Abstract
Purpose
To describe the retinal imaging findings in the index patient with Heimler syndrome (OMIM #234580).
Design
Non-interventional case report.
Methods
A 29-year-old woman with Heimler syndrome developed bilateral vision loss. Fluorescein angiography (FA), fundus autofluorescence (FAF), spectral domain optical coherence tomography (SD-OCT) and electroretinography (ERG) were performed to assess the retinal anatomy and function.
Results
FA showed mottling of the retinal pigment epithelium (RPE) in the posterior pole and periphery of the retina. FAF revealed hyper and hypoautofluorescent dots corresponding to the RPE mottling observed on FA. SD-OCT documented loss of the inner/outer segments boundary, and RPE thinning. ERG testing excluded generalized rod-cone dysfunction.
Conclusion
We report an adult-onset macular dystrophy in one of the previously reported patients with Heimler syndrome and hypothesize that this syndrome is probably an expression of a ciliopathy.
Keywords: Amelogenesis imperfecta, Ciliopathy, Deafness, Heimler syndrome, Nail abnormalities, Retina
INTRODUCTION
In 1991 Heimler described a new syndrome in a pair of siblings born to healthy and non-consanguineous parents.1 They had enamel hypoplasia, nail malformations and deafness. In 1999 the third patient2 a 12-year-old female, was found to have unilateral deafness, nail abnormalities and amelogenesis imperfecta of her teeth. Two other patients, 8- and 15-year-old female siblings, were reported in 20033 and presented with the same combination of hearing loss, nail abnormalities and amelogenesis imperfecta. The last two cases of Heimler syndrome were described in 2006.4 We describe the previously unknown association of a macular dystrophy in one of the index patients with Heimler syndrome (patient #2).1
CASE REPORT
The index case is now a 29-year-old woman with diminished visual acuity in both eyes for 1 year. She was born in the 39th week of an uneventful pregnancy. The patient had normal audiological tests until age 3 when she developed profound bilateral sensorineural hearing loss. At age 8, she was noted to have dental anomalies. Additional examination revealed Beau’s lines on her toe nails which represent transverse depressions of the nail plates and leukonychia of the fingernails. She was otherwise in good health with no other pathological findings and her intellectual development had been normal.
One year before presentation the patient’s ophthalmic examination was unremarkable. Both eyes were emmetropic and had a normal pupillary reaction without a relative afferent papillary defect. At presentation the visual acuity was 20/200 in the right eye and 20/40 in the left eye. Ocular motility, slit-lamp biomicroscopy results, and intraocular pressures were unremarkable. Fundus examination of both eyes showed extensive “salt-and-pepper”-like mottling of the retinal pigment epithelium (RPE) involving the posterior pole and extending beyond the temporal vascular arcades (Figure 1). The optic disc appeared to be normal in both eyes.
FIGURE 1.

(A and B). Color fundus photographs of both eyes showing extensive “salt and pepper”-like mottling of the retinal pigment epithelium of the posterior pole and beyond the temporal vascular arcades.
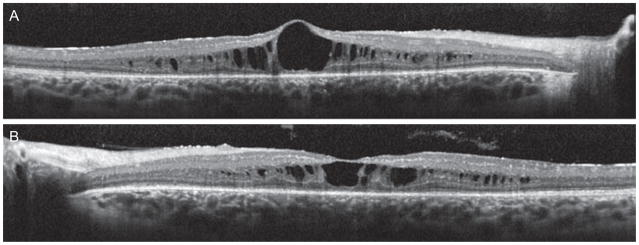
Fluorescein angiography (FA) showed normal retinal circulation. There was mottled fluorescence throughout the posterior pole as well as non-leaking cystoid macular edema bilaterally. Fundus autofluorescence (FAF) showed hyper- and hypo-autofluorescent dots in the posterior pole and beyond the temporal vascular arcades sparing the fovea in both eyes (Figure 2). Spectral domain optical coherence tomography (SD-OCT) revealed multiple inner retinal cystoid spaces, thinning of the RPE, and loss of the inner segment/outer segment (IS/OS) junction in both eyes (Figure 3).
FIGURE 2.
(A and B). Fundus autofluorescence showed hyper- and hypo-autofluorescent dots in the posterior pole and beyond the temporal vascular arcades sparing the fovea in both eyes.
FIGURE 3.
(A and B). Optical coherence tomography revealed multiple inner retinal cysts, thinning of the retinal pigment epithelium and disorganization of the inner segment/outer segment junction in both eyes.
Full-field ERGs were performed with silver-impregnated fiber electrodes (DTL; Diagnosys LLC, Littleton, MA) using extended testing protocols incorporating the International Society for Clinical Electrophysiology of Vision (ISCEV) standards. The minimum protocol incorporates the rod-specific and standard bright flash ERGs, both recorded after a minimum of 20 min dark adaptation. Following 10 min of light adaptation, the photopic 30 Hz flicker cone and transient photopic cone ERGs were recorded. The scotopic rod specific ERG b-wave amplitudes (normal range: 266.1 ± 170.2 μV), maximal ERG b-wave amplitudes (normal range: 335 ± 273.2 μV), transient photopic ERG (normal range: 178.8 ± 116.9 μV), photopic 30 Hz flicker ERG (normal range: 131 ± 82 μV), and the pattern ERG (p50 normal >2 μV) showed tracings within average (Figure 4). The multifocal ERG was abnormal with decreased sensitivity in the macular area in both eyes.
FIGURE 4.
Full-field electroretinography (ERG) was normal with tracings within average in both eyes. The multifocal ERG showed decreased sensitivty in the macular area in both eyes.
Because of hearing loss and “salt and pepper”-like retinopathy changes, laboratory tests for rubella (antibody tests IgM and IgG by Western blot), syphilis (VDRL and FTA-ABS), and Lyme disease (antibody tests IgM and IgG by Western blot) were ordered. However, the serology results for such diseases were negative. She was subsequently treated with acetazolamide 250 mg daily for her macular edema and her vision remained stable as of the last follow up (2 months after the treatment initiation).
DISCUSSION
Our case presented with the systemic features seen in Heimler syndrome, namely sensorineural hearing loss, nail abnormalities and enamel hypoplasia with normal primary dentition. The patient’s retinal fundus examination revealed a “salt-and-pepper” mottling of the RPE in the posterior pole and beyond the vascular arcades with sparing of the fovea and the retinal periphery in both eyes. FAF showed hyper- and hypo-autofluorescent dots at the same topography, and SD-OCT revealed cystoid macular edema, thinning of the RPE, and loss of the IS/OS junction in both eyes. The normal full-field ERG excluded generalized retinal dysfunction. The patient’s normal pattern ERG, nerve fiber layer within normal limits by OCT, and normal looking of optic discs reviewed previously by several seasoned clinicians indicate that there is absence of optic neuropathy. These features along with the absence of prior visual complaints and retinal abnormalities documented by several seasoned clinicians suggest an adult-onset macular dystrophy. The patient’s ERG findings are not consistent with conditions that cause generalized retinal dysfunction such as Usher syndrome, retinitis pigmentosa and enhanced S-cone syndrome. Mitochondrial diseases, such as maternal inherited diabetes and deafness (MIDD), mitochondrial myopathy, lactic acidosis, and stroke-like episodes (MELAS), and Kearns-Sayre syndrome share “salt-and-pepper” pigmentary retinopathy and normal or mildly abnormal full-field ERG. However, the lack of extra-retinal features typically associated with these diseases such as diabetes, cardiomyopathy and encephalomyopathy make them improbable diagnoses in our case. Additionally, there is no electronegative ERG or implicit time delays on photopic 30 Hz flicker which makes inflammatory disease unlikely.
Heimler syndrome was hypothesized to be the consequence of a single gene involving derivatives of the ectoderm because the defects described have a common embryological source in the ectodermal tissue.1–4 As retina also originates from the ectodermal tissue, the development of retinal degeneration could possibly coincide with deafnesss, nail abnormalities and amelogenesis imperfecta. The specific combination of IS/OS junction absence, hearing loss, nail abnormalities, and enamel hypoplasia seen in our patient could classify Heimler syndrome as a ciliopathy that is caused by dysfunction of the primary cilium with different phenotypes and overlapping disease expression.5,6 Primary cilia take action as sensory organelles and as coordinators of a variety of signal transduction pathways. Ciliary defects could result in different human diseases that may reflect the involvement of cilia in such diverse sensory modalities and signaling pathways.7,8 The wingless protein (WNT) and sonic hedgehog protein (SHH) represent the signaling pathways proven to be controlled by cilia.9 Since SHH and WNT pathways are implicated in the formation of ectodermal elements such as the retina,10 teeth,11,12 nails13 and auditory system14 which are involved in our case, SHH and WNT signaling defects should be considered. Abnormal responses to SHH have been linked to ciliopathies associated with teeth and nail dysplasia such as Ellis-van Creveld syndrome.15 Similarly, the enamel hypoplasia and nail malformations observed in Heimler syndrome could also be due to a SHH signaling malfunction. However, cilia are also related to other pathways such as platelet-derived growth factor and fibroblast growth factor16 and the genetic defect in Heimler syndrome could be a result of other specific cilia protein abnormality. The categorization of a specific signaling pathway or protein defect in Heimler syndrome is complex given that most ciliopathies are genetically heterogeneous disorders and mutations of the same gene can cause widely varying phenotypes.
We report a unique case of Heimler syndrome associated with adult-onset macular dystrophy. Based on the presented case and a possible ciliopathy involvement we would recommend that patients presenting with the combination of hearing loss, nail abnormalities and amelogenesis imperfect undergo regular retinal examinations.
Acknowledgments
Funding/Support: This work was supported by the LuEsther T. Mertz Retina Research Center, Manhattan Eye, Ear, and Throat Hospital, The Macula Foundation Inc., Foundation Fighting Blindness, Hirschl Trust, Schneeweiss Stem Cell Fund, Joel Hoffmann Foundation, Crowley Research Fund, Jahnigen/Hartford/American Geriatrics Society, Eye Surgery Fund, Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness (RPB), and EY018213.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Heimler A, Fox JE, Hershey JE, Crespi P. Sensorineural hearing loss, enamel hypoplasia, and nail abnormalities in sibs. Am J Med Genet. 1991;39:192–195. doi: 10.1002/ajmg.1320390214. [DOI] [PubMed] [Google Scholar]
- Tischkowitz M, Clenaghan C, Davies S, et al. Amelogenesis imperfect, sensorineural hearing loss, and Beau’s lines: a second case report of Heimler’s syndrome. J Med Genet. 1999;36:941–943. [PMC free article] [PubMed] [Google Scholar]
- Pollack C, Floy M, Say B. Sensorineural hearing loss and enamel hypoplasia with subtle nail findings: another family with Heimler’s syndrome. Clin Dysmorphol. 2003;12:55–58. doi: 10.1097/00019605-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Ong KR, Visram S, McKaig S, Brueton LA. Sensorineural deafness, enamel abnormalities and nail abnormalities. Eur J Med Genet. 2006;49:187–193. doi: 10.1016/j.ejmg.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ramamurthy V, Cayouettte M. Development and disease of the photoreceptor cilium. Clin Genet. 2009;76:137–145. doi: 10.1111/j.1399-0004.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151:263–280. doi: 10.1002/ajmg.c.30227. [DOI] [PubMed] [Google Scholar]
- Toriello HV, Parisi MA. Cilia and the ciliopathies: an introduction. Am J Med Genet C Semin Med Genet. 2009;151:261–262. doi: 10.1002/ajmg.c.30230. [DOI] [PubMed] [Google Scholar]
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopa-thies. Am J Med Genet C Semin Med Genet. 2009;151:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- D’Angelo A, Franco B. The dynamic cilium in human disease. Pathogenetics. 2009;2:3. doi: 10.1186/1755-8417-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Satterfield R, Muhunthan K, et al. Age-related cone abnormalities in zebrafish with genetic lesions in sonic hedgehog. Invest Ophthalmol Vis Sci. 2008;49:4631–4640. doi: 10.1167/iovs.07-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, et al. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, et al. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 2009;136:897–903. doi: 10.1242/dev.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW. Development and evolution of the mammalian limb: adaptive diversification of nails, hooves and claws. Evol Dev. 2001;3:355–363. doi: 10.1046/j.1525-142x.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, et al. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet. 2009;151C:341–351. doi: 10.1002/ajmg.c.30226. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]