Summary
The negative selection of self-reactive thymocytes depends on the induction of tissue-specific antigens by medullary thymic epithelial cells. The autoimmune regulator (Aire) protein plays an important role in turning on these antigens, and the absence of even one Aire-induced tissue-specific antigen in the thymus can lead to autoimmunity in the antigen-expressing target organ. Recently, Aire protein has been detected in peripheral lymphoid organs, suggesting that peripheral Aire plays a complementary role here. In these peripheral sites, Aire was found to regulate the expression of a group of tissue-specific antigens that is distinct from those expressed in the thymus. Furthermore, transgenic antigen expression in extrathymic Aire-expressing cells (eTACs) can mediate deletional tolerance, but the immunological relevance of Aire-dependent, endogenous tissue-specific antigens remains to be determined.
Keywords: autoimmunity, transcriptional activator, thymic selection, peripheral tolerance
Introduction
T cells play a major role in guiding the immune system to specifically recognize and remove infectious agents, while also providing lasting immunological memory of previously encountered pathogens. Their ability to recognize a wide variety of foreign antigens is a result of random recombination events that generate T-cell receptors (TCRs) capable of recognizing both self and foreign antigens. While the wide range of foreign antigen specificities generated ensures a nearly unlimited potential to recognize pathogens, the production of self-reactive T cells has the potential to result in debilitating autoimmunity, as seen in experimental conditions in which mice lack critical regulatory pathways due to loss of genes encoding autoimmune regulator protein (Aire) or forkhead box protein 3 (Foxp3). The classical function of these proteins prevents autoimmunity in healthy organisms at two distinct stages during the development and function of T cells. Central tolerance is established within the thymus by purging self-reactive thymocytes, and thus reducing the propensity for autoreactivity among mature T cells in the periphery. Nevertheless, the incompleteness of central tolerance in removing the mature T cells with self-antigen specificity necessitates additional mechanisms to maintain peripheral tolerance, including further clonal deletion of mature autoreactive T cells in the periphery or active suppression of their activation by regulatory T-cell populations.
Positive selection promotes central tolerance by setting the baseline T-cell receptor signaling threshold
T cells are ultimately derived from hematopoietic precursors that arise in the bone marrow, but unlike their B cell cousins, T-cell precursors migrate to the thymus to undergo maturation. Upon entering the thymus, most T-cell precursors make a commitment to the αβ T-cell lineage, while in the double-negative stage, defined by the lack of expression of CD4 or CD8 T-cell coreceptors (1–3). As lineage commitment progresses, successful TCR β chain rearrangement is first validated through a ligand-independent process, followed by a rearrangement of the TCR α chain and upregulation of both CD4 and CD8 (4, 5). Expression of αβ TCR heterodimers on the cell surface of developing double-positive thymocytes allows cells with functional TCR complexes to be positively selected by peptide-major histocompatibility complex (MHC) complexes in the thymic cortex (6). Double positive thymocytes with a minimal threshold of reactivity to the particular self-MHC haplotypes in the organism’s genetic background survive; thymocytes failing to express a TCR without this basal self-recognition undergo apoptosis. Survival of positive selection allows thymocytes to make a commitment to either the CD4 or CD8 single positive lineage.
The importance of creating a TCR repertoire with appropriate signaling thresholds is highlighted by the development of autoimmunity when genetic mutations alter the process of positive selection. ζ-chain (TCR)-associated protein kinase of 70 kDa (ZAP-70) is a kinase whose activity is critical for TCR signal transmission (7). A mutation of this gene was subsequently found to be the causative defect in a spontaneous model of rheumatoid arthritis, the SKG mouse, due to attenuated TCR signaling (8). This and other ZAP-70 mutants with intermediate function were found to have defects in both positive selection and removal of strongly self-reactive thymocytes through negative selection, as reduced signaling ability first necessitates a stronger positively selecting signal from self-ligands and then reduces sensitivity to deletion by a broader set of self-ligands during negative selection. Alteration of TCR signaling thresholds in both these settings contributes to the formation of a TCR repertoire that has increased propensity for self-recognition (9, 10).
Negative selection prevents maturation of thymocytes with strong self-reactivity
After receiving a positively selecting signal and migrating to the thymic medulla, thymocytes must survive the process of negative selection in which thymocytes bearing strongly self-reactive TCRs undergo apoptosis, thus preventing their maturation and subsequent ability to mount an autoimmune response in the periphery. Negative selection can be observed in experimental systems at both the double positive and single positive stages in the cortex and medulla, respectively, depending on the location of the negatively selecting signal (6, 11). This process serves to reduce the frequency of strongly self-reactive T cells in the periphery. The absence of negatively selecting self-peptide-MHC complexes in the medulla leads to an increase in maturation of autoreactive T cells with the potential to cause autoimmunity (12, 13).
The first reports of tissue-specific antigen transcription resulted from the creation of transgenic mice expressing target genes under control of the rat insulin promoter, although these systems were originally designed to target gene expression to the pancreas. Surprisingly, these transgenes and several others with tissue-specific promoters were activated in the thymic stroma, leading to the deletion of antigen-specific T cells and establishment of tolerance (14). Other experiments suggested that promiscuous expression of tissue-specific antigens within the thymus and subsequent negative selection of antigen-specific T cells could play a physiological role in the prevention of experimental autoimmune encephalomyelitis (15). Later, expression of tissue-specific genes within the thymus was found to be particularly enriched in medullary thymic epithelial cells (mTECs), in contrast to cortical medullary epithelial cells (cTECs), CD11c+ dendritic cells, and F4/80+ macrophages (16). Taken together, these studies demonstrated the potential for self-antigen expression within the thymus to mediate deletion of autoreactive thymocytes and suggested that mTECs may have particularly important role in this process of guiding the thymocyte repertoire.
Aire prevents autoimmunity by directing thymic expression of tissue-specific antigens
Autoimmune polyglandular syndrome type 1 (APS1), which has also been called autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), is a monogenic autoimmune disease characterized by multi-organ endocrinopathy such as hypoparathyroidism or adrenocortical failure, chronic mucocutaneous candidiasis, and ectodermal dystrophies (17, 18). Though the pattern of inheritance was noted to be monogenic, patients with APS1 develop an array of organ-based autoimmune diseases with significant heterogeneity between individuals. In 1997, two groups identified a novel gene responsible for this condition, the Autoimmune Regulator (AIRE). Sequence analysis suggested that AIRE likely participated in protein-protein interactions and DNA binding based on the presence of conserved domains and clustering of disease-inducing mutation within those domains (19–21). Later, Aire was found to be expressed within the thymic medulla, suggesting that its mechanism of autoimmune prevention could be to facilitate immune tolerance within the thymus, perhaps by maintaining normal thymic architecture or regulating interactions with thymocytes undergoing negative selection (22, 23). Furthermore, the detection of Aire specifically within nuclear speckles suggested that Aire might function by directly regulating gene expression within the thymus (22). Interestingly, mRNA in situ hybridization also showed the presence of Aire transcripts within both the lymph nodes and spleen (24), and reverse transcriptase polymerase chain reaction (RT-PCR) of peripheral blood showed that human AIRE message was detected in CD14+ monocytes and dendritic cells (25).
Two independent strains of Aire-deficient mice were made and found to have multi-organ autoimmunity with lymphoid infiltration of target tissues and serum autoantibodies (26, 27). While the pattern of autoimmunity was slightly different than the clinical symptoms seen in patients with AIRE mutations, the overall pattern of multi-organ autoimmunity was consistent between mice and humans. Like APS1 patients, the autoimmunity was predominantly targeted against endocrine organs. Autoimmunity against the salivary gland and retina was the most penetrant phenotype of Aire knockout mice on the C57BL/6 background, and infiltrates increased in an age-dependent manner (26). Further work showed that pattern of Aire-controlled autoimmunity was highly strain dependent (28). The Aire knockout mouse was bred onto the non-obese diabetic (NOD) strain, resulting in the expansion of the autoimmune attack to the pancreas and thyroid gland, and an acceleration of infiltration of targets previously identified in C57BL/6 mice (28). Also, Aire knockout mice on the BALB/c background displayed an intermediate phenotype. Curiously, Aire knockout mice on the NOD background were completely protected from diabetes, and pancreatic infiltration was restricted to exocrine tissue (29).
The autoimmunity observed in the Aire knockout mouse was found to map primarily to the thymus itself, and not to peripheral tissues. Bone marrow chimerism experiments showed that Aire deficiency precipitated disease when it was absent from the radioresistant stroma, but did not substantially affect autoimmune processes when lacking from the hematopoietic compartment (26). Similarly, thymus transplant experiments demonstrated that the primary contribution of Aire to the prevention of autoimmunity occurred through its thymic expression (26, 30). More recently, further insight into Aire’s role in the prevention of autoimmunity has come from the creation of a transgenic mouse in which Aire expression is repressed by doxycycline administration (31). In this study, the authors demonstrated that the presence of Aire was most critical during a perinatal window. Transgenic mice on the NOD background developed the characteristic autoimmunity of Aire knockout mice only if Aire was absent before weaning; repression of Aire after 21 days of age did not provoke autoimmunity. Experiments in which Aire was experimentally repressed during recovery from lethal irradiation or antibody-mediated T-cell depletion showed the absence of Aire promoted autoimmunity in these settings, thus suggesting that its perinatal requirement is a result of relative peripheral lymphopenia in the perinatal mouse (31). Alternatively, the observed temporal importance of Aire may more directly reflect decreased thymic output of mature thymocytes as the mice age, a phenomenon may be accelerated in mice on the NOD background due to relatively elevated systemic inflammation (32, 33).
Having demonstrated that Aire prevents autoimmunity primarily through its actions in the thymus, Anderson et al. (26) demonstrated that Aire promotes the expression of a multitude of tissue-specific genes within the thymus, and both Aire and its controlled genes are expressed primarily in mTECs. Comparisons of Aire wildtype and knockout mice found that thymic transcription of some previously identified targets including insulin was Aire dependent, while expression of other targets such as GAD67 was not (26, 34). The identification of interphotoreceptor retinoid-binding protein (IRBP) as a target of eye-specific autoimmune disease in the Aire knockout mice led to further investigation of whether or not expression of an individual self-antigen within the thymus could be responsible for the prevention of autoimmunity to a particular target organ in Aire-sufficient mice (35). IRBP was expressed within the thymus in an Aire-dependent fashion, and its targeted absence from the thymus through transplantation of IRBP knockout thymi into athymic IRBP sufficient recipients led to IRBP-directed autoimmunity. Thus, the absence of a single self-antigen in the thymus was sufficient to lead to autoimmune disease, highlighting the role of this individual thymic TSA in maintaining tolerance. Later, adoptive transfers and depletion of lymphocyte subsets showed that CD4+ T cells are sufficient to mediate autoimmune disease resulting from lack of Aire activity within the thymus, consistent with defective T-cell negative selection in Aire-deficient mice (36). Further examination of Aire-deficient mice has recently led to the identification of odorant binding protein 1a as a target of lacrimal gland autoimmunity (37) and vomeromodulin as a target of lung autoimmunity (38). These recent findings provide additional support for the importance of individual TSAs in preventing autoimmunity, and in the case of vomeromodulin, show the usefulness of examining autoimmunity in Aire-deficient mice as a tool to identify clinically relevant autoantigens.
Mechanistic control of tissue-specific antigen expression by Aire
Chromatin immunoprecipitation assays have shown that Aire can directly interact with its target TSAs in vivo, but the mechanism by which target specificity is determined and transcriptional activation occurs is still poorly understood (39, 40). While some investigators have found evidence for direct DNA binding by multimers of Aire, a DNA consensus sequence consistent with the observed activity of Aire in the thymus has not been found (41, 42). A bioinformatics approach to identifying Aire-induced genes found a broad representation of targets across the genome, with individual targets tending to occur together in clusters (34, 43). When analyzing transcripts in individual Aire-expressing mTECs by single-cell qPCR, Aire-induced transcripts seem to be present in only a fraction of these cells in a stochastic manner (34,44). Aire is thus likely to exert its transcriptional activation by promoting the opening of chromatin, allowing transcription of otherwise-repressed target genes. Such a role is consistent with the previously hypothesized properties of Aire’s individual domains.
Closer examination of the conserved domains of Aire provides some insight into the mechanisms by which it might function. At its N-terminus, a CARD/HSR (caspase-recruitment domain/homogenously staining region) domain is critical for proper Aire function and may serve as a site of Aire homodimerization, as CARD domain mutants fail to aggregate into characteristic nuclear speckles in an in vitro transfection system (45). Furthermore, studies analyzing the effects of transfected variants of Aire and target antigens in HeLa cells showed that cyclic-AMP response element binding protein (CBP), a transcriptional activator (46), colocalizes with Aire and amplifies the transcriptional activation activity of Aire in a CARD-dependent manner, suggesting that CBP may also interact with the CARD domain of Aire in vivo. These data are consistent with previously reported ability of CBP to interact with Aire and activate transcription (47). Aire also contains a SAND domain, which contributes to transcriptional activation in other proteins that bear this domain, such as DEAF-1 or the Sp100 protein family (48). Finally, Aire contains two plant homeodomain (PHD) fingers that contribute to transcriptional regulation by Aire, possibly through recognition of histones bearing silenced methylation profiles at the H3K4 lysine (40, 49). Mutations in the PHD domains are associated with reduced Aire activity and altered nuclear dimerization. In fact, mice harboring Aire mutations in their PHD domain that abolish H3K4 binding were found to have a global dampening in transcription of Aire-induced TSAs (50). Consistent with reduced thymic TSA expression, these mice also developed characteristic multiorgan autoimmunity.
Though several domains suggest a role in transcriptional activation, the mechanisms of Aire-mediated transcription likely depend on its interactions with other transcriptional complex proteins rather than direct DNA binding. In addition to its domains, Aire contains four LXXLL motifs that are often important for protein-protein interactions. Several Aire-interacting cofactors have been identified, but the relative importance of these cofactors in vivo has yet to be established. In addition to the previously mentioned CBP, positive transcription elongation factor b (P-TEFb) may play a role in Aire-mediated transcriptional activation. The proposed function of P-TEFb binding complements that of CBP; while CBP may help direct Aire to sites of repressed transcription, P-TEFb and Aire were shown to associate with the elongation phase of transcription, suggesting that Aire might stabilize this process (51). DNA-PK, a DNA repair enzyme, can also interact with and phosporylate Aire at regions outside of its previously described domains to promote in vitro transcriptional activation (52). Recent co-immunoprecipitation experiments conducted by Abramson et al. (53) during a systematic approach to identify new Aire-interacting proteins found that DNA-PK was the most reliable binding partner of Aire. As further support for the relevance of DNA-PK in vivo, Aire-dependent TSAs were reduced in the thymi of mice bearing the SCID mutation among the stromal cells of their thymi. Aire was suggested to increase the frequency of double-stranded breaks as part of its transcriptional initiation, thus necessitating the activity of DNA repair enzyme such as DNA-PK. These observed double-stranded breaks may also be associated with Aire’s proposed pro-apoptotic role (54). Additional binding partners for Aire were found by co-immunoprecipitation, and functions of many of them can be broadly assigned to the processes of pre-mRNA processing, transcription, chromatin binding, and nuclear export of mRNA, all of which could conceivably enhance the transcription of otherwise repressed TSAs (53). RNA interference was used to knockdown the function of these novel Aire-interacting proteins in vitro, and it was found that several of these proteins did in fact promote the transcriptional activity of Aire. Some of these targets were suggested to have a role in proper Aire nuclear localization. For example, knockdown of Ran binding protein 2 prevented nuclear localization of Aire, and it was instead found to reside in the nucleus. Thus, multiple binding partners of Aire have now been described, and several have been directly shown to influence transcriptional activation by Aire. While some in vivo relevance has been demonstrated by experiments with SCID thymi, for example, more work is warranted to clarify the in vivo relevance of these Aire-interacting factors and determine the mechanisms by which they assist Aire-directed TSA transcription in the thymus.
Aire expression is restricted to a mature subset of mTECs
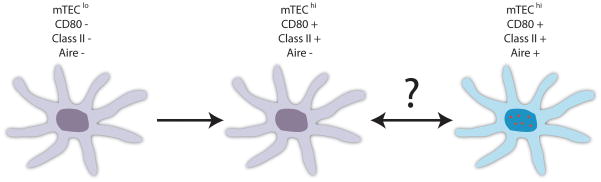
Aire was initially found to be present within mTECs, and it has since been determined that its expression is specific to a subset of mTECs expressing high levels of both CD80 and MHC class II (34) (Fig. 1). Further experiments used bromodeoxyuridine (BrdU) labeling to determine the proliferative status of mTEC subsets and found that while mTEClo (CD80 low, MHC class II low) and Aire−mTEChi (CD80 high, MHC class II high) populations both appeared to be dividing, Aire+ mTEChi cells were not (54). Instead, the Aire+ mTEChi cells were non-dividing and replaced by maturing Aire−mTECs as part of a cycle of continual apoptosis and replacement. Consistent with the above cycle, transfected Aire was found to promote apoptosis in vitro in the mTEC cell line 1C6. Furthermore, an experimental system in which Aire+ mTECs were indirectly depleted as a result of cyclosporine treatment showed that these cells repopulated the thymus at the expense of the mTEClo fraction, suggesting a precursor-product relationship between mTEClo and mTEChi cells (55). Fletcher et al. (55) also found that the mTEClo population had the highest proportion of MTS24+ cells, a marker present on immature epithelial cells in the fetal thymus that can give rise to a functioning thymus with cortical and medullary architecture in fetal thymic organ cultures (56). Nevertheless, this proposed relationship among mTECs has not been conclusively demonstrated and may be at odds with the finding that transcription factors associated with multipotency, such as Nanog and Oct4, seem to be expressed among mTECs in an Aire-dependent fashion (57). More recently, using an Aire-driven Cre transgene and floxed green fluorescence protein (GFP)-reporter to mark past and present Aire-expressing cells, Nishikawa et al. (58) found more GFP expression activated by their Cre system than in another transgenic mouse that expressed GFP controlled directly by the Aire promoter. If in fact Aire+ mTECs are able to give rise to other mTEC subsets, this may be consistent with the findings of Gray et al. (54), as the absence of proliferation does not strictly prohibit the differentiation of Aire+ mTECs into other lineages, and the apoptotic activity of transfected may not reflect its in vivo role.
Fig. 1. Aire+ mTECs may represent a terminally differentiated TEC population.
mTECs expressing low levels of CD80 and MHC class II (mTEClo) appear to differentiate into mTECs expressing high levels of both CD80 and MHC class II (mTEChi). Aire expression (represented by depiction of its characteristic histological nuclear speckling) is restricted to a non-dividing subset of the mTEChi subset.
Thymocyte-mTEC interactions are required for proper mTEC maturation and Aire expression
Many signaling components required for normal Aire expression have been identified, but the mechanism responsible for directly activating Aire transcription is unknown. Perturbations of NF-κB signaling can prevent appropriate differentiation of mTECs, resulting in reduced Aire representation within the thymus (59). For example, lymphotoxin β receptor (LTβR) knockout mice develop thymi with a markedly reduced mTEC population, and the lack of UEA-1+ mTECs is even more severe in mice bearing the aly/aly point mutation in NIK, a downstream signaling mediator of lymphotoxin activation of NF-κB (60). Ligands for LTβR were detected on thymocytes by LTβR-Fc staining, and mice lacking the ligands LTβ or LIGHT also showed defective development of the mTEC populations. While Aire itself was not specifically examined in these studies, the authors suggest a functional inhibition of Aire based on the development of autoantibodies against stomach, pancreas, and salivary gland in LTβR−/− mice. However, it was later suggested that these deficiencies were due to indirect effects on Aire through a broad inhibition of proper mTEC differentiation, and it was demonstrated that negative selection in LTβR−/− mice proceeded more or less normally in the OT-II-RIP-mOVA model of Aire-dependent negative selection (61).
More recent work has found a role for tumor necrosis factor (TNF) family receptor-ligand interactions in promoting NF-κB activation and proper mTEC maturation. Both CD40 and RANK were found to be important mediators of mTEC differentiation. The absence of either of the TNF signaling pathways mildly reduced mTEC populations within the thymus, and a combined deficiency of both receptors drastically reduced UEA-1+ events within the thymic medulla and nearly abolished thymic Aire expression (62, 63). RANK signaling, through interactions with RANK-L on lymphoid tissue inducer cells, seemed to be especially important during organogenesis of the thymus, as its absence resulted in marked delay in the appearance of mature mTECs. CD40, on the other hard, was postulated to have a more prominent role in maintaining mTEC populations in the maturating thymus in response to CD40L signaling coming from maturing thymocytes. The findings of these two studies, together with the data showing LTβR signaling promotes proper mTEC differentiation, suggest that thymocytes deliver multiple signals to enforce proper mTEC differentiation. In fact, it has recently been shown that a reduction in mTEC cellularity, including Aire+ mTECs, occurs in response to cyclosporine mediated thymocyte ablation (55).
Sin is a signaling component that has recently been identified as another critical regulator of mTEC differentiation. Within the thymic stroma, it is expressed most highly in mTECs, and its absence in thymic stroma leads to their improper maturation and subsequent deficiencies in Aire expression and immune tolerance (64). The decreased immune tolerance was suggested to result from defective negative selection, as OT-II TCR transgenic thymocytes in Sin−/− mice failed to be properly negatively selected in response to RIP-mOVA produced antigen. The authors also found that the global architecture of Sin−/− thymi was altered; there was an increased proportion of mTEC islets within the thymus, suggesting a failure to properly to fuse into a distinct thymic medulla as is normally seen in thymic development. Their final observation in support of a role of Sin in mTEC maturation was the observation that Sin deficiency preferentially impacted the more mature mTEChi population, as opposed to the mTEClo cells. This finding of a NF-κB-independent signaling pathway with relevance to mTEC differentiation should lead to a greater understanding of the control of mTEC development, and elucidation of the Sin signaling pathway may provide new insight into important signaling events between thymocytes and TEC populations. White et al. (65) have also provided fresh evidence that thymocytes themselves promote proper differentiation and function of mTECs, including Aire+ mTECs. Specifically, using an inducible ZAP70 TCR signaling component, the authors show that T-cell signaling and subsequent maturation of thymocytes is required for normal development of Aire+ mTECs and that lymphotoxin signaling is likely a component of this communication between mature thymocytes and mTECs. These findings echo previous work by Irla et al. (66) showing that the mTEC compartment fails to develop normally in the absence of CD4+ thymocytes.
While proper thymocyte interactions are important for regulating correct development of the thymic epithelium, Aire might also play a more direct role in its own regulation. An increase in the number of mTECs was noted in Aire knockout mice and has subsequently been confirmed with the use of a transgenic mouse in which GFP is driven under control of the Aire promoter (26, 67, Metzger et al., unpublished observations). Other experiments have suggested that Aire knockout mice may have thymi with mildly altered medullary architecture (57). An Aire-GFP knockin mouse, where GFP was inserted and interrupts the Aire locus, was used to show that Aire knockout mTECs themselves may have differences in their architectural characteristics in comparison to Aire-sufficient mTECs, including increased representation of GFP-expressing mTECs in the Aire knockout mouse (68). The mechanism behind the observed differences in the frequencies of Aire-expressing mTECs between Aire wildtype and knockout mice remains unknown. While Aire may directly interact with factors controlling mTEC differentiation and perhaps contribute to a negative-feedback back loop controlling the development of Aire-expressing mTECs, the observed changes in Aire-expressing mTEC frequencies could also be an indirect result of Aire’s control of tolerance. Aire knockout mice develop autoimmunity in peripheral tissues, and increased inflammatory cytokine levels in the circulation might influence thymic microarchitecture. In summary, Aire seems to play a role in its own regulation, although it is not clear whether this role is direct or indirect, and it remains to be determined how Aire itself collaborates with thymocyte-derived signals and other unknown factors to regulate levels of Aire expression within the thymus.
Dendritic cells contribute to presentation of Aire-driven antigens
The major antigen-presenting cells (APCs) within the thymus include mTECs, cTECs, and dendritic cells (DCs). cTECs reside in the cortex of the thymus and are primarily involved in providing positively selecting ligands to double positive thymocytes. Expression of many TSAs occurs only in Aire-expressing mTECs, and, while mTECs synthesize and display high levels of MHC class I and class II complexes, they are not necessarily responsible for directly interacting with and inducing deletion of self-reactive thymocytes. Rather, mTECs could serve as an antigen reservoir, with DCs playing the major role in directly presenting antigen to developing thymocytes. Using RIP-mOVA mice that express the model antigen ovalbumin (OVA) in the thymus, it was found that OVA-specific CD8+ T cells underwent thymic deletion even in the absence of class I expression among bone marrow-derived APCs (69). Conversely, the authors also found that CD4+ T cells with OVA-specificity required bone marrow-derived APCs for thymic deletion, suggesting that mTECs can directly mediate negative selection of CD8+ but not CD4+ single positive thymocytes.
More recently, antigen handoff from mTECs to DCs has been directly demonstrated. Using an MHC class I promoter to drive expression of OVA tagged with a nuclear localization sequence, the authors found that while mTECs transcribed the transgene most efficiently, the thymic DCs displayed the strongest stimulatory capacity, suggesting a handoff of antigen from mTECs to thymic DCs (70). The need for mTECs to transcribe the antigen and hand it off for maximum T-cell stimulation was confirmed by using bone marrow chimeras to restrict transcription of the antigen to the radioresistant mTECs. The finding was also extended to endogenous antigens, confirming the relative strengths of mTECs and DCs to transcribe and present antigen, respectively. Finally, Foxn1-GFP mice expressing GFP in thymic stroma were used to visualize the transfer of membrane bound protein from mTECs to DCs, and mismatched MHC bone marrow chimeras were used to suggest that MHC complexes could be directly transferred from radioresistant mTECs to hematopoietically derived DCs.
Aire-directed tissue-specific antigens appear to promote tolerance primarily by serving as ligands for negative selection
After discovering that Aire directs expression of self-antigens within the thymus and prevents autoimmunity, the next step was to determine how self-antigen expression was accomplishing disease prevention. Negative selection of thymocytes specific for Aire-directed TSAs and induction of regulatory T cells (Tregs) responsive to these self-antigens were two potential ways by which this might be accomplished. Tregs, the majority of which are generated within the thymus as natural Tregs, are required to control autoreactive T cells in the periphery; their absence leads to lethal autoimmunity (71, 72). Furthermore, Tregs seem to depend on the presence of self-antigen recognition for thymic development. TCR transgenic mice fail to develop Tregs when the absence of RAG recombinase prevents thymocytes from rearranging endogenous TCRs, which, when allowed to occur, can result in recognition of self-ligands through expression of a second TCR chain (73, 74). Furthermore, Tregs appear to have a higher propensity for recognition of self-ligands than conventional T cells (75). Also, their development is promoted by thymic expression of cognate ligand in a transgenic system using hemagglutinin as a model antigen (76), thus suggesting that the self-antigens whose transcription is directed by Aire in the thymus might also serve as Treg-inducing ligands.
To address the question of how Aire expression promotes tolerance, Hen egg lysozyme (HEL), a model antigen, was expressed in the thymus using the insulin promoter, and Aire’s affect on the selection of antigen-specific T cells was examined in HEL-specific TCR transgenic mice expressing the neo-self-antigen (77). In this system, negative selection of cognate T cells was dependent on Aire expression, but roughly equivalent numbers of CD25+ Tregs were generated regardless of the presence of Aire. Similar results were also seen in the ovalbumin model antigen system, in which OT-II OVA-specific T cells undergo Aire-dependent negative selection in response to the RIP-mOVA transgene (78). The authors also found that Foxp3+ CD25+ ratios in the thymus and periphery do not significantly change in Aire knockout mice with polyclonal TCR repertoires. Furthermore, co-transplantation of Aire wildtype and knockout thymi into athymic nude recipients still resulted in autoimmune disease, which could be prevented if a primary role of Aire wildtype thymi is to induce Tregs that dominantly maintain tolerance. Recently, experiments in mice with a Foxp3 promoter-driven GFP reporter and a restricted TCR repertoire suggested that TCR usage by Foxp3+ cells did not significantly differ between Aire-sufficient and -deficient mice, further supporting the observation that Aire-driven antigens do not play a large role in shaping the TCR repertoire (79).
Providing further evidence for the role of Aire in providing negatively selecting ligands, Liston et al. (80) found in two independent model antigen systems that Aire-induced transgenic antigens were less efficiently transcribed among Aire+/− mice than their wildtype counterparts, and that the heterozygous mice experienced an intermediate level of cognate T-cell negative selection in comparison to Aire wildtype and knockout mice. This dose-dependent effect of Aire on its target antigens was later confirmed by the creation of a mouse with a point mutation in the SAND domain of Aire. The mutant Aire caused a reduction in Aire activity in a dominant negative manner, which led to a milder form of autoimmunity than that observed in the complete Aire knockout mouse (81). The finding that the amount of negative selection occurring to Aire-driven antigens is proportional to the activity of Aire further support negative selection as the primary mechanism by which Aire promotes central tolerance.
Aire may also promote central tolerance through additional mechanisms
While the above work demonstrated that Aire is important for negative selection and that global defects among peripheral Treg populations do not result from a lack of Aire in the thymus, it has not been demonstrated that Aire-induced TSAs cannot contribute to Treg selection. On the contrary, transgenic expression of an Aire-driven neo-self antigen was shown to provide positively selecting ligands to support the formation of natural Tregs which would otherwise not encounter any positively selecting antigen in the thymus (82). More recently, Hinterberger et al. (83) provided evidence for a role of Aire-expressing mTECs in guiding natural Treg development in a polyclonal setting. Using a bacterial artificial chromosome approached to drive a transgene under control of the Aire promoter, the authors directed expression of a siRNA that inhibited expression of the MHC class II transactivator (CIITA), a master regulator of MHC class II expression. The resulting mice had a partial reduction in CIITA target expression among mature mTECs, and the strongest target knockdown was observed in the subset of mature mTECs that also express Aire, resulting in an Aire+ mTEC population that maintained its TSA transcription activity but had a reduced surface expression of MHC class II molecules. The result of this deficiency was an increased representation of CD4+ single positive thymocytes and an increased ratio of Foxp3+ cells among that fraction. Furthermore, using two systems of Aire-driven antigen and cognate TCR transgenes, negative selection was shown to be impeded by CIITA siRNA, while Treg induction was increased. Therefore, while this work is consistent with the well-known role of Aire+ mTECs in mediating negative selection, it also suggests that antigens presented by Aire+ mTECs affects the induction of Tregs, possibly by affecting their negative selection.
Providing further contrast with experimental systems which identify a role of Aire-driven antigen expression in providing negatively selecting ligands to cognate T cells, autoimmunity against α-fodrin, a ubiquitously expressed actin-binding protein, was observed in Aire-deficient mice, suggesting a lack of proper negative selection despite finding equivalent thymic expression of this antigen between wildtype and knockout mice (30). A similar result was obtained in response to RIP-mOVA directed antigen expression; reduced negative selection was observed in the Aire-deficient context despite a maintenance of detectable transcript levels (78). These findings suggest that, in addition to its role in promoting transcription of tissue-specific antigens (TSAs), Aire may control the process of antigen presentation at a post-transcriptional stage and may also affect negative selection through ligand-independent pathways. In fact, the work of Anderson et al. (78) in 2005 suggested a list of chemokine receptors and molecules involved in antigen processing events with different expression between Aire-sufficient and -deficient mTECs (78). Consistent with the above microarray data, non-transgenic mTECs from Aire wildtype and knockout mice were loaded with exogenous SIINFEKL peptide, and mTECs lacking Aire exhibited a reduced ability to stimulate proliferation among cognate OT-I CD8+ T cells.
Another suggestion that Aire’s role in promoting central tolerance involves more than driving negative selection comes from investigation of human APS1 patients. These patients with AIRE mutations appear to diverge from their mouse counterparts with regard to the importance of Aire in controlling the Treg pool, as two studies suggest that APS1 patients may in fact have defects within their populations of Tregs. In an analysis of APS1 patients and controls, it was found that proportion of circulating CD25+ T cells was the same between conditions, but that the APS1 patients’ CD25+ T cells had impaired suppressive ability in vitro (84). Looking specifically at Foxp3 expression, it was found that T cells from control patients had in fact twofold higher expression levels than samples from APS1 patients, and thus defective Treg induction in human APS1 patients was offered as an explanation for the more severe disease manifestations in patients with defective AIRE, in comparison with knockout mice. A later study also found a relative deficiency in Foxp3+ Tregs resulting from AIRE mutations in humans and further described this phenomenon as being due to an increased conversion of naive Tregs that had recently emigrated from the thymus to an activated phenotype, and corresponding inability of these Tregs from APS1 patients to fully upregulate Foxp3 during activation (85). The authors conclude that altered Treg ratios in humans are a result of a response to autoreactivity through altered Treg homeostasis in the periphery and not thymic selection events. However, due to the inherent limitations of studying human disease, these studies do not conclusively rule out altered induction of natural Tregs in the thymus.
Aire expression in peripheral lymphoid tissues can promote tolerance
While the role of Aire in contributing central tolerance is well established, its role in secondary lymphoid tissues has been less well known. Initial investigations of Aire expression showed that it was present at the transcript level in both lymph nodes and spleen (22, 26). This finding was challenging to confirm at the protein level (86), but the creation of a BAC transgenic mouse in which the Aire locus was used to drive GFP facilitated the identification of Aire protein in both lymph nodes and spleen in its characteristic nuclear speckles (67). The level of protein expression was consistent with differences in Aire transcript; its expression was noticeably lower in peripheral tissues, both by immunofluorescence and flow cytometry (67, Metzger et al., unpublished observations). Aire was reported to be undetectable in the periphery in mice on the C57BL/6 background (86), while the peripheral Aire observed by Gardner et al. (67) used mice on the autoimmune prone NOD background, suggesting that these different outcomes could be due differences in strain background. However, we have found equivalent activation of Aire-driven GFP among transgenic mice between mice on the C57BL/6 and NOD backgrounds and were able to detect nuclear Aire protein by immunofluorescence in C57BL/6 with the aid of the Adig GFP reporter (Metzger et al., unpublished observations).
The transgenic Aire reporter created by Gardner et al. (67) contained not only GFP for the purposes of identifying Aire-expressing cells but also a type I diabetes autoantigen, islet-specific glucose-6-phosphatase-related protein (IGRP) (67). This allowed for the determination of whether or not extrathymic Aire-expressing cells (eTACs) could present antigen to cognate T cells and whether or not such interactions had functional consequences. Adoptive transfer of cognate 8.3 T cells revealed that these cells did indeed proliferate in all secondary lymphoid organs in response to transgenic antigen expression, and that such proliferation ultimately led to their deletion. Furthermore, the ability of eTACs to directly interact with cognate T cells was demonstrated by two methods. First, bone marrow chimerism experiments in which class I was removed from hematopoietically derived cells, including DCs, demonstrated that direct cognate T cell-eTAC interactions could result in T-cell proliferation and deletion. Second, two-photon imaging experiments allowed direct visualization of specific, long-lasting contacts between cognate T cells and antigen-bearing eTACs. While not strictly ruling out the possibility that DCs or other hematopoietically derived components may also participate in peripheral Aire-driven antigen expression and/or presentation, these results show that presentation of endogenously derived antigen by radioresistant eTACs is sufficient to mediate deletional tolerance.
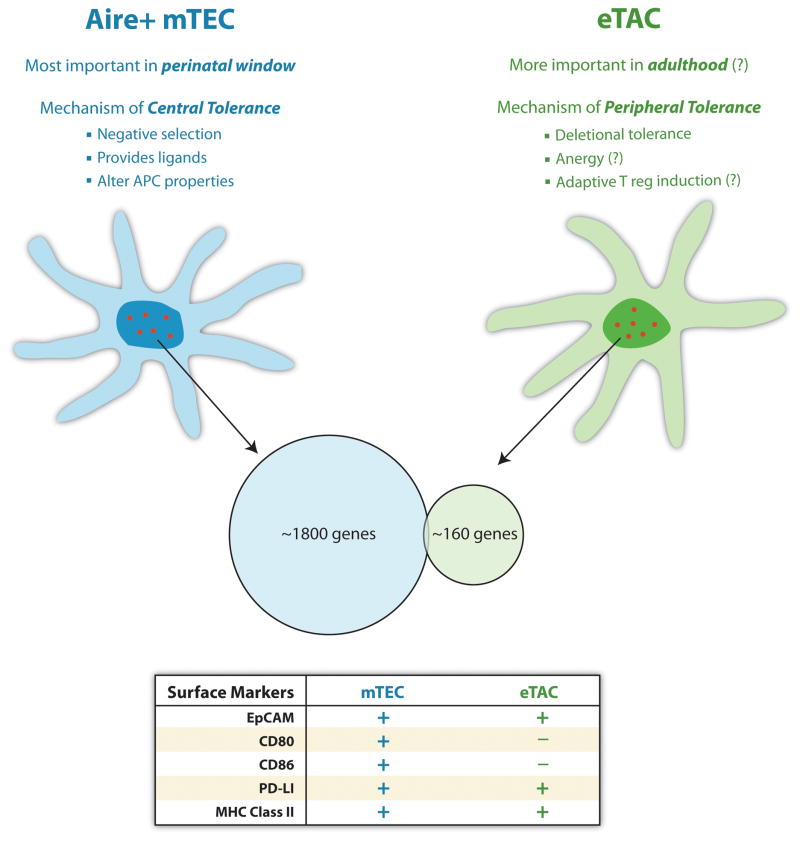
The potential ability of these eTACs to tolerize peripheral T cells through a process of deletional tolerance is analogous to the removal of self-reactive thymocytes through negative selection in the thymus. This prompted the question of which signaling pathways other than specific antigen signals might be relevant to the observed phenomenon, and expression of a panel of important costimulatory molecules by these Aire-expressing cells was analyzed. Flow cytometry revealed that, unlike their thymic counterparts, eTACs surprisingly lacked both CD80 and CD86, surface markers required to provide an activating costimulatory signal through the CD28 receptor on T cells undergoing activation. Like Aire+ mTECs, eTACs expressed both EpCAM, and the inhibitory PD-L1 costimulatory ligand. Finally, eTACs in the lymph nodes and spleen displayed high levels of ICOS-L, while mTECs did not, suggesting that this costimulatory ligand may play a role in guiding the outcome of T cell-eTAC interactions. Also, immunofluorescent staining was conducted to determine whether Aire was present among previously identified stromal or hematopoietic fractions of the secondary lymphoid tissues. eTACs were reported to be negative for the B cell marker B220, the fibroblastic reticular cell markers gp 38 and ER-TR7, and the DC marker CD11c (67). Interestingly, eTACs were found to have high levels of MHC class II, suggesting that they may also have a functional capacity to interact with CD4+ T cells, and, because of the relative restriction of class II expression to cells of the immune system, that eTACs are likely to have a fundamental role in contributing to immune regulation. Future experiments are needed to determine whether or not eTACs are capable of inducing tolerance in CD4+ T-cell populations, and, if so, whether such tolerance involves the processes of clonal deletion, anergy induction, and Treg induction.
Recently, Poliani et al. (87) have shown that similar to the findings in mouse, AIRE is detectable at the protein level in humans. In contrast to the mouse findings, AIRE was only detected in the lymph nodes and not the spleen. A closer examination of peripheral AIRE-expressing cell frequency also found that abdominal lymph nodes such as the mesenteric lymph nodes had more AIRE+ cells than the more superficial inguinal lymph nodes, for example, likely reflective slight differences in inductive signals between the different environments. Surface marker analyses showed some overlap with those expressed by mouse eTACs; human AIRE+ cells failed to stain for CD45 and also did not appear to express CD86. Also, while no direct data on tolerance induction by human peripheral AIRE+ cells is available, the authors did show that AIRE+ cells may be inherently tolerogenic through their synthesis of anti-inflammatory IL-10 and through the transcription of tissue-specific antigens such as insulin. Human AIRE+ cells stained positive for CD11c, a DC marker, and CD83, a marker for activation of dendritic and other cell types. In contrast, immunofluorescent analyses of Aire and Aire-driven GFP in mice have shown that mouse eTACs appear to lack CD11c expression (67). More investigation of peripheral human AIRE+ cells and their murine counterparts is warranted to clarify this discrepancy.
Peripheral Aire expression may complement the function of Aire in the thymus
In addition to determining the potential ability of eTACs to interact with cognate T cells, an analysis of the role of Aire itself in directing expression of tissue specific antigens in eTACs was conducted by sorting out eTAC-enriched fractions from Aire wildtype and knockout mice and comparing their transcripts by microarray analyses (67). Tissue-specific antigens were identified by being previously detected in 5 or fewer tissues analyzed during a whole organism genetic screen. The results were compared with those obtained earlier for mTECs, and it was found that there were roughly 160 genes differentially regulated by Aire, that these targets were enriched for TSAs, and that the identified targets were distinct from those which were turned on in the thymus by Aire (Fig. 2). A few of the most strongly Aire-regulated TSAs were confirmed to be differentially expressed between Aire wildtype and knockout eTACs using qPCR. This novel set of targets suggests that peripheral Aire may complement Aire’s role in the thymus, rather than simply reinforcing deletion to the same TSAs that are already expressed in the thymus.
Fig. 2. Central and peripheral Aire expression promotes tolerance through complementary mechanisms.
Central Aire expression is most important during a perinatal window, and promotes tolerance through negative selection against tissue-specific antigens within the thymus. Peripheral Aire expression is likely to promote tolerance throughout life, and may induce deletional tolerance, Foxp3+ regulatory T cells, or anergy through the expression of a distinct set of peripheral tissue-specific antigens. The lack of CD80 and CD86 on peripheral eTACs is likely to be an important component of their tolerance induction.
The ability of eTACs to induce deletional tolerance among cognate T cells and their expression of putative tissue-specific antigens imply that they may have a physiological role in maintaining peripheral tolerance, although the experiments required to formally make that determination have yet to be conducted. While the surface marker profile of eTACs suggests that their ability to induce tolerance may be due to the lack of CD80 and CD86 on their surface, this question should also be investigated more rigorously. Also, another outstanding question is how inflammation would affect the tolerogenic properties of eTACs, and how the cells themselves would respond to inflammatory stimuli in terms of homeostatic expansion or contraction. DCs are known to upregulate CD80 and CD86 in response to inflammatory stimuli, and it would be interesting to see whether the lack of CD80 and CD86 on eTACs is an inherent property or whether they too would upregulate these molecules in response to inflammation (88). To address the second question, experiments conducted to identify eTACs in mice on the NOD or C57BL/6 background have not found significant differences in eTAC representation, suggesting that eTACs as a whole are not overly sensitive to the relative differences in systemic inflammation between these strains.
While signals regulating peripheral Aire are unknown, recent data has suggested temporal regulation of Aire. Poliani et al. (87) found that AIRE seemed to be absent from fetal lymph nodes and was only present in postnatal tissues. The finding that peripheral AIRE expression does not appear in humans until after birth contrasts with the appearance of Aire-expressing cells within the fetal thymus. Moreover, Poliani et al.(87) also described an increase in AIRE+ peripheral cells throughout middle age, although the finding did not reach statistical significance. Likewise, preliminary data also suggest that eTACs increase in absolute number with the age of mice (Metzger et al., unpublished data). In contrast, the prevention of autoimmunity by Aire expression in the thymus seems to be most important during a perinatal window leading up to the age of weaning or during conditions in which perinatal-like settings are experimentally induced through ablation of peripheral lymphocytes (31). Therefore, peripheral Aire may represent a distinct method of establishing tolerance that becomes more important in aging organisms as the burden of tolerance shifts from the thymus into the periphery.
Aire-independent peripheral tissue-specific antigens may also contribute to peripheral tolerance
In addition to the presence of Aire-driven peripheral TSAs as a possible mechanism of peripheral tolerance, other Aire-independent antigens with tolerogenic properties have been identified. Lee et al. (89) found an unexpected activation of their iFABP-OVA transgene in all peripheral lymph nodes investigated. Adoptively transferred cognate OT-I T cells experienced deletional tolerance in the lymph nodes but not spleens of iFABP-OVA mice and failed to cause inflammatory lesions in the intestine, the standard site of iFABP transcription. This was likely to due deletional tolerance, as initial proliferation of transferred OT-I T cells gave way to a later contraction among the same population. Further experiments were done to characterize the lymph node cells responsible for transcribing the iFABP driven transgene, and it iFABP-OVA was found to be activated specifically in CD45−, radioresistant stromal cells in the lymph nodes but not spleen. Furthermore, activation did not occur among CD11c+ cells, consistent with the lack of OVA transcription among cells of the hematopoietic lineage. Transcripts of other tissue-specific antigens were found among the lymph node stroma, including GAD67, and Aire transcript was also detected among this population, suggesting that peripheral Aire could be playing a role in driving these antigens. In fact, UEA-1 staining, which marks a subset of mTEC in the thymus including those that express Aire, was found among the lymph node stroma, along with the fibroblastic reticular cell marker gp38. These cells were found to preferentially interact with cognate OT-I T cells following their transfer when the stroma expressed cognate antigen.
While the work by Gardner et al. showed that Aire-expressing cells are gp38−, this finding was confirmed by later work using the iFABP-OVA mouse. These later experiments found that OVA was most highly transcribed by gp38+CD31− fibroblastic reticular cells (FRC), and not by the gp38−CD31− fraction of lymph node stroma, where Aire transcript was most enriched (90). Another piece of information demonstrating that eTACs and the iFABP-transcribing cells are distinct entities is the fact that Aire expression is detected at roughly equivalent levels in the both the lymph nodes and spleen, but iFABP activation appears to occur exclusively in lymph nodes and not in the spleen. Fletcher et al. (90) also found that the FRC fraction of the lymph node was able to stimulate OT-I T cell proliferation ex vivo when it contained the iFABP-OVA transgene, and furthermore, that this proliferation was subject to regulation by TLR3 signaling. Treatment of transgenic mice with a TLR3 ligand prior to analysis of their FRCs resulted in decreased expression of OVA and subsequently milder activation of cognate OT-1 T cells. Interestingly, no particular lymph node stromal fraction seemed to be uniquely responsible for the presence of the analyzed antigens. While iFABP-OVA activation occurs in cells that are distinct from the eTACs described by Gardner et al. (67), these independent systems suggest that tissue-specific antigen transcription in the periphery capable of inducing clonal deletion may be an underappreciated phenomenon.
Tyrosinase is another antigen found to be naturally expressed in lymph nodes and, similarly to iFABP-OVA, can trigger deletional tolerance among cognate CD8+ T cells (91). Furthermore, tyrosinase presentation by stromal elements within the lymph nodes was shown to be sufficient to mediate this effect. Two groups found that gp38+CD31+ lymphatic endothelial vessels were uniquely responsible for transcribing the tyrosinase antigen, at least in comparison with FRCs, blood endothelium, and the gp38−CD31− fraction of the lymph node fraction, of which eTACs are a rare constituent (90, 92). Therefore, the findings of the tyrosinase system complement those of the iFABP-OVA system; Aire-independent stromal cells can transcribe antigens capable of mediating deletional tolerance, at least through an MHC class I pathway. Further work on peripheral tissue-specific antigens is warranted to determine whether these antigens are in fact representative of a larger transcriptional profile within the lymph node with the function of inactivating cognate T cells or whether these represent proteins whose transcription within lymph nodes serves a previously unappreciated function related to the function of the antigen itself. The applicability of these findings to MHC class II antigens should also be investigated, as it is unclear whether peripheral tissue-specific antigen transcription contributes to tolerance among CD4+ T cells.
The possibility that peripheral antigen induction may occur through a transcriptional activator other than Aire must be considered. In fact, evidence was recently put forth that Deaf1, a structural cousin of Aire through their shared presence of a SAND domain, may contribute to peripheral tissue-specific antigen activation in lymph nodes (93). Transcripts of genes representing autoantigens in type I diabetes such as insulin were detected in the pancreatic lymph node of NOD mice, and their expression level was found to fluctuate in parallel with those of Deaf1. Furthermore, a comparison of Deaf1 knockout to wildtype mice by microarray revealed that while Deaf1 deficiency does not affect peripheral Aire transcription, it does appear to affect transcription of tissue-specific antigens, and Deaf1 knockout mice develop eye-reactive autoantibodies on the BALB/c background. Also, the authors found a splice variant of Deaf1 that seemed to be particularly enriched among NOD mice and suggested that the presence of this non-functional isoform, through sequestration of the standard Deaf1 isoform in the cytoplasm, could contribute to a reduction in Deaf1-controlled antigen expression. As a result of reduced peripheral tolerance induction to Deaf1-controlled antigens, the development of type I diabetes in the NOD mouse may be favored. Furthermore, the human equivalent of this variant Deaf1 isoform was shown to be 20-fold higher in pancreatic lymph node sections from type I diabetics in comparison with control tissues, suggesting a possible maintenance of tolerance in humans by the standard DEAF1 isoform. An independent investigation found that Deaf1 was expressed in all lymph node stromal subsets investigated, and was most highly expressed among FRCs (90), suggesting that this putative transcriptional activator might represent another set of tissue-specific antigens. In tandem with Aire-directed antigens in eTACs and the class I antigens tyrosinase and iFABP in lymphatic endothelium and FRCs, respectively, DEAF-1 may promote physiologically relevant peripheral TSA expression.
Concluding remarks
Since the initial identification of the AIRE gene in 1997, significant progress has been made in understanding its critical contribution to processes of central tolerance. However, future work is required to expand our knowledge of Aire and its function, and critical questions still remain. The mechanisms by which Aire specifically targets TSAs for transcription have not been sufficiently identified. While the binding of an Aire-containing complex to histones bearing transcriptional repression marks may account for some specificity, the role of known and novel Aire-interacting components should be further defined. Also, while many pathways responsible for the differentiation of mTEC populations as whole have been identified, the critical signals for directly initiating Aire expression have yet to be discovered. Furthermore, while much work has been done to describe the role of Aire in promoting central tolerance, its contribution to peripheral tolerance necessitates further investigation; specifically, it will be important to understand which mechanisms contribute to peripheral tolerance induction by Aire, and, of these mechanisms, which may be most physiologically relevant. Finally, it remains to be seen whether or not the striking ability of Aire to control immune responses endogenously might also be harnessed for therapeutic purposes. The ability of central and peripheral Aire-expressing cells to induce clonal deletion of autoreactive T cells in mouse models suggests that these cells may be useful to purge humans of autoreactive T cells.
Acknowledgments
This work was supported by the JDRF, NIH, Helmsley Foundation, and Burroughs Wellcome Fund. We also thank Una Fan for creating the figure illustrations and Mickie Cheng and Eileen McMahon for helpful comments on this manuscript. The authors have no conflict of interest to disclose.
References
- 1.Washburn T, et al. Notch activity influences the αβ versus γδ T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 2.Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F. Inactivation of Notch1 impairs VDJβ rearrangement and allows pre-TCR-independent survival of early αβ lineage thymocytes. Immunity. 2002;16:869–879. doi: 10.1016/s1074-7613(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg EV, Dionne CJ. Lineage plasticity and commitment in T-cell development. Immunol Rev. 2002;187:96–115. doi: 10.1034/j.1600-065x.2002.18709.x. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α in development of αβ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 5.Irving BA, Alt FW, Killeen N. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science. 1998;280:905–908. doi: 10.1126/science.280.5365.905. [DOI] [PubMed] [Google Scholar]
- 6.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 7.Negishi I, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi N, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–60. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 9.Siggs OM, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–926. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu LY, Tan TX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009;206:2527–2541. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 12.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 13.Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J Immunol. 1999;162:5078–5084. [PubMed] [Google Scholar]
- 14.Hanahan D. Peripheral-antigen-expressing cells in the thymic medulla: factors in self-tolerance and autoimmunity. Curr Opin Immunol. 1998;10:656–662. doi: 10.1016/s0952-7915(98)80085-x. [DOI] [PubMed] [Google Scholar]
- 15.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nature Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 16.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 17.Peterson P, Peltonen L. Autoimmune polyendocrinopathy syndrome type 1 (APS1) and AIRE gene: New views on molecular basis of autoimmunity. J Autoimmun. 2005;25:49–55. doi: 10.1016/j.jaut.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Waterfield M, Anderson MS. Clues to immune tolerance: the monogenic autoimmune syndromes. Ann NY Acad Sci. 2010 doi: 10.1111/j.1749-6632.2010.05818.x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaltonen J. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. [Google Scholar]
- 20.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. [Google Scholar]
- 21.Bjorses P, et al. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66:378–392. doi: 10.1086/302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heino M, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in the thymus medulla. Biochem Biophys Res Commun. 1999;257:821–825. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 23.Zuklys S, Balciunaite G, Agarwal A, Fasler-Kan E, Palmer E, Hollander GA. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) J Immunol. 2000;165:1976–1983. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 24.Heino M, et al. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur J Immunol. 2000;30:1884–1893. doi: 10.1002/1521-4141(200007)30:7<1884::AID-IMMU1884>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Kogawa K, et al. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80:195–198. doi: 10.1016/s0165-2478(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 26.Anderson MS, et al. Projection of an Immunological Self Shadow Within the Thymus by the Aire Protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey C, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Gen. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 28.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niki S, et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J Clin Invest. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 31.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semkowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2001;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 33.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 34.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeVoss J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2736. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeVoss JJ, et al. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular sydrome type 1. J Immunol. 2008;181:4072–4079. doi: 10.4049/jimmunol.181.6.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeVoss JJ, et al. An autoimmune response to odorant binding protein 1a is associated with dry eye in the Aire-deficient mouse. J Immunol. 2010;184:4236–4246. doi: 10.4049/jimmunol.0902434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shum AK, et al. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2010;1:1–9. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan QG, et al. The autoimmune regulator directly controls the expression of genes critical for thymic epithelial function. J Immunol. 2007;178:7173–7180. doi: 10.4049/jimmunol.178.11.7173. [DOI] [PubMed] [Google Scholar]
- 40.Org T, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–376. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar PG, et al. The autoimmune regulator (AIRE) is a DNA-binding protein. J Biol Chem. 2001;276:41357–41364. doi: 10.1074/jbc.M104898200. [DOI] [PubMed] [Google Scholar]
- 42.Purohit S, Kumar PG, Laloraya M, She JX. Mapping DNA-binding domains of the autoimmune regulator protein. Biochem Biophys Res Commun. 2005;327:939–944. doi: 10.1016/j.bbrc.2004.12.093. [DOI] [PubMed] [Google Scholar]
- 43.Johnnidis JB, Venanzi ES, Taxman DJ, Ting JP, Benoist CO, Mathis DJ. Chromosomal clustering of genes controlled by the Aire transcription factor. Proc Natl Acad Sci USA. 2005;102:7233–7238. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci USA. 2008;105:657–662. doi: 10.1073/pnas.0707486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson BJ, et al. AIRE’s CARD revealed, a new structure for central tolerance provokes transcriptional plasticity. J Biol Chem. 2008;283:1723–1731. doi: 10.1074/jbc.M707211200. [DOI] [PubMed] [Google Scholar]
- 46.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 47.Pitkanen J, et al. Cooperative activation of transcription by autoimmune regulator AIRE and CBP. Biochem Biophys Res Commun. 2005;333:944–953. doi: 10.1016/j.bbrc.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 48.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 49.Musco G, Peterson P. PHD finger of autoimmune regulator. Epigenetics. 2008;3:310–314. doi: 10.4161/epi.3.6.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koh AS, Kingston RE, Benoist C, Mathis D. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci USA. 2010;107:13016–13021. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oven I, Brdickova N, Kohoetek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liiv I, et al. DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochim Biophys Acta. 2008;1783:74–83. doi: 10.1016/j.bbamcr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 54.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher AL, et al. Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol. 2009;183:823–831. doi: 10.4049/jimmunol.0900225. [DOI] [PubMed] [Google Scholar]
- 56.Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic microenvironment by MTS24+ thymic epithelial cells. Nat Immunol. 2002;3:635–642. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- 57.Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol. 2007;178:3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 58.Nishikawa Y, et al. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med. 2010;207:963–971. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derbinski J, Kyewski B. Linking signalling pathways, thymic stroma integrity and autoimmunity. Trends Immunol. 2005;26:503–506. doi: 10.1016/j.it.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic Medullary Epithelial Cell Differentiation, Thymocyte Emigration, and the Control of Autoimmunity Require Lympho-Epithelial Cross Talk via LTβR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venanzi ES, Gray DH, Benoist C, Mathis D. Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J Immunol. 2007;179:5693–5700. doi: 10.4049/jimmunol.179.9.5693. [DOI] [PubMed] [Google Scholar]
- 62.Akiyama T, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Hikosaka Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Danzl NM, Donlin LT, Alexandropoulos K. Regulation of medullary thymic epithelial cell differentiation and function by the signaling protein Sin. J Exp Med. 2010;207:999–1013. doi: 10.1084/jem.20092384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White AJ, et al. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol. 2010;185:4769–4776. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irla M, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Gardner JM, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yano M, et al. Aire controls the differentiation program of thymic epithelial cells in the medullar for the establishment of self-tolerance. J Exp Med. 2008;205:2827–2838. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 72.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 73.Itoh M, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally angeric and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 74.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the Peripheral Self by Naturally Arising CD25+ CD4+ T Cell Receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 77.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CG. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 78.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Daniely D, Kern J, Cebula A, Ignatowicz L. Diversity of TCRs on natural Foxp3+ T cells in mice lacking Aire expression. J Immunol. 2010;184:6865–6873. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liston A, et al. Gene dosage-limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su MA, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118:1712–1726. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self-antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 83.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat Immunol. 2010;11:512–521. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 84.Kekalainen E, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 85.Laasko SM, et al. Regulatory T cell defect in APECED patients in associated with loss of naïve FOXP3+ precursors and impaired activated population. J Autoimmunity. 2010;35:351–357. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Hubert FX, et al. A specific anti-Aire antibody reveals Aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 87.Poliani PL, et al. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am J Path. 2010;176:1104–1112. doi: 10.2353/ajpath.2010.090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 89.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 90.Fletcher AL, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional Self-Tolerance to a Melanocyte/Melanoma Antigen Derived from Tyrosinase Is Mediated by a Radio-Resistant Cell in Peripheral and Mesenteric Lymph Nodes. J Immunol. 2008;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 92.Cohen JN, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yip L, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10:1026–1033. doi: 10.1038/ni.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]