Abstract
The gastrointestinal epithelium is anatomically positioned to provide a selective barrier between the anaerobic lumen and lamina propria, which has a high rate of metabolism. Supported by a complex vasculature, this important barrier is affected by reduced blood flow and resultant tissue hypoxia, particularly during the severe metabolic shifts associated with active inflammation in individuals with inflammatory bowel disease (IBD). Activation of hypoxia-inducible factor (HIF) under these conditions promotes resolution of inflammation in mouse models of disease. Protective influences of HIF are attributed, in part, to the complex regulation of a barrier protection with the intestinal mucosa. Reagents that activate HIF, via inhibition of the prolyl hydroxylase enzymes, might be developed to induce hypoxia-mediated resolution in patients with intestinal mucosal inflammatory disease.
Keywords: hypoxia, inflammation, cytokine, epithelia, transcription
Introduction
The gastrointestinal (GI) tract constitutes the largest mucosal surface found in multicellular organisms. Intestinal epithelia line the entire GI tract, covering an area of some 300m2 in adult humans. This monolayer of cells comprise a highly dynamic barrier that must be intricately regulated to accommodate fluid and nutrient transport and to exclude antigenic material at the luminal interface 1, 2. As such, the intestinal mucosa has a unique, adaptive metabolic profile that is regulated by many sources (e.g. enteric microbiota, intestinal perfusion, and tissue oxygenation) and is subject to profound fluctuations even under physiologic, steady-state conditions 3. For instance, marked increases in intestinal blood flow following food ingestion significantly shift local oxygen partial pressure. This metabolic profile is altered under conditions of active inflammation, such as those characterized in inflammatory bowel disease (IBD). Recent studies have associated hypoxia-regulated pathways with barrier function in patients with IBD; these pathways might help resolve mucosal inflammation. We review hypoxia-regulated pathways and discuss the therapeutic potential of modifying hypoxic signaling in patients with IBD.
Energy metabolism and the mucosal immune response
Oxygenation of the mucosa
Intestinal mucosae are characterized by a uniquely dynamic oxygenation profile; they undergo multiple, large fluctuations in blood perfusion and metabolism per day. Even in the basal state, the component epithelial cells that line the mucosa exist in a relatively low oxygen-tension environment, described as ‘physiologic hypoxia’. In the small intestine, this has been attributed to a countercurrent oxygen exchange mechanism, whereby oxygen from arterial blood that supplies the villi diffuses to adjacent venules, travelling from villous tip to base, resulting in graded hypoxia 4. However, a steep oxygen gradient has also been documented in more distal, colonic regions of the GI tract, spanning from the anaerobic lumen, across the epithelium, to the richly vascularized subepithelial mucosa. Because of the high energy requirements of the GI tract and the integral role of the epithelium in maintaining intestinal homeostasis, these cells have evolved many molecular mechanisms to regulate the challenging metabolic conditions. The intestinal epithelium is remarkably resistant to hypoxia; even low levels of oxygenation within this cell layer can be altered to regulate barrier function and integrity 5.
A role for epithelial barrier dysregulation in IBD is supported by observations of increased intestinal permeability in a subset of first-degree relatives of patients with Crohn’s disease (CD) 6. Barrier function of the epithelial monolayer is mediated by a number of specialized anatomical features that confer selective permeability to luminal contents 1. Epithelia are polarized, with apical surface functions optimized for luminal interaction and enteric bacterial exclusion (e.g. intercellular junctions, vectorial membrane transport systems and mucus secretion) and basolateral surfaces adapted for interface with the underlying mucosa and immune cell repertoire. Absorptive and barrier epithelial functions are regulated by oxygen 7. Intestinal epithelia also actively participate as innate immune sensors of microbial pathogens and commensal organisms 8, 9. In fact, a state of low-grade inflammation at the GI mucosal surface is sustained by omnipresent luminal antigens and is important for development of oral tolerance priming of the mucosal immune system, should antigenic material penetrate the epithelial barrier. Studies with gnotobiotic mice have shown that the enteric microbiota influence epithelial cell metabolism, barrier function, and survival 10. Increased epithelial permeability and resultant mucosal inflammation and injury underly the pathology of IBD; improving our understanding of microenvironmental metabolic factors that influence initiation, perpetuation, and resolution of overt disease could lead to new therapeutic approaches.
Inflammation and oxygen metabolism
Large metabolic shifts occur at sites of active mucosal inflammation; nutrients and local oxygen become rapidly depleted, resulting in hypoxia, hypoglycemia, lactate accumulation, and acidosis 7. Over the past decade, much work has focused on establishing the microenvironmental metabolic cues and signaling mechanisms for leukocyte recruitment to these sites, and the metabolic consequences that ensue. Adaptive immune responses to GI tract inflammation are characterized by high rates of local T- and B-cell proliferation and requirements for large amounts of glucose, amino acids, and lipids to fuel oxidative phosphorylation 11, 12. Unlike resident lymphocytes however, innate immune myeloid cells such as neutrophils (polymorphonuclear cells [PMN]), macrophages, and dendritic cells must be actively recruited to inflammatory lesions 13. Cell migration to these lesions is induced by complex cytokine, chemokine, and adhesion molecule expression. PMNs, for example, are mobilized by chemical signals generated at sites of active inflammation, such as interleukin-8, N-formylated peptides, leukotriene B4, and platelet-activating factor. Cell migration requires large amounts of energy, partly because high levels of ATP are required to sustain turnover of actin filaments 14. Upon arrival at the inflammatory site, energy and oxygen demands of recruited cells increase to facilitate phagocytosis and microbial killing. The predominantly glycolytic form of metabolism shared by PMNs is thought to ensure their survival and function in the hypoxic, often anoxic environment of deep inflammatory foci 15. PMNs have unique mitochondria that maintain transmembrane potential via the glycerol-3-phosphate shuttle, which regulates aerobic glycolysis and promotes energy production 16.
Phagocytic functions are controlled by antimicrobial peptides, proteases, and reactive oxygen species (ROS) generated in response to bacterial engulfment 17. ROS are short-lived reactive molecules derived from the incomplete reduction of oxygen, such as superoxide anion, hydrogen peroxide, and hydroxyl radical. Rapid generation of ROS by phagocytes is mediated by a powerful respiratory or oxidative burst, commensurate with large increases in oxygen and glucose consumption that in turn activate further ROS production 18. Upon activation, it is estimated that PMN oxygen demands increase by as much as 50-fold, eliciting consumption of up to 10 times more oxygen than any other cell in the body. The PMN oxidative burst is not inhibited by oxygen concentrations as low as 4.5% 18, so ROS are still generated in the hypoxic environment of intestinal inflammatory lesions. There is evidence for ROS induction in other cell types, including intestinal epithelial cells, in response to microbial signals 19. Though ROS is usually considered to be a microbicide because of its role in the phagocytic immune response, it is also an important second messenger that is involved in mucosal injury in IBD 20.
The metabolic changes that occur during mucosal inflammation in IBD provide might also be studied during development of hypoxia in inflammatory lesions. The presence of hypoxia at sites of mucosal inflammation was first identified in mouse models of IBD using 2-nitroimidazole dyes 21, a class of compounds that undergo intracellular metabolism in an oxygen-dependent manner 22. Tissue staining with these dyes revealed intriguing features of the mucosal oxygenation profile. First, basal hypoxia is detectable in normal, non-inflamed intestinal epithelial cells, particularly in the colon epithelium. So, low oxygen tension might regulate basal gene expression in these cells (i.e. physiologic hypoxia). Second, inflammatory mucosal lesions observed in colitic mice were highly hypoxic or even anoxic, similar to those observed in large tumors. Several clinical studies have further defined the occurrence of hypoxia in IBD 23–25. Although mechanisms for local energy and oxygen depletion in the microenvironment of active inflammatory lesions have been partially elucidated, there is a growing body of data to indicate that microvascular deficits in IBD might contribute to mucosal hypoxia, through reduced intestinal blood supply and oxygen delivery; these are further discussed below. Notably, analyses of inflamed colon samples from IBD patients revealed prominent immunohistochemical staining for the hypoxia-inducible factors (HIFs) HIF-1 and HIF-2 23—transcription regulators of genes that control cell survival and functionality under hypoxic conditions. Some staining differences were noted between HIF-1 and HIF-2 in samples from patients with CD or ulcerative colitis (UC). For example, although HIF-1 was expressed focally within various stromal cells, HIF-2 appeared to be expressed more diffusely in CD samples. These studies also found that vascular density was significantly higher in samples from patients with CD or UC, compared with normal tissues, and that increased vascular density correlated with the expression of VEGF, a gene that is regulated by HIF 26, 27.
HIF–transcriptional regulators in response to hypoxia
HIFs
HIF is a member of the Per-ARNT-Sim family of basic helix-loop-helix transcription factors that binds hypoxia response elements (HREs) at target gene loci under hypoxic conditions 28. Functional HIF is a heterodimer that comprises a constitutive subunit (HIF-1β) and a hypoxia-inducible ‘α’ component; stabilization of this α-subunit is regulated, in part, by a family of oxygen- and iron-dependent prolyl hydroxylase (PHD) enzymes 29. Three subunits have been identified (HIF-1α, HIF-2α, and HIF-3α), with the highest level of sequence homology conserved between HIF-1α and HIF-2α 30. Analyses of genetic mouse models indicate that HIF-1 and HIF-2 have non-redundant functions, 28 despite their concurrent expression in many cell types, including intestinal epithelial cells 31. Several studies have indicated that these proteins modulate the transcription of an overlapping but distinct set of genes (Table 1) and that transcriptional responses might be integrated in ways that support specific adaptations to hypoxia. For instance, transcriptional regulation of genes that encode glycolytic enzymes appears to be more specifically mediated by HIF-1 than HIF-2 32, whereas HIF-2 selectively regulates gene expression of factors involved in duodenal iron homeostasis 31 and in early erythropoiesis. The N-terminal transactivation domain of HIF proteins has been proposed to mediate specificity for target genes, via interactions with auxiliary transcription factors 28, but compelling evidence for this aspect remains elusive.
TABLE I.
Influence of HIF signaling on intestinal mucosal functions implicated in IBD pathogenesis
| Compartment | Function | Isoform Specificity | Reference |
|---|---|---|---|
| Epithelial | Barrier | HIF-1 |
Furuta et al., 2001 Louis et al., 2006 Karhausen et al., 2004 |
| Nucleotide metabolism | HIF-1 | Synnestvedt et al., 2002 | |
| Iron transport | HIF-2 | Mastrogiannaki et al., 2009 | |
| Cytokines/Chemokines | HIF-1 | Shah et al., 2008 | |
| Migration/Wound healing | HIF-1 |
Keely et al., 2009 Robinson et al., 2008 |
|
| Apoptosis/Barrier | HIF-1 | Cummins et al., 2008 | |
| Endothelial | Barrier | HIF-1 | Kong et al., 2006 |
| Nucleotide metabolism | HIF-1 |
Kong et al., 2006 Eltzschig et al., 2003 |
|
| Angiogenesis | HIF-1/HIF-2 | Pugh et al., 2003 | |
| Myeloid | Bacterial killing | HIF-1 | Peyssonnaux et al., 2005 |
| ATP generation | HIF-1 | Cramer et al., 2003 | |
| Cytokine production | HIF-1/HIF-2 | Acosta-Iborra et al., | |
| Colitis-associated tumor infiltration | HIF-2 | Imtiyaz et al., 201079 | |
| T-cell | TCR signaling | HIF-1 | Neumann et al., 2005 |
| Hepatic | Erythropoietin production | HIF-2 | Rankin et al., 2007 |
| General | Glucose metabolism | HIF-1 | Hu et al., 2003 |
| Glycolysis | HIF-1 | Vermeulen et al., 201080 |
During mucosal inflammation, HIF has a protective role 5, 33–35; microarray analyses of differentially expressed mRNAs in cultured epithelial cells subjected to hypoxia and animal models of inflammation showed that the HIF-regulated transcriptional profile promotes intestinal epithelial barrier function. Further investigation of mechanisms related to hypoxia-elicted barrier protection revealed interesting features. First, expression of the functional proteins encoded by these transcripts was localized to the most luminal apical aspect of polarized epithelia 5, 33, 35. Second, molecular dissection of the hypoxia-elicited pathway(s) for this apical gene cluster revealed a high propensity for regulation by HIF. Third, HIF-dependent, epithelial barrier protective pathways induced by hypoxia tend to be more non-conventional regulators of barrier function than prototypical junction proteins such as occludin or claudin(s). Rather, HIF-regulated signaling promotes overall tissue integrity, influencing functions that range from increased mucin production 36 by molecules that modify mucins, such as intestinal trefoil factor 5, to xenobiotic clearance by P-glycoprotein, 33 to nucleotide metabolism by 5′-ectonucleotidase (CD73) 34, 35, 37, 38 and nucleotide signaling through the adenosine A2B receptor 34, 39, 40. More recent work has indicated that HIF-1 induces the integrin β1 subunit, which regulates fibroblast contraction, epithelial migration, and might mediate restitution of the mucosal barrier after wounding 41. Interestingly, HIF-2α might specifically regulate duodenal iron uptake through the apical iron uptake pathway, via discrete regulation of Dcytb and DMT1, rather than via basolateral iron transport 42. These findings indicate that HIF-2 is an important component of mechanisms of local changes in enterocyte iron or oxygen, altered duodenal transporter expression, and dietary iron absorption. Anemia is the most prevalent extraintestinal complication of IBD 43, so it is important to further investigate these mechanisms.
To investigate the physiologic functions of intestinal epithelial HIF, Karhausen et. al. generated transgenic mouse lines with intestinal epithelial-targeted expression of either mutant Hif1α, leading to repression of HIF-1, or mutant von Hippel-Lindau gene (Vhlh), resulting in constitutive overexpression of HIF (HIF-1 and HIF-2) 21. Studies of trinitrobenzene sulfuric acid (TNBS)-induced colitis in these mice revealed that the loss of epithelial HIF-1 caused more severe symptoms, including increased weight loss, intestinal epithelial permeability, and mortality. By contrast, constitutively active epithelial HIF was protected against these parameters. However, results vary among models—epithelial HIF-1-based signaling promoted inflammation in another study 44. Nonetheless, these findings confirm that intestinal epithelial cells can adapt to hypoxia and that HIF mediates the adaptation.
Cross-talk between hypoxia and inflammation
Given the integration of intestinal epithelia and mucosal immune cells and our enhanced understanding of the inflammatory tissue microenvironment, there is much interest in the influence of hypoxia and HIF signaling on immune-cell metabolism and effector function in inflammatory diseases such as IBD. HIF supports the innate immune functions of dendritic and mast cells and promotes the activities of phagocytes by mechanisms that range from increased killing of bacteria to antigen presentation 45. Pro-inflammatory signals (e.g. cytokines, lipopolysaccharide) promote stabilization of HIF proteins, even under normoxic conditions, indicating the interaction between hypoxic and immune responses to infection and tissue damage 46. Survival of CD3+ T cells under hypoxic conditions is thought to partially depend on HIF-1α-mediated expression of the vasoactive peptide adrenomedullin 47. T-cell expression of functional HIF-1α protein is, in turn, likely influenced by hypoxia and by T-cell receptor (TCR)-mediated signaling through PI3K, via the mammalian target of rapamycin 48. Experiments in which HIF-1α was constitutively stabilized in thymocytes demonstrated the role for HIF in modulating signaling events downstream of TCR activation 49. Studies of chimeric mice with HIF1α-deficient T and B cells revealed lineage-specific defects that induce autoimmunity, including auto-antibody production, increased rheumatoid factor, and kidney damage 50.
HIF function has also been studied in some detail in myeloid cells. Cre-LoxP based deletion of Hif-1α in cells of the myeloid lineage revealed multiple features that implicate HIF signaling in metabolic control of myeloid function (see 45 for review). These findings are attributable, at least in part, to the inability of HIF1α-deficient myeloid cells to mount an appropriate metabolic response to decreased oxygen concentrations that are characteristic of infection sites. These studies have shown that the capacity of PMNs and macrophages to kill bacteria is severely limited in the absence of HIF-1α, because HIF-1 is required for production of antimicrobial peptides and granule proteases 45 and generation of pro-inflammatory cytokines that facilitate this process 51. HIF-1α mediates transcription of the gene that encodes the integrin β2 subunit, which is required for myeloid cell adhesion and transmigration; β2 upregulation is accompanied by increased adhesion of leukocytes to activated vascular endothelial cells 52, 53. These findings have increased our appreciation for the role of innate immune cells, such as PMNs, in innate host defense and IBD. PMN depletion techniques have been used to demonstrate the role of PMNs in the resolution of inflammation in several mouse models of IBD; 54 this process is likely to include release of soluble mediators of resolution and antimicrobial activity.
Intestinal microvascular metabolism in IBD
There is controversy over the roles of intestinal microvascular deficits in the etiology of IBD—in part because vascular changes in the submucosa in active disease could be secondary to transmural inflammation that originates in the mucosa 55. However, evidence indicates that the microvasculature contributes to chronic mucosal inflammation through several diverse mechanisms 56 (Figure 1), and that a major consequence of these alterations is impaired intestinal perfusion and attenuated oxygen delivery. Reduced generation of nitric oxide (NO) by chronically inflamed IBD intestinal endothelia is thought to mediate inappropriate and sustained leukocyte adherence 57, whereas increased production of the eicosanoid thromboxane A2, detected in cultured IBD biopsy samples, could potentiate proinflammatory effects, in vivo, such as neutrophil adhesion to endothelia, apoptosis, platelet aggregation, and vasoconstriction 56, 58.
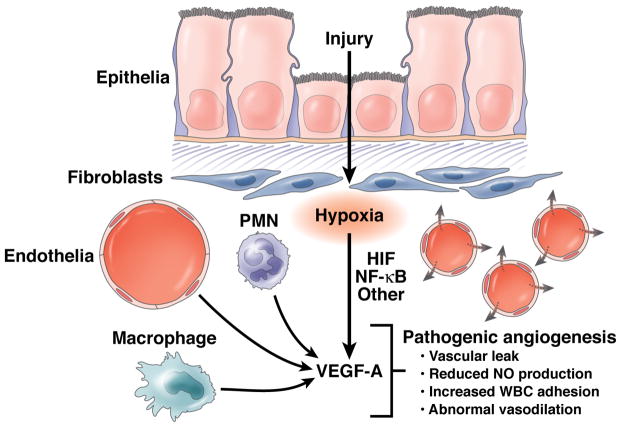
Figure 1. Contributions of inflammation and hypoxia to angiogenesis.
Inflammation and hypoxia each contribute to angiogenesis during pathogenesis of IBD, partly by induction of VEGF-A expression in multiple cell types that include submucosal fibroblasts, macrophages, neutrophils (PMNs), and endothelial cells. VEGF-A-induced angiogenesis is likely to be pathogenic and result in abnormal vessel formation and poorly functioning vasculature.
Some studies have indicated that angiogenesis is involved in development of microvascular dysfunction in IBD 59, 60. Neovascularization is an established feature of chronic intestinal inflammation 61 and might arise as a compensatory response to the extensive hypoxia and increased metabolism of inflammatory lesions. Studies of tissue samples from patients with IBD and mouse models of colitis have shown that VEGF signaling mediates angiogenic and inflammatory processes during disease progression 60. Increased expression of VEGF-A in colitic tissue correlates with increased angiogenesis, leukocyte recruitment, and vascular leaks. Moreover, dynamic amplification of pathophysiologic processes (e.g. altered leukocyte adherence, attenuated NO generation, and impaired vasodilation) in the expanded microvascular bed might perpetuate the inflammatory process, to a point that angiogenesis and inflammation become chronically co-dependent processes 62, 63. Overall, these features could collectively contribute to the impaired perfusion dynamics observed in IBD.
Therapeutic approaches to alter mucosal metabolism
Researchers are investigating therapeutic approaches to manipulate hypoxia pathways, to promote resolution of inflammation in patients with IBD. Most preclinical studies have been performed using mice with acute colitis as a model of IBD.
Stabilization of HIF
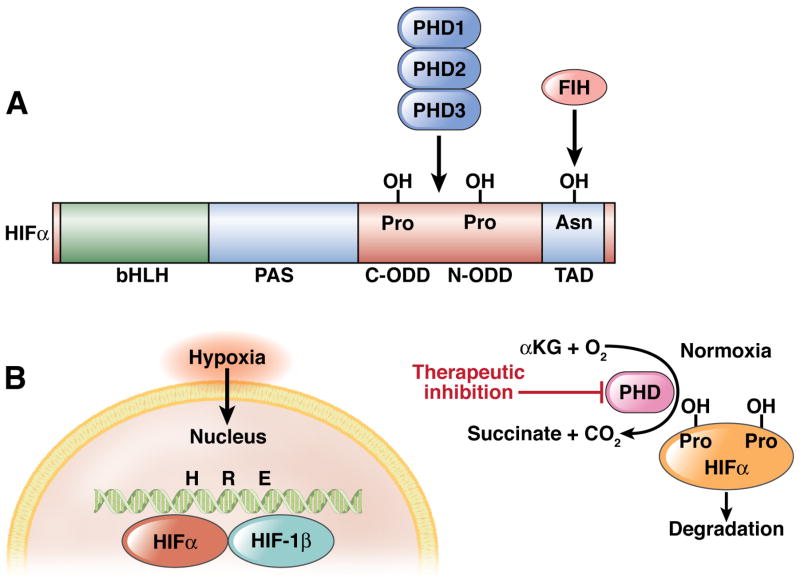
Reagents that affect activities of HIF-selective prolyl hydroxylase enzymes might be developed as of therapeutics to alter function of HIF 64, 65. These enzymes were first identified using a candidate molecular approach, based on conserved structural features shared by well-characterized mammalian hydroxylases that target extracellular collagen 66. A family of 3 prolyl hydroxylases has been characterized—PHD1, 2, and 3 (known also as egl nine homolog [EGLN] 1, 2, and 3) facilitate hypoxic regulation of the HIF pathway 66. In the presence of 2-oxoglutarate, iron, and oxygen, PHDs hydroxylate the α-subunit of HIF, leading to ubiquitylation and degradation of HIF 67. Specifically, PHDs target prolines 402 and 564 within the oxygen-dependent degradation domain of the HIF-1α subunit (Figure 2). Reactions conducted in vitro, in an environment of limited oxygen, revealed that the activities of purified PHDs are sensitive to reduced levels of oxygen 68, 69. The enzymes have different tissue distributions and, when overexpressed, have distinct patterns of subcellular localization. Expression of PHD family members was not observed to differ among tissues or cells of the GI tract; all 3 PHDs are found in the intestinal epithelium, with a distribution differential of PHD1<PHD2=PHD3 in mouse intestinal mucosa 70, 71.
Figure 2. Functional features of hypoxia-inducible factor (HIF) and mechanism of HIF stabilization.
HIF is hydroxylated by the combination of α–ketoglutarate (αKG), molecular oxygen (O2), and the PHD enzymes in normoxic conditions. When O2 becomes limiting (hypoxia), the HIF-1 α subunit is stabilized and binds to the HIF-1 β subunit in the nucleus; the complex binds to the hypoxia-response element (HRE) in target genes to regulate their transcription.
HIF prolyl hydroxylases– potential therapeutic agents?
Although HIF1 inhibitors might be developed as cancer therapies, reagents that selectively stabilize HIF, such as PHD inhibitors, might support mucosal barrier function and promote inflammatory resolution in patients with IBD 71. Several PHD inhibitors, including direct inhibitors, have been described 72. In addition, analogues of naturally occurring cyclic hydroxamates 73 and antagonists of α-ketoglutarate 65 are competitive inhibitors of PHDs (Figure 2). Within the GI tract, the PHD inhibitors DMOG and FG-4497 reduce features of colitis in mice 70, 71. These studies showed that PHD inhibition affected parameters of disease, including weight loss, colon length, and disease activity index. These effects are most likely due to their barrier-protective function and enhancement of wound healing at the site of inflammation.
It is important to note that HIF is not the only hypoxia-responsive transcription factor that regulates mucosal homeostasis and disease, and not the only oxygen-dependent regulator of hydroxylase activity45. Nuclear factor (NF) κB is regulated in a similar manner to HIF; hypoxia activates NF-κB, partially through altered hydroxylation of factors in this signaling pathway 74, 75. Like conditional HIF-1α-null mice, disruption of NF-κB signaling in intestinal epithelial cells of mice increases their susceptibility to mucosal inflammation, indicating that epithelial NF-κB protects against colitis 76. This effect is likely mediated by increased expression of anti-apoptotic genes in the intestinal epithelium, which increases epithelial barrier function. Hydroxylase inhibition might protect against colitis in mice by increasing the activity of NF-κB in the intestinal epithelium70. Conditional knockout mice are being used to study whether the ability of hydroxylase inhibitors to protect against colitis require HIF and/or NF-κB pathways.
Therapeutic reagents designed to stabilize HIF could have adverse effects. PHD inhibitors can substantially increase hematocrit values, by increasing HIF-mediated erythropoietin production—for this reason they are used to treat patients with anemia 77 In mice with colitis, high doses of FG-4497 (>60 mg/kg), administered daily for 5 days, occasionally caused vascular occlusions in the intestine (S.P. Colgan, unpublished ). These adverse effects likely resulted from erythrocyte aggregation, determined by high hematocrit values. This problem was rectified by reducing the dose and interval of dosing of the PHD inhibitor. Furthermore, long-term stabilization of HIF-1 and HIF-2 could promote tumor growth 78. It is not clear whether pharmacological stabilization of HIF could initiate or promote tumor development, these affects should be investigated. Until proven otherwise, the safest use of PHD inhibitors might be for short-term treatment of IBD or as an adjunct therapy with other drugs over a short period of time.
Conclusions
The GI mucosa is a unique setting for studying changes in tissue oxygenation and metabolism during disease progression. Because this mucosal surface has relatively low baseline oxygen tension and high energy demands, along with sustained physiologic inflammatory activity, it could be affected by HIF-based therapies. Studies in animal models of IBD have demonstrated the protective and anti-inflammatory effects of hydroxylase inhibition. It will be important to determine the role of HIF-2α, and the genes it regulates, in this protective response, as well as the interaction between the HIF and NF-κB pathways. Because hypoxia regulates myeloid and lymphocyte functions, a significant but important challenge is to elucidate innate and adaptive immune responses that are mediated by HIF signaling; these responses might affect barrier function in inflammation. In sum, the endogenous adaptive metabolic pathways activated in response to hypoxia represent potentially important new windows of opportunity for treatment of IBD.
Acknowledgments
This work was supported by National Institutes of Health Grants DK50189 and HL60569 and by a grant from the Crohn’s and Colitis Foundation of America.
Footnotes
The authors declare no financial interests in any of the work submitted here.
Contributions:
LEG: drafted manuscript content, reviewed manuscript
SPC: drafted manuscript content, reviewed manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–7. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 3.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 7:281–7. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc. 1982;41:2084–9. [PubMed] [Google Scholar]
- 5.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–34. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–5. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 7.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 8.Clavel T, Haller D. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr Issues Intest Microbiol. 2007;8:25–43. [PubMed] [Google Scholar]
- 9.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–91. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 11.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 12.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 184:4062–8. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 14.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 15.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–7. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS One. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–89. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 18.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979;53:1133–9. [PubMed] [Google Scholar]
- 19.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 20.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–41. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans SM, Hahn S, Pook DR, Jenkins WT, Chalian AA, Zhang P, Stevens C, Weber R, Weinstein G, Benjamin I, Mirza N, Morgan M, Rubin S, McKenna WG, Lord EM, Koch CJ. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–24. [PubMed] [Google Scholar]
- 23.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–13. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariani F, Sena P, Marzona L, Riccio M, Fano R, Manni P, Gregorio CD, Pezzi A, Leon MP, Monni S, Pol AD, Roncucci L. Cyclooxygenase-2 and Hypoxia-Inducible Factor-1alpha protein expression is related to inflammation, and up-regulated since the early steps of colorectal carcinogenesis. Cancer Lett. 2009;279:221–9. doi: 10.1016/j.canlet.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Matthijsen RA, Derikx JP, Kuipers D, van Dam RM, Dejong CH, Buurman WA. Enterocyte shedding and epithelial lining repair following ischemia of the human small intestine attenuate inflammation. PLoS One. 2009;4:e7045. doi: 10.1371/journal.pone.0007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 28.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117:862–5. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 30.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005:2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 31.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–66. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–94. [PubMed] [Google Scholar]
- 34.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–96. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–27. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 37.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–55. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 38.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–64. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. Faseb J. 2006;20:2242–50. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 41.Keely S, Glover LE, MacManus CF, Campbell EL, Scully MM, Furuta GT, Colgan SP. Selective induction of integrin beta1 by hypoxia-inducible factor: implications for wound healing. Faseb J. 2009;23:1338–46. doi: 10.1096/fj.08-125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson RJ, McKie AT. Regulation of intestinal iron absorption: the mucosa takes control? Cell Metab. 2009;10:84–7. doi: 10.1016/j.cmet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, Gonzalez FJ. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–48. 2048, e1–3. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–15. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makino Y, Nakamura H, Ikeda E, Ohnuma K, Yamauchi K, Yabe Y, Poellinger L, Okada Y, Morimoto C, Tanaka H. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J Immunol. 2003;171:6534–40. doi: 10.4049/jimmunol.171.12.6534. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174:7592–9. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 49.Neumann AK, Yang J, Biju MP, Joseph SK, Johnson RS, Haase VH, Freedman BD, Turka LA. Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc Natl Acad Sci U S A. 2005;102:17071–6. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 51.Acosta-Iborra B, Elorza A, Olazabal IM, Martin-Cofreces NB, Martin-Puig S, Miro M, Calzada MJ, Aragones J, Sanchez-Madrid F, Landazuri MO. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-gamma production through the HIF-1 alpha transcription factor. J Immunol. 2009;182:3155–64. doi: 10.4049/jimmunol.0801710. [DOI] [PubMed] [Google Scholar]
- 52.Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc Natl Acad Sci U S A. 2004;101:10440–5. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong T, Scully M, Shelley CS, Colgan SP. Identification of Pur alpha as a new hypoxia response factor responsible for coordinated induction of the beta 2 integrin family. J Immunol. 2007;179:1934–41. doi: 10.4049/jimmunol.179.3.1934. [DOI] [PubMed] [Google Scholar]
- 54.Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, Loddenkemper C, Heimesaat MM, Grollich K, Zeitz M, Farkas S, Hoffmann JC. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–92. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 55.MacDonald TT. Aetiology of Crohn’s disease. Arch Dis Child. 1993;68:623–5. doi: 10.1136/adc.68.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatoum OA, Heidemann J, Binion DG. The intestinal microvasculature as a therapeutic target in inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:78–97. doi: 10.1196/annals.1326.003. [DOI] [PubMed] [Google Scholar]
- 57.Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology. 2003;125:58–69. doi: 10.1016/s0016-5085(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 58.Rampton DS, Collins CE. Review article: thromboxanes in inflammatory bowel disease--pathogenic and therapeutic implications. Aliment Pharmacol Ther. 1993;7:357–67. [PubMed] [Google Scholar]
- 59.Chidlow JH, Jr, Greer JJ, Anthoni C, Bernatchez P, Fernandez-Hernando C, Bruce M, Abdelbaqi M, Shukla D, Granger DN, Sessa WC, Kevil CG. Endothelial caveolin-1 regulates pathologic angiogenesis in a mouse model of colitis. Gastroenterology. 2009;136:575–84. e2. doi: 10.1053/j.gastro.2008.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J, Yla-Herttuala S, Fiocchi C, Danese S. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–95. e5. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 61.Binion DG, Rafiee P. Is inflammatory bowel disease a vascular disease? Targeting angiogenesis improves chronic inflammation in inflammatory bowel disease. Gastroenterology. 2009;136:400–3. doi: 10.1053/j.gastro.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 62.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. Faseb J. 1997;11:457–65. [PubMed] [Google Scholar]
- 63.Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035–9. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci. 2003;116:3041–9. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 65.Mole DR, Schlemminger I, McNeill LA, Hewitson KS, Pugh CW, Ratcliffe PJ, Schofield CJ. 2-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett. 2003;13:2677–80. doi: 10.1016/s0960-894x(03)00539-0. [DOI] [PubMed] [Google Scholar]
- 66.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–23. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 67.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–8. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 69.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 70.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–65. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–55. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nwogu JI, Geenen D, Bean M, Brenner MC, Huang X, Buttrick PM. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation. 2001;104:2216–21. doi: 10.1161/hc4301.097193. [DOI] [PubMed] [Google Scholar]
- 73.Schlemminger I, Mole DR, McNeill LA, Dhanda A, Hewitson KS, Tian YM, Ratcliffe PJ, Pugh CW, Schofield CJ. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg Med Chem Lett. 2003;13:1451–4. doi: 10.1016/s0960-894x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 74.Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, Pugh CW, Oldham NJ, Masson N, Schofield CJ, Ratcliffe PJ. Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc Natl Acad Sci U S A. 2006;103:14767–72. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–9. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 77.Jelkmann W. Control of erythropoietin gene expression and its use in medicine. Methods Enzymol. 2007;435:179–97. doi: 10.1016/S0076-6879(07)35010-6. [DOI] [PubMed] [Google Scholar]
- 78.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vermeulen N, Vermeire S, Arijs I, Michiels G, Ballet V, Derua R, Waelkens E, Lommel LV, Schuit F, Rutgeerts P, Bossuyt X. Seroreactivity against glycolytic enzymes in inflammatory bowel disease. Inflamm Bowel Dis. 2010 Jul 13; doi: 10.1002/ibd.21388. [DOI] [PubMed] [Google Scholar]