
The initial literature search produced 1209 papers and 469 conference titles and abstracts. There was near perfect agreement between BR and DCC on choosing the potentially relevant 42 papers and 21 abstracts from this larger set. Of these, 20 papers [26]–[45] and 6 conference abstracts [46]–[51] were judged relevant for our review (See Figure 1). There was perfect agreement on the final papers and abstracts selected (kappa = 1). All were published in English. The majority of included papers initially were identified in PubMed via Medline (n = 15, 75%) [26]–[30], [32], [33], [35], [37]–[42], [45]. Of the remaining 5 papers, 4 were from Embase [31], [34], [36], [43] and one was identified from the CAB Abstracts database [44]. All included abstracts were identified through the International AIDS Society conference abstract database.
Figure 1. Flow Chart of Studies Included in Review.
All included papers and abstracts employed a quantitative methodology and used structured questionnaires (n = 8) [26], [35], [37], [42], [45], [48], [50], [51] or structured interviews (n = 18) [27]–[34], [36], [38]–[41], [43], [44], [46], [47]. Seventeen of the papers [26]–[42] and 3 of the conference abstracts [46]–[48] were cross-sectional studies and 3 papers [43]–[45] and 3 abstracts [49]–[51] were longitudinal studies, following up patients over time. Almost all studies (n = 19) used logistic regression analysis to measure the association between livelihoods and adherence. One study [43] used Cox's proportional hazards to assess the relative hazard of non-adherence. Although detailed information on the nature of statistical analysis was poorly described, all abstracts reported conducting multivariable analysis.
Quality Assessment
Tables 1 and 2 display the quality criteria results. There was no improvement in quality over time and no studies reported contacting non-responders. The proportion of studies meeting our quality criteria ranged from 0–100%.
Table 1. Quality Criteria for included cross-sectional studies [n = 17, (26–42)].
| Describe setting, time period | Include eligibility criteria, sources, selection | Include sample size calculations | Include and describe response rates | Describe variables outcomes, exposures, covariates | Describe data sources, measures | Survey pre-tested | Survey tool tested for validity | Describe consent process, ethics approval | Train interviewer | Translate survey tool | Describe analysis | Include participant descriptions | Include events or outcome measures | Main results: include un-adjusted and adjusted results | Main results- describe adjustment | |
| Aboubacrine/2007 | √ | √ | √ | √ | √ | √ | √ | |||||||||
| Boyer/2009 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Byakika-Tusiime/2005 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Carlucci/2008 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Iliyasu/2005 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Malangu/2008 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||
| Nachega/2004 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Nemes/2007 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Pinheiro/2002 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Ramadhani/2007 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Sarna/2008 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Silva/2009 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||
| Stout/2004 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Uzochukwu/2009 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Wang/2007 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Weiser/2003 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Williams/2007 | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| TOTAL/17 | 17 | 16 | 8 | 13 | 17 | 17 | 5 | 10 | 15 | 9 | 8 | 13 | 16 | 14 | 9 | 8 |
Table 2. Quality Criteria for included longitudinal studies [n = 3, (43–45)].
| Describe setting, time periods | Describe eligibility criteria, sources, methods of selection, methods of follow-up | Include sample size calculations | Include and describe response rate | Describe variables-outcomes, exposures, covariates | Describe data sources, survey tool | Survey pre-tested | Survey tested for validity | Describe consent process, ethics approval | Train interviewer | Translate survey tool | Describe analysis | Describe proportion followed at each stage | Include reasons for drop out at each follow-up | Include participant description | Include events or outcome measure | Main results-Include un-adjusted and adjusted results | Main results-describe adjusted | |
| Bonolo/2005 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| Erah/2008 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||
| Orrell/2003 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| Total/3 | 3 | 3 | 1 | 2 | 3 | 3 | 2 | 1 | 3 | 0 | 0 | 2 | 1 | 1 | 3 | 3 | 2 | 2 |
All cross-sectional studies adequately described the setting, variables included, and data measurement sources, and provided descriptive characteristics of included participants. Ten studies (58.9%) noted use of a previously validated survey [28]–[32], [34]–[38], [41], but few studies reported pre-testing their survey instrument (n = 5, 29.4%) [30], [31], [35], [38], [41]. Nine (52.9%) studies reported both unadjusted and adjusted estimates [27], [28], [33]–[38], [40] and eight (47.1%) justified the inclusion of any covariates [27], [28], [33], [34], [36]–[38], [40] (no studies reported stratification so we only assessed the use of adjustment).
All longitudinal studies described the study setting, the populations sampled, the variables and data measurement sources used, provided information on informed consent, provided descriptive characteristics of participants, and provided data on outcome events and summary measures. One study used a previously validated questionnaire [44] yet none of the two studies conducting interviews provided details on whether the interviewer had been trained. Only one study provided detail on the follow-up of participants and the reasons for losses-to-follow-up [45]. Two studies (66.7%) reported unadjusted and adjusted estimates and also justified their inclusion of covariates [43], [45].
Settings and Populations (Tables 3 and 4)
Table 3. Characteristics of included cross-sectional papers [n = 17, (26–42)] and conference abstracts [n = 3, (46–48)].
| Author | Yr | Country | N | Setting | Female (%) | Median Age, y | Response Rate | Assessor | Adherence, % threshold for measurement | Adherence Proportion (%) |
| Aboubacrine | 2007 | Mali, Burkina Faso | 270 | Public hospital, community based clinics | 65 | 36–40 (median range) | 100 | patient | less than 100%, no. doses missed in past 7 days | 58.5 |
| Boyer | 2009 | Cameroon | 532 | Public hospital | 70.9 | mean (SD): 38 (9) | 83.9 | patient | high, moderate, low score in past 4 days | 56.6 |
| Byakika-Tusiime | 2005 | Uganda | 304 | ARV delivery centres | 53 | 39 | unknown | patient | ≥95%, No. doses taken/no.prescribed in last 3 days | 68 |
| Carlucci | 2008 | Zambia | 409 | Rural mission hospital | 63 | 39 (32–47) | 78.2 | patient | ≥95%, No. doses taken/no. doses prescribed for total time | 83.7 |
| Iliyasu | 2005 | Nigeria | 263 | Teaching hospital | 34 | 36.2 (3.3) | 94 | patient | ≥95%, based on previous 7 days | 54 |
| Malangu | 2008 | South Africa | 180 | Hospital | 68.8 | 36.7 (8.1) | 63.2 | patient | ≥95%, No. doses taken/no. doses in past 7 days | 57.2 |
| Nachega | 2004 | South Africa | 66 | public outpatient clinic | 77 | 36.1 (10.1) | Unknown | patient | ≥95%, No. doses taken/no. doses in past 30 days | 88 |
| Nemes | 2004 | Brazil | 1972 | Health service sites | 38 | 39.3 | 97 | patient | ≥95%, No. doses taken/no. doses in past 3 days | 75.1 |
| Pinheiro | 2002 | Brazil | 195 | Publicly funded specialist clinic | 39 | 35 (17–67) | 84 | patient | ≥95%, No. doses taken/no. doses prescribed in past 2 days | 56.9 |
| Ramadhani | 2007 | Tanzania | 150 | Infectious disease clinic | 63 | 41 (19–69) | Unknown | patient | 100, >2 days without dose | 84 |
| Sarna | 2008 | India | 310 | Public and private outpatient clinic | 16 | 36 (23–70) | 96 | patient | ≥90, no. Doses taken/no. prescribed in past 4 days | 84 |
| Silva | 2009 | Brazil | 412 | Clinics at referral hospital | 21.8 | 36 (17–67) | Unknown | patient | ≥90, no. Doses taken/no. prescribed in past 5 days | 74.3 |
| Stout | 2004 | Costa Rica | 88 | Social security hospital | 15 | 38.2 (18–79) | 87 | patient | 100, no. Doses taken/no. prescribed past 3 days | 85 |
| Uzochukwu | 2009 | Nigeria | 174 | Teaching hospital | 37.5 | 34.6 (7.2) | 95.6 | patient | 100, miss at least 1 dose in past 30 days | 25 |
| Wang | 2007 | China | 181 | Clinic | 59.7 | 47.8 (11.3) | 100 | patient | ≥95, no. Doses taken/no. Doses prescribed past 3 days | 81.8 |
| Weiser | 2003 | Botswana | 109 | Private clinic | 50 | Not available | 97.3 | patient or clinician | ≥95, previous year of missing < 1 dose in 10day period or 1 dose/week | 54 |
| Williams | 2007 | West Indies | 96 | Clinic | 54.2 | 35.6 | 95 | patient or provider | ≥95, no. Doses taken/no. prescribed in past 7 days | 87.7-patient, 87.0-provider |
| Abstracts | ||||||||||
| Dahab | 2006 | South Africa | 69 | Workplace ART programme | 1% | 43.1 | Unknown | patient | <1 log drop in viral load at 6 weeks after treatment start | 86 |
| Shah | 2006 | India | 279 | Private clinic | 27 | Unknown | patient | >95, doses missed in past 4 days | 73 | |
| Warley | 2006 | Brazil | 71 | clinic | 58 | 37.9 | Unknown | patient | >95, doses missed in past 4 days | 70.4 |
| Summary (median, range) | 51.5 (1–77) | 37.9 (34.6–47.8) | 95 (63.2–100) | 73.6 (25–88) |
Table 4. Characteristics of included longitudinal papers [n = 3, (43–45)] and conference abstracts [n = 3, (49–51)].
| Author | Year | Country | N | Setting | Female (%) | Median Age, y | Response Rate | Follow-up (FU) | Assessor | Adherence, % threshold for measurement | Adherence Proportion (%) |
| Bonolo | 2005 | Brazil | 306 | Public referral hospital | 35 | 35 | 73.4% | Median overall FU time: 247 days | patient | ≥95%, number doses taken in past 3 days | cumulative: 36.9% |
| Erah | 2008 | Nigeria | 102 | HIV treatment centre | 64 | mean: 36.3 (7.9) | 81.6 | Unknown | patient | ≥95%, number doses taken in past 30 d | 58.1 |
| Orrell | 2003 | South Africa | 289 | University HIV clinic | 43 | 33.4 (8.7) | 96.2 | 87.5% after 4 wk; 83.7% after 48 wk | pharmacy refill and pill count | ≥90, medication dispensed minus pills returned/no. Pills prescribed over 48 weeks | 63 |
| Abstracts | |||||||||||
| Abah | 2006 | Nigeria | 130 | Teaching hospital | N/A | N/A | N/A | N/A | patient and pharmacy | ≥95, % of doses prescribed over 6 month period | 85.1 |
| Darder | 2004 | South Africa | 192 | Clinic | N/A | N/A | N/A | N/A | patient | ≥95, % of doses | 88 |
| Sidle | 2007 | Kenya | 7381 | clinics | patient | 100 | 77 | ||||
| Summary (median, range) | 43 (35–64) | 35 (33.4–36.3) | 81.6 (73–96.2) |
N/A: Not Available.
Sixteen studies (13 papers, 3 abstracts) were conducted in Africa [26]–[32], [35], [39], [41], [44], [45], [46], [49]–[51]. Seven studies (6 papers, 1 abstract) were conducted in Central and South America [33], [34], [37], [38], [42], [43], [48], with the majority in Brazil [33], [34], [37], [43], [48]. Two studies (1 paper, 1 abstract) [36], [47] were conducted in India and 1 study in China [40]. Since the most recent systematic review which examined factors affecting adherence [15], eleven cross-sectional (64.7%) [26], [27], [29], [31], [35]–[37], [39], [40], [42] and 2 longitudinal studies (66.7%) have been published [44], [51].
Included studies reflect a diverse range of settings and study populations. Studies were conducted primarily in public, teaching, or referral hospitals (n = 9), public outpatient or community-based clinics (n = 10), and specialist/HIV clinics or treatment centres (n = 6), although private clinics (n = 3) and a workplace ARV programme (n = 1) were also reported. The proportion of women included in cross-sectional studies ranged from 1–77% (median 51.5%) and between 35–64% (median 43%) in longitudinal studies. The median age was 37.9 and 35 for participants in cross-sectional and longitudinal studies respectively. The response rate was unknown for 4 cross-sectional papers [28], [32], [35], [37] and all conference abstracts.
Adherence Threshold Measurements
Twenty-two studies (84.6%) assessed adherence using patient-reported adherence levels over a specified period. One study used pharmacy claims and three used a combination of patient and clinician/provider assessment. Twelve papers [28]–[34], [40]–[42], [43], [44] and 4 abstracts [47]–[50] defined adherence as greater or equal than 95% during the measurement period, which ranged from 2 days to 6 months. Five studies [26], [35], [38], [39], [51] defined adherence as being 100% during the measurement period. Three studies [36], [37], [45] assessed adherence as greater than 90% over the measurement period. Median adherence proportions in cross-sectional studies were 74.3% (Range 25–88%) for papers alone and 73.6% when the three conference abstracts were added. While the median adherence levels among the 3 longitudinal studies were 58.1%, it increased to 70% when the three conference abstracts were added. Overall range differed little from the cross-sectional studies (36.9–88%).
Financial, Human, and Social Capital Factors Affecting Adherence: (Tables 5– 9)
Table 5. Financial, Human, Social Capital factors associated* with adherence or non-adherence to antiretroviral therapy in included cross-sectional papers [n = 7, (26–32)].
| Financial Capital | Human Capital | Social Capital | Other | ||||||||
| Author/Year | Financial constraints/ARV payment | Household Income | Distance to clinic/transport costs | Education | Employment Status | Food-related restrictions | Marital Status | Household size | Social support | Fear of Stigma/non-disclosure | Reasons for missing doses |
| Aboubacrine/2007 | work with no stable salary vs. no work associated with adherence: OR: 3.15 (1.15–11.13) | Having children vs. not associated with adherence OR: 2.36 (1.08–5.15) | |||||||||
| Boyer/2005 | Difficulty buying ARV and reporting high adherence: OR: 0.24 (0.15–0.4) | ||||||||||
| Byakika-Tusiime/2005 | Monthly < $US 50 associated with non-adherence 0R: 2.42 (1.24–4.0), AOR: 2.77 (1.64–4.67) | Education level attained, ns | ns | being single associated with non-adherence: OR: 1.19 (0.73–1.95) AOR: 2.93 (1.32–6.5) | ns | lack of money (72.4%), away from home (11.2%) | |||||
| Carlucci/2008 | Travel duration: ns Transport cost: ns | Stigma vs. none, ns | |||||||||
| Iliyasu/2005 | formal vs. no formal education associated with adherence: OR: 3.97 (1.75–9.24) | lack of funds (15.8%) | |||||||||
| Malangu/2008 | Having a tertiary education vs. other, ns | Being employed vs. other, ns | Eating well associated with adherence (p = 0.03) | Away from home (15.6%) | |||||||
| Nachega/2004 | Ns | Ns | Being employed vs. unemployed: ns | Fear of stigma from partner vs. no associated with adherence OR: 0.13 (0.02–0.70) | being away (30%), stigma (75%) | ||||||
*OR: Odds Ratio, AOR: Adjusted Odds Ratio, (n1–n2): 95% Confidence Intervals, sig: significant, ns: not significant.
Table 6. Financial, Human, Social Capital factors associated* with adherence or non-adherence to antiretroviral therapy in included cross-sectional papers [n = 4, (33–36)].
| Financial Capital | Human Capital | Social Capital | |||||||||
| Author/Year | Financial constraints/ARV payment | Household Income | Distance to clinic/Transport costs | Education | Employment Status | Food-related restrictions | Marital Status | Household size | Social support | Fear of Stigma/non-disclosure | Reasons for missing doses |
| Nemes/2004 | 0–2 years schooling associated with non-adherence OR: 1.51 (1.12–2.02), AOR: 1.48 (1.16–1.89) | ||||||||||
| Pinheiro/2002 | Monthly income, ns | ≥8 years of schooling vs. 0–4 associated with adherence AOR: 2.26 (1.02–5.02) | |||||||||
| Ramadhani/2007 | Paying for treatment associated with non-adherence OR: 4.9 (1.92–25.9), AOR: 23.5 (1.2–444.4) Sacrifice health for other needs OR: 20.7 (3.9–110.3), AOR: 19.8 (3.1–127.8) | Walking time to the clinic associated with non-adherence OR: 1.2 (1–1.5) | Disclosure of HIV associated with non-adherence OR: 0.23 (0.05–1.1), AOR: 0.16 (0.02–1.1) | ||||||||
| Sarna/2008 | free ARV vs. paid out-of- pocket associated with non-adherence: 5.71 (2.94–11.10), AOR: 4.05 (1.42–11.54) | < 5 years education vs. university associated with non-adherence: OR: 4.28 (1.49–12.33), 6–12 years, OR: 2.83 (1.29–6.19) | Unemployed vs. employed associated with non-adherence: OR: 2.35 (1.22–4.88). AOR: ns | travel, financial difficulties | |||||||
*OR: Odds Ratio, AOR: Adjusted Odds Ratio, (n1–n2): 95% Confidence Intervals, sig: significant, ns: not significant.
Table 7. Financial, Human, Social Capital factors associated* with adherence or non-adherence to antiretroviral therapy in included cross-sectional papers [n = 6, (37–42)].
| Financial Capital | Human Capital | Social Capital | |||||||||
| Author/Year | Financial constraints/ARV payment | Household Income | Distance to clinic/Transport costs | Education | Employment Status | Food-related restrictions | Marital Status | Household size | Social support | Fear of Stigma/non-disclosure | Reasons for missing doses |
| Silva/2009 | Higher income associated with adherence: p = 0.08, AOR: 2.33 (1.17–4.66) | 8 years of schooling vs. 11 years, ns | |||||||||
| Stout/2004 | Difficulty finding transport vs. other associated with non-adherence OR: 6.3, (1.5–26.9) | Difficulty taking meds on empty stomach vs. other OR: 6.7 (1.3-35.7) | travel away from home: 17% | ||||||||
| Uzochukwu/2009 | Ns | Living 20+Km associate with adherence, p = 0.038 | formal education associated with non-adherence (p = 0.0394) | being single associated with non-adherence p = 0.02 | Cost and transport (30.1%), sold drugs because need money (28.2%) | ||||||
| Wang/2007 | HIV knowledge associated with adherence OR: 5.59, (2.48–12.57), AOR: 3.20, (1.24–8.26) | Support as reminder tool associated with adherence: OR: 4.22, (1.90–9.39), AOR: 3.49, (1.36–8.96) | Community/social activities (16.3), food restrictions (11.2%), stigma (14.3%) | ||||||||
| Weiser/2003 | Cost as a barrier to treatment associated with adherence: OR: 0.15 (0.06–0.35), AOR: 0.11 (0.04–0.30) | Incomplete secondary ed compared to complete associated with adherence OR: 3.87 (1.21–12.40) | ns | Disclosure to others associated with adherence OR: 3.55 (0.91–13.92) | Financial difficulties (48%), travelling (12%), distance to clinic (5%), stigma (3%) | ||||||
| Williams/2007 | positive, ns | positive, ns | No food, social problems | ||||||||
*OR: Odds Ratio, AOR: Adjusted Odds Ratio, (n1–n2): 95% Confidence Intervals, sig: significant, ns: not significant.
Table 8. Financial, Human, Social Capital factors associated* with adherence or non-adherence to antiretroviral therapy in included cross-sectional abstracts [n = 3, (46–48)] and summary of associations in all cross-sectional studies [n = 20, (26–42, 46–48)].
| Financial Capital | Human Capital | Social Capital | ||||||||
| Author/Year | Financial constraints/ARV payment | Household Income | Distance to clinic/Transport costs | Education | Employment Status | Food-related restrictions | Marital Status | Household Size | Social Support | Fear of stigma/non-disclosure |
| Dahab/2006 | Educated vs. other associated with adherence OR: 2.4 (1.2–4.7) | |||||||||
| Shah/2006 | Positive association, sig. | Fear of stigma, Negative association, sig. | ||||||||
| Warley/2006 | ns | ns | ns | |||||||
| SUMMARY of associations with adherence across all cross-sectional studies (n = 20) | n = 4 Positive: 1 Negative: 3 | n = 5 Positive: 2 No assoc: 3 | n = 4 Positive:1 Negative:2 No assoc: 1 | n = 15 Positive: 7 Negative: 2 No Assoc: 6 | n = 7 Positive: 2 No Assoc: 5 | n = 2 Negative:2 | n = 3 Negative (single): 2 No assoc: 1 | n = 1 Positive: 1 | n = 3 positive: 1 No assoc: 2 | n = 5 No Assoc: 1 Positive (disclosure):3 Negative (stigma):1 |
*OR: Odds Ratio, AOR: Adjusted Odds Ratio, (n1–n2): 95% Confidence Intervals, sig: significant, ns: not significant.
Table 9. Financial, Human, and Social capital associated* with adherence or non-adherence to antiretroviral therapy in included longitudinal studies and summary of all associations [n = 6, (43–45, 49–51)].
| Financial Capital | Human Capital | Social Capital | Other | |||||||
| Author/Year | Financial constraints/payment of ARV | Household Income | Distance from Clinic | Education | Employment Status | Food-Related Restrictions | Marital status | Household size | Social support | Reasons for missing doses: |
| Bonolo/2005 | Individual ≤US$80 vs. greater, associated with non-adherence RH: 1.61 (1.08–2.39) | ≤4 years school vs. > 8 associated with non-adherence RH: 1.80 (1.08–2.29) | Unemployed vs. employed associated with non-adherence: RH: 2.16 (1.20–3.91), ARH: 2.17 (1.19–3.96) | Does not participate in religious activities vs. regular activity associated with non-adherence ARH: 2.27 (1.58–3.25) | ||||||
| Erah/2008 | None or primary education associated with non-adherence OR: 1.81 (1.25–2.51), AOR: 2.23 (1.02–2.89) | ns | poor financial status and inadequate family support (15.9%), occupational factors (25%) | |||||||
| Orrell/2003 | ns | ns | Restrictions associated with adherence: ns | |||||||
| Abstracts | ||||||||||
| Abah/2006 | ns | ns | ns | Married associated with adherence, p = 0.02 | lack of money (17.1%) | |||||
| Darder/2006 | Positively associated with adherence, sig. | |||||||||
| Sidle/2007 | Level of education associated non- adherence: AOR: 0.96, p = 0.0269 | More children associated adherence, AOR = 1.19, p = 0.0352 | ||||||||
| Summary of associations with adherence | n = 1, No assoc:1 | n = 2 Positive: 1 No assoc: 1 | n = 1, No assoc: 1 | n = 5 Positive: 4 No Assoc. : 1 | n = 2 Positive: 1 No assoc.: 1 | n = 1 No assoc.: 1 | n = 1 Positive (married): 1 | n = 2 Positive: 1 No Assoc.: 1 | n = 1 Positive: 1 | |
*OR: Odds Ratio, AOR: Adjusted Odds Ratio, RH: Relative Hazard, ARH: Adjusted Relative Hazard.
Financial Capital
Five studies, 4 cross-sectional [27], [35], [36], [41] and 1 longitudinal [45], measured the association between financial constraints/ability to pay for treatment and adherence. Two reported lower levels of adherence associated with increasing financial difficulties [27], [41]. One study reported that the need to sacrifice health to pay for other resources such as housing was associated with non-adherence (Odds Ratio (OR): 19.8, 95% Confidence Intervals (CI): 3.1–122.7) [35]. One study reported that non-adherence was associated with having access to free treatment (OR: 4.05, 95% CI: 1.42–11.54) [36] while another reported that the association between financial constraints and adherence was not-significant when examined over time [45].
Five cross-sectional [28], [32], [34], [37], [39] and 2 longitudinal [43], [45] studies examined the association between household income and adherence, 4 of which demonstrated a non-significant association [32], [34], [39], [45]. One study reported an increase in non-adherence associated with a monthly income of <$50 US (Adjusted OR (AOR): 2.77, 95% CI: 1.64–4.67) [28] while one cross-sectional and one longitudinal study reported that adherence was associated with an increase in household (AOR: 2.33, 95% CI: 1.17–4.66) [37] or individual income (Relative Hazard (RH): 1.61, 95% CI: 1.08–2.39) [43].
Five studies (4 cross-sectional [29], [35], [38], [39], 1 longitudinal [49]) examined how distance from the clinic and the ability to pay for transport impacted adherence. One large study showed a non-significant association [29]. One study demonstrated that living more than 20 km away was positively associated with better adherence [39], while two studies demonstrated a negative association, with non-adherence increasing with distance (OR: 1.2, 95% CI 1.0–1.5) [35] or difficulty finding transport (AOR: 6.3, 95% CI: 1.5–26.9) [38].
There was a statistically significant positive association demonstrated between overall financial livelihood and adherence proportions (exponentiated beta coefficient = 1.53, 95% CI: 1.03–2.29, p = 0.04).
Human Capital
Fifteen cross-sectional [28], [30]–[34], [36], [37], [39]–[42], [46]–[48], and five longitudinal [43], [44], [49]–[51] studies examined the association between education and adherence. One study examined HIV knowledge and reported that increasing education and knowledge about HIV was associated with adherence (AOR: 3.20, 95% CI: 1.24–8.26) [40]. Six cross-sectional [30], [33], [34], [36], [46], [47] and four longitudinal studies [43], [44], [50], [51] reported a positive association between education and adherence. Two cross-sectional studies reported a negative association: one reporting that a formal education was associated with non-adherence [39] and another reporting higher levels of adherence among individuals who had not completed secondary school compared to those who had (OR: 3.87, 1.21–12.40) [41].
Nine studies, seven cross-sectional [26], [28], [31], [32], [36], [42], [48] and two longitudinal [43], [49], examined the association with employment status. Of these, one cross-sectional [26] and one longitudinal [43] study reported a positive and significant association between employment and adherence.
Three studies, 2 cross-sectional [31], [38] and one longitudinal [45], measured the association with food-related restrictions. One study reported that adherence was positively associated with eating well [31] while another demonstrated that non-adherence was associated with not having enough food to take with medications (OR: 6.7, 95% CI: 1.3–35.7) [38].
No statistically significant association between human capital and adherence was found (exponentiated beta coefficient = 1.04, 95% CI: 0.71–1.53, p = 0.81).
Social Capital
Four studies (3 cross-sectional [28], [39], [41], 1 longitudinal [49]) examined the role of marital status: one study demonstrated that being single was positively associated with adherence (AOR: 2.93, 95% CI: 1.32–6.5) [28] while another demonstrated that being single was negatively associated with adherence [39]. Being married was positively associated with adherence in one longitudinal study [49].
One cross-sectional (OR: 2.36, 95% CI: 1.08–5.15) [26] and 1 longitudinal study (AOR: 1.19, p = 0.0352) [51] reported a positive association between household size, specifically the number of children, and adherence.
Three studies, 3 cross-sectional [28], [40], [48] and one longitudinal [43]), examined the role of social support: One study found that using support networks as reminder tools was positively associated with adherence (AOR: 3.49, 95% CI: 1.36–8.96) [40]. A longitudinal study reported that not participating in any religious activities was associated with non-adherence (Adjusted RH (ARH): 2.27, 95% CI: 1.58–3.25) [43].
Fear of stigma and disclosure of HIV status was examined in 5 cross-sectional studies [29], [32], [35], [41], [47]. Two studies reported that stigma was negatively associated with adherence to ARVs [32], [47].
Overall social livelihood and adherence were positively associated but not significant for this set of studies (exponentiated beta coefficient: 1.79, 95% CI: 0.63–5.08, p = 0.21).
Patient-report reasons for missing doses/non-adherence
Ten cross-sectional studies [28], [30]–[32], [36], [38]–[42] and two longitudinal studies [44], [50] reported additional patient-identified barriers to treatment adherence (i.e., reasons for missing doses). Reported barriers included: financial difficulties (n = 7) [28], [30], [36], [39], [41], [44], [50], being or travelling away from home (n = 7) [28], [31], [32], [36], [38], [39], [41], fear of stigma (n = 3) [32], [40], [41], the need to participate in social activities (n = 2) [40], [42], food restrictions (n = 2) [40], [42], inadequate family support (n = 1) [44] and occupational factors (n = 1) [44].
Discussion
The diversity of studies included in this review and the lack of consistency between them suggests that the literature on livelihood and HIV treatment outcomes is still in its infancy. Studies were conducted in numerous settings and the measurement tools used to assess and define both livelihood factors and adherence varied substantially. Adherence proportions ranged from 25% to 88% and were tested for associations with ten different livelihood factors related to financial, human, or social capital.
Education level was the most commonly measured livelihood factor. While almost all studies indicated that a higher level of education was associated with adherence, two studies reported a negative association [39], [41]. Higher levels of education have previously been associated with increased risky behavior and risks of HIV infection [52]. The reasons for this remain unclear. Talam et al. (2008) argue that better educated patients may be too busy with their professional activities to take their pills regularly [53]. In contrast, others have argued that greater access to information as a result of higher education, likely helps individuals to make more informed decisions about the need to remain adherent [44], [54]. Higher educated patients may also be better equipped to plan, organize, and integrate new realities into their daily lives [55]. Furthermore, education level has also been considered an important determinant of self-efficacy which previously has been positively associated with adherence to ARVs [34], [55].
Financial capital was one key factor impacting on adherence to ARVs and the only type of capital which demonstrated a significant association with adherence. The inability to afford medication was one of the most frequently reported reasons for non-adherence both in included studies [27], [28], [35], [39], [41] as well as in others [56]–[58]. While access to free ARVs was associated with non-adherence in one study [36], individuals receiving free treatment may be more likely to be highly impoverished and facing numerous obstacles (e.g., lack of food, shelter) which impact on their ability to adhere [59]. Importantly, user fees and charges for treatment are widespread in resource-poor settings [39] although it has been suggested that optimal levels of adherence can be achieved with access to subsidized ARVs [41], [45]. A 2005 meta-analysis focused on ARV programmes in resource-poor settings reported that, in fact, when medications were provided free-of-charge, there was a higher probability of achieving adherence and undetectable viral loads compared to situations where patients were required to pay for treatment [18].
Even in the context of free drugs however, the cost of transportation to obtain ARVs can still remain a barrier to adherence. Research from sub-Saharan Africa has demonstrated that patients often have to choose between using their limited income on paying for transportation to the clinic versus being able to adequately feed their families [60]. As a result, individuals may miss their scheduled clinic appointments and thus not receive their ARVs at the regular time intervals critical for optimal adherence [38], [61]. Therefore, increasing access to affordable transportation as well as expanding the number and location of ARV clinics may help to facilitate HIV treatment adherence [38], [62].
Findings related to food-related restrictions i.e. inability to take medications on an empty stomach, and greater adherence when eating well, were also associated with adherence in two studies. Food insecurity has been well-documented in Africa and has been linked with decreased adherence to ARVs and poor clinical outcomes [61], [63]–[65]. Medication-related food restrictions place an additional burden on patients, in many ways, increasing the complexity of the treatment regimen itself [62], [66]. The perception that ARVs need to be taken with food may lead to non-adherence [40], [42], [67], [68] suggesting that access to adequate food via self-production or, at the very least, food supplements, bolsters human capital, recognizing that taking pills on an empty stomach may lead to gastrointestinal upset.
Social stability and social capital have both been associated with medication adherence in various settings [69]–[73]. Importantly, social support can take the form of direct reminders, financial help, and emotional backing [71]. Qualitative research from South Africa suggests that treatment supporters (i.e., clinic buddies) are a valuable aid in promoting adherence [74]. As identified in this review, social factors such marital status and having children can impact on adherence. The desire to be alive and be able to support their families and see their children grow up may be a strong motivator for patients to adhere [39], [69], [71], [75]. However, disclosure to one's sexual partner has been recognized as a double edged sword [74]- it has the potential to yield much needed social support [35] but may also result in stigmatization, discrimination, and potentially abandonment [74], [76], [77]. This may partly explain why adherence was higher for single individuals in one study [28]. Issues of stigma and discrimination related to HIV/AIDS remain a real concern in many settings [78] and may lead to social isolation, limit sources of social capital, and undermine relationships that are essential for survival [70], [78]. Further research in this area is still needed to help elucidate the type and nature of social capital that impacts on adherence across settings.
Limitations
Limitations in our review reflect the quality and nature of included studies. While our search was extensive and we did seek clarification from various study authors, it is possible we missed unpublished studies measuring the association between relevant livelihood factors and adherence. There is no gold standard for measuring adherence -patient recall and pill count, both commonly used, have inherent biases in their use [79]–[81]. For example, there is a tendency for self-reported adherence to be positively skewed (i.e., patients overestimating adherence levels) increasing the risk of patient misclassification [79]. While reporting bias, specifically social desirability bias, may have a profound impact here, other influences may include question misinterpretation and issues of recall [82]. Additionally, the lack of methodological standards makes assessments and comparisons between levels of adherence difficult. As such there remains the need for validation of adherence monitoring tools capable of measuring real-time behaviour of patients in various settings [81]. While numerous livelihood factors were examined, few were measured using standardized or validated instruments for particular constructs, again reflecting the dearth of research conducted in this area to-date and perhaps the lack of experience among clinical investigators with use of standard measures more out of social science traditions.
Unmeasured or unidentified features of included studies may also have a large impact on either apparent or real adherence [15]. Detailed population descriptions (e.g., education level) and the regional and political conditions under which a study was conducted would assist interpretation of future studies in this field. For example, patients with access to private or non-governmental health services may have additional benefits including better access to laboratory equipment and testing [43] which may ultimately affect treatment outcomes. Furthermore, patients in care and on ARVs may differ meaningfully from patients who either lack access to ARVs or who refused treatment. The experiences of livelihood insecurity for these individuals may, in part, explain why they are not or did not remain in care, but such populations were rarely included. Finally, the majority of included studies were cross-sectional in study design, limiting the ability to establish temporal causality (i.e., livelihood to adherence) validly.
Conclusions
We found only one significant association that was consistent across settings. We demonstrated a positive association between financial capital and adherence whereas no statistically significant relationship was found for human or social capital and adherence. Importantly, the included studies reflect a range of experiences in the association between various livelihood factors and adherence to ARVs. This heterogeneity and diversity can also be considered an important strength of this review. More longitudinal studies that can effectively measure and monitor the dynamic interactions between livelihood security and adherence across settings are needed. Linked to these could be additional qualitative work able to explore the lives and treatment challenges of PLWHA [83]. Both study designs are essential for understanding adherence, the way adherence changes over time, and the reasons for non-adherence. Through their incorporation in structural policies or programs, findings from such research can contribute to improved patient outcomes [84]. Furthermore, clinicians and other health providers can actively work with their patients and help them to prioritize adherence while addressing potential obstacles to care [84], [85]. As many of these obstacles and challenges lie beyond the control of the individual patient, addressing adherence in low- and middle-income settings, therefore, may require eliminating or lowering user fees and patient costs, bringing care closer to the patients, and implementing community-based livelihood development strategies.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Drimie S, Mullins D. Gillespie, S, editors. Mainstreaming HIV and AIDS into livelihood and food security programs: The experience of CARE Malawi. 2006. pp. 283–303. AIDS, poverty, and hunger: Challenges and responses. Highlights of the International Conference on HIV/AIDS and Food and Nutrition Security, Durban South Africa, April 14–16, 2005. Washington, DC: International Food Policy Research Network.
- 2.Elasha BO, Elhassan NG, Ahmed H, Zakeildin S. Sustainable livelihood approach for assessing community resilience to climate change: case studies from Sudan. 2005. Assessments of Impacts and Adaptation to Climate Change working paper No 17. August 2005. Available: http://www.aiaccproject.org/working_papers/Working%20Papers/AIACC_WP_No 017.pdf. Accessed on 3 February, 2010.
- 3.Carney B. Sustainable Rural Livelihoods-what contributions can we make? 1998. Paper presented at the Department of International Development's Natural Resources Advisers' Conference London.
- 4.De Waal A, Whiteside A. New variant famine: AIDS and food crisis in Southern Africa. Lancet. 2003;362:1234–1237. doi: 10.1016/S0140-6736(03)14548-5. [DOI] [PubMed] [Google Scholar]
- 5.Masanjala W. The poverty-HIV/AIDS nexus in Africa: a livelihood approach. Soc Sci Med. 2007;64:1032–1041. doi: 10.1016/j.socscimed.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Seeley J. Thinking with the livelihood framework in the context of the HIV/AIDS epidemic. 2002. Research paper, Livelihoods Connect, Institute of Development Studies, University of Sussex.
- 7.Ellis F, Kutengule M, Nyasulu A. Livelihoods and rural poverty education in Malawi. World Dev. 2003;31:1495–1510. [Google Scholar]
- 8.Hogg RS, O'Shaughnessy MV, Gataric N, Yip B, Craib K, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 9.Mannheimer SB, Matts J, Telzak E, Chesney M, Child C, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care, 2005;1:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- 10.Joint United Nations Programme on HIV/AIDS. 2009. Report on the Global AIDS Pandemic. Available: http://www.unaids.org/en/KnowledgeCentre/HIVData/Global-Report/2009/. Accessed on 3 Jan 2010. [PubMed]
- 11.Gavian S, Galaty D, Kombe, K Gillespie, S, editors. Multisectoral HIV/AIDS approaches in Africa: How are they evolving? 2006. pp. 221–243. AIDS, poverty, and hunger Challenges and responses. Highlights of the International Conference on HIV/AIDS and Food and Nutrition Security, Durban South Africa, April 14–16, 2005. Washington, DC: International Food Policy Research Network.
- 12.Bangsberg DR, Perry S, Charlesbois ED, Clark RA, Robertson M, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, et al. Is there a baseline CD4 count that precludes a survival response to modern antiretroviral therapy? AIDS. 2003;17:711–720. doi: 10.1097/00002030-200303280-00009. [DOI] [PubMed] [Google Scholar]
- 14.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America. A meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 15.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. JAIDS. 2001;1:47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Loevinsohn M, Gillespie SR. HIV/AIDS, rural livelihoods and food security: understanding and responding. 2003. RENEWAL Working paper No 2. Available: www.isnar.org/renewal. Accessed on 10 Jan 2010.
- 18.Ivers L, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: A meta-analyses of the published literature. HIV/AIDS Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 19.UK Department of Development. Sustainable livelihoods guidance sheets. 2004. London, Department for International Development. Available: http://www.ennonline.net/pool/files/ife/section2.pdf. Accessed on 20 July 2009.
- 20.Stroup DF, Berlin JA, Morton CS, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA. 2000;282:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21. World Bank. Country Classification Table. Available: http://go.worldbank.org/K2CKM78CC0. Accessed on 10 Jan 2010.
- 22.Katrak P, Bialocerkowski AE, Massy-Westropp N, Kumar VSS, Grimmer KA. A systematic review of the content of critical appraisal tools. BMC Med Res Method. 2004;4:22. doi: 10.1186/1471-2288-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. STROBE Initiative. The strengthening of reporting of observational studies in epidemiology (STROBE) statement guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Public Health Resource Unit. Critical appraisal skills programme: making sense of evidence. 12 Questions to help you make sense of a cohort study. Available: http://www.phru.nhs.uk/Doc_Links/cohort%2012%20questions.pdf. Accessed on 31 January 2010.
- 26.Aboubacrine SA, Niamba P, Bioleau C, Zunzunegui MV, Machouf N, et al. Inadequate adherence to antiretroviral treatment and prevention in hospital and community sites in Burkina Faso and Mali: a study by the ATARAO group. Int J STD AIDS. 2007;18:741–7. doi: 10.1258/095646207782212243. [DOI] [PubMed] [Google Scholar]
- 27.Boyer S, Marcellin F, Ongolo-Zogo P, Abega SC, Nantchouang R, et al. Financial barriers to HIV treatment in Yaoundé, Cameroon: first results of a national cross-sectional survey. Bull World Health Organ. 2009;87:279–87. doi: 10.2471/BLT.07.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, et al. Adherence to HIV antiretroviral therapy in HIV+Ugandan patients purchasing therapy. Int J STD AIDS. 2005;16:38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- 29.Carlucci JG, Kamanga A, Sheneberger R, Shepherd BE, Jenkins CA, et al. Predictors of adherence to antiretroviral therapy in rural Zambia. JAIDS. 2008;47:615–622. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iliyasu Z, Kabir M, Abubakar IS, Babashani M, Zubair ZA. Compliance to antiretroviral therapy among AIDS patients in Amino Kano Teaching Hospital, Kano, Nigeria. Niger J Med. 2005;14:290–4. [PubMed] [Google Scholar]
- 31.Malangu NG. Self-reported adverse effects as barriers to adherence to antiretroviral therapy in HIV-infected patients in Pretoria. SA Family Practice. 2008;50:49. [Google Scholar]
- 32.Nachega JB, Stein DM, Lehman DA, Hlatshwayo D, Mothopeng R, et al. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res Human Retro. 2004;20:1053–1056. doi: 10.1089/aid.2004.20.1053. [DOI] [PubMed] [Google Scholar]
- 33.Nemes MI, Carvalho HB, Souza MF. Antiretroviral therapy adherence in Brazil. AIDS 18. 2004;(Suppl 3):S15–20. doi: 10.1097/00002030-200406003-00004. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro CAT, de-Carvalho-Leite JC, Drachler ML, Silveria VL. Factors associated with adherence to antiretroviral therapy in HIV/AIDS patients: a cross-sectional study in Southern Brazil. Br J Med Biological Research. 2002;35:1173–1181. doi: 10.1590/s0100-879x2002001000010. [DOI] [PubMed] [Google Scholar]
- 35.Ramadhani HO, Thielman NM, Landman KZ, Ndosi EM, Gao F, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45:1492–8. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 36.Sarna A, Pujari S, Sengar AK, Garg R, Gupta I, et al. Adherence to antiretroviral therapy & its determinants amongst HIV patients in India. Ind J Med Res. 2008;127:28–36. [PubMed] [Google Scholar]
- 37.Silva MCF, Ximenes RAA, Mirando Filho DB, Arraes LWMS, Mendes M, et al. Risk factors for non-adherence to antiretroviral therapy. Rev Inst Med Trop S. Paulo. 2009;51:135–139. doi: 10.1590/s0036-46652009000300003. [DOI] [PubMed] [Google Scholar]
- 38.Stout BD, Leon MP, Niccolai LM. Nonadherence to antiretroviral therapy in HIV-positive patients in Costa Rica. AIDS Patient Care STDS. 2004;18:297–304. doi: 10.1089/108729104323076034. [DOI] [PubMed] [Google Scholar]
- 39.Uzochukwu BSC, Onwujekwe OE, ONoka AC, Okoli C, Uguru NP, et al. Determinants of non-adherence to subsidized anti-retroviral treatment in southeast Nigeria. Health Policy Plan. 2009;24:189–96. doi: 10.1093/heapol/czp006. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Wu Z. Factors associated with adherence to antiretroviral therapy among HIV/AIDS patients in rural China. AIDS 21. 2007;(Suppl 8):S149–S155. doi: 10.1097/01.aids.0000304711.87164.99. [DOI] [PubMed] [Google Scholar]
- 41.Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. JAIDS. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Williams M, Clarke T, Williams P, Barton EN. The mean levels of adherence and factors contributing to non-adherence in patients on highly active antiretroviral therapy. West Indian Med J. 2007;56:270. doi: 10.1590/s0043-31442007000300016. [DOI] [PubMed] [Google Scholar]
- 43.Bonolo P, Cesar CC, Acurcio FA, Ceccato MGB, de Padua CM, et al. Non-adherence among patients initiating antiretroviral therapy: a challenge for health professionals in Brazil. AIDS. 2005;19:S5–13. doi: 10.1097/01.aids.0000191484.84661.2b. [DOI] [PubMed] [Google Scholar]
- 44.Erah PO, Arute JE. Adherence of HIV/AIDS patients to antiretroviral therapy in a tertiary health facility in Benin City. Afr J Pharmacy Pharmacology. 2008;2:145–152. [Google Scholar]
- 45.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17:1369–75. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 46.Dahab M, Charalambous S, Hamilton R, Fielding K, Tsimele J, et al. A quantitative study of barriers & facilitators to adherence in an ART programme in South Africa. 2006. AIDS 2006-XVI International AIDS Conference: Abstract no. TUPE0151.
- 47.Shah B, Walshe L, Saple DG, Mehta S, Kharkar JP, et al. Adherence to antiretroviral therapy among Indian HIV-infected persons seeking care in the private sector in Mumbai, India. 2006. AIDS 2006-XVI International AIDS Conference: Abstract no. THPE0194. [DOI] [PubMed]
- 48.Warley E, Shield D, Salas M, Vienie I, Moneti S, et al. Adherence to antiretroviral therapy in a population of the low-income suburbs of Buenos Aires, Argentina. 2006. AIDS 2006-XVI International AIDS Conference: Abstract no. CDB0805.
- 49.Abah IO, Falang K, Finangwi A, Iyaji P, Wakdet L, et al. Adherence to antiretroviral therapy in HIV-infected adults in Jos, Nigeria. 2006. AIDS 2006-XVI International AIDS Conference: Abstract no. TUPE0105.
- 50.Darder M, Michaels D, Boulle A, Ncobo N, MacLean E, et al. Determinants of short and long-term adherence to antiretroviral treatment in resource-poor settings. 2004. CD Only: The XV International AIDS Conference 2004: Abstract no. B11852.
- 51.Sidle J, Kimaiyo S, Monahan P, Nyandiko W, Wools-Kaloustian K, et al. Patterns of antiretroviral adherence among Kenyan patients and factors related to non-adherence during the first year of treatment. 2007. Poster exhibition: 4th IAS Conference no HIV Pathogenesis, Treatment, and Prevention 2007: Abstract no. WEPEB099.
- 52.Shelton JD. Ten myths and one truth about generalised HIV epidemics. Lancet. 2007;370:1809–11. doi: 10.1016/S0140-6736(07)61755-3. [DOI] [PubMed] [Google Scholar]
- 53.Talam NC, Gatongi P, Rotich J, Kimaiyo S. Factors affecting antiretroviral drug adherence among HIV/AIDS adult patients attending HIV/AIDS clinic at Moi Teaching and referral hospital, Eldoret, Kenya. East Afr J Public Health. 2008;5:74–78. [PubMed] [Google Scholar]
- 54.Nwauche CA, Erhabor O, Ejele OA, Akani C. Adherence to antiretroviral therapy among HIV-infected subjects in resource-limited setting in the Niger Delta of Nigeria. Afr J Health Sci. 2006;13:13–17. [Google Scholar]
- 55.Bandura A. Self-efficacy: the exercise of control. 1997. W.H. Freeman & Company. New York, NY, USA.
- 56.Daniel OJ. Adherence pattern to ARV drugs among patients on self-purchased drugs and those on free medications in Sagamu, Nigeria. XV International AIDS Conference, Thailand. Abstract. 2004;WePeB5768 [Google Scholar]
- 57.Laniece I, Ciss M, Desclaux A, Diop K, Mbodje F, et al. Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS 17. 2003;(Suppl 3):S103–108. doi: 10.1097/00002030-200317003-00014. [DOI] [PubMed] [Google Scholar]
- 58.Kumaraswamy N, Safren SA, Raminani SR, Pickard R, James R, et al. Barriers and facilitators to antiretroviral medication adherence among patients living with HIV in Chennai, India; A qualitative study AIDS Patient Care STDS. 2005;19:526–37. doi: 10.1089/apc.2005.19.526. [DOI] [PubMed] [Google Scholar]
- 59.Moss AR, Hahn JA, Perry S, Charlesbois ED, Guzman D, et al. Adherence to highly active antiretroviral therapy in the homeless population of San Francisco: A prospective study. Clin Infect Dis. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 60.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, et al. Transportation costs impeded sustained adherence and access to HAART in a clinic population in Southwestern Uganda: a qualitative study. AIDS Behav. 2010;14:778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anema A, Vogenthaler N, Frongillo EA, Kadiyala S, Weiser SD. Food insecurity and HIV/AIDS: current knowledge, gaps, and research priorities. Curr HIV/AIDS Res. 2009;6:224–231. doi: 10.1007/s11904-009-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cauldbeck MB, O'Connor C, O'Connor MB, Saunders JA, Rao B, et al. Adherence to antiretroviral therapy among HIV patients in Bangalore India. AIDS Res Ther. 2009;6:7. doi: 10.1186/1742-6405-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Au JT, Kayitenkore K, Shutes E, Karita E, Peters PJ, et al. Access to adequate nutrition is a major potential obstacle to antiretroviral adherence among HIV-infected individuals in Rwanda. AIDS. 2006;20:2116–18. doi: 10.1097/01.aids.0000247580.16073.1b. [DOI] [PubMed] [Google Scholar]
- 64.Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, et al. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2008;24:14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiser SD, Fernandes K, Brandson EK, Lima VD, Anema A, et al. The association between food insecurity and mortality among HIV-infected individuals on HAART. JAIDS. 2009;52:342–9. doi: 10.1097/QAI.0b013e3181b627c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers AIDS Care. 2002;14:309–318. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- 67.Sanjobo N, Frich JC, Freitheim A. Barriers and facilitators to patients' adherence to antiretroviral adherence to antiretroviral treatment in Zambia. SAHARA J. 2008;5:136–43. doi: 10.1080/17290376.2008.9724912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Unge C, Johansson A, Zachariah R, Some D, Van Engelgem I, et al. Reasons for unsatisfactory acceptance of antiretroviral treatment in the urban Kibera slum, Kenya. AIDS Care. 2006;20:146–49. doi: 10.1080/09540120701513677. [DOI] [PubMed] [Google Scholar]
- 69.Remien RH, Hirky AE, Johnson MO, Weinhardt LS, Whittier D, et al. Adherence to medication treatment: a qualitative study of facilitators and barriers among a diverse sample of HIV+ men and women in four US cities AIDS Behav. 2003;7:61–72. doi: 10.1023/a:1022513507669. [DOI] [PubMed] [Google Scholar]
- 70.Ware NC, Idoko J, Kaaya S, Biararo IA, Wyatt MA, et al. Explaining adherence successes in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6:e1000011. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watt MH, Maman S, Earp JA, Eng E, Setel PW, et al. “It's all the time in my mind”: Facilitators of adherence to antiretroviral therapy in a Tanzanian setting. Soc Sci Med. 2009;68:1793–1800. doi: 10.1016/j.socscimed.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diabete S, Alary M, Koffi CK. Determinants of adherence to highly active antiretroviral therapy among HIV-1 infected patients in Cote D'Ivoire. AIDS. 2007;21:1799–1803. doi: 10.1097/QAD.0b013e3282a5667b. [DOI] [PubMed] [Google Scholar]
- 73.Nam SL, Fielding K, Avalos A, Dickenson D, Gaoathe T, et al. The relationship of acceptance or denial of HIV-status to antiretroviral adherence among adult HIV patients in urban Botswana. Soc Sci Med. 2008;67:301–10. doi: 10.1016/j.socscimed.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 74.Birbeck GL, Chomba E, Kvalsund M, Bradbury R, Mang'ombe C, et al. Antiretroviral adherence in Rural Zambia; the first year of treatment availability. Am J Trop Med. 2009;80:669–74. [PubMed] [Google Scholar]
- 75.World Health Organization. Geneva: WHO; 2008. Priority interventions: HIV/AIDS prevention, treatment and care in the health sector. [Google Scholar]
- 76.Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. J Gen Intern Med. 1999;14:267–73. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norman A, Chopra M, Kadiyala S. Factors related to HIV disclosure in 2 South African communities. Am J Public Health. 2007;97:1775–81. doi: 10.2105/AJPH.2005.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ncama BP, McInerney PA, Bhengu BR, Corless IB, Wantland DJ, et al. Social support and medication adherence in HIV disease in KwaZulu-Natal, South Africa. Int J Nurs Stud. 2008;45:1757–63. doi: 10.1016/j.ijnurstu.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. JAIDS. 2006;43:S79–S87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;137:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 81.Nachega JB, Mills EJ, Schechter M. Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: current status of knowledge and research priorities. Curr Opin HIV AIDS. 2010;5:70–77. doi: 10.1097/COH.0b013e328333ad61. [DOI] [PubMed] [Google Scholar]
- 82.Wagner G, Miller LG. Is the influence of social desirability on patients' self-reported adherence overrated? JAIDS. 2004;35:203–204. doi: 10.1097/00126334-200402010-00016. [DOI] [PubMed] [Google Scholar]
- 83.Castro A. Adherence to antiretroviral therapy: merging the clinical and social course of AIDS. PLoS Med. 2005;2:e338. doi: 10.1371/journal.pmed.0020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naik E, Casanas B, Pazare A, Wabale G, Sinnott J, et al. Cost of treatment: the single biggest obstacle to HIV/AIDS treatment adherence in lower-middle class patients in Mumbai, India. Ind J Sex Transmit Dis. 2009;40:23–27. doi: 10.4103/2589-0557.55476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you'll do? A systematic review of the evidence for a relation between patients' recovery expectations and health outcomes. CMAJ. 2001;165:174–79. [PMC free article] [PubMed] [Google Scholar]