Abstract
Introduction
We describe longitudinal trends in surgeons’ adoption of laparoscopic radical nephrectomy (LRN) and assess whether use of this technique is associated with specific surgeon and/or practice setting characteristics.
Methods and Materials
We used SEER-Medicare data to identify patients who underwent LRN or open radical nephrectomy (ORN) as treatment for early-stage kidney cancer from 1995 through 2005. We assessed long-term trends in surgeon adoption of LRN and fit multilevel logistic regression models to estimate the association between surgeon or practice setting characteristics and patient receipt of LRN.
Results
The annual proportion of patients receiving LRN increased from 1.4% in 1995 to 44.9% in 2005 (p<0.001). Among patients treated by recent medical school graduates (i.e., year of graduation ≥ 1991), the likelihood of receiving LRN was more than 2-fold higher for those with urologists practicing at NCI-designated Cancer Centers (OR 2.37, 95% CI 1.11–5.06) or in urban settings (OR 2.92, 95% CI 1.10–7.75). Patients treated by urologists who graduated before 1991 and had a major academic affiliation (OR 1.78, 95% CI 1.34–2.38) or who were in group practices (OR 1.99, 95% CI 1.51–2.63) were significantly more likely to be treated with a minimally-invasive surgical approach, compared to non-academic and solo practices respectively.
Conclusions
Urologists’ adoption of LRN increased progressively from 1995 through 2005 and has been influenced by their proximity to training, academic affiliation, and rural/urban status. These data may clarify residual barriers to surgeons’ adoption of LRN and potentially other innovative surgical therapies.
Keywords: renal cell carcinoma, laparoscopy, practice patterns, SEER-Medicare, technology adoption
Introduction
Based on easier convalescence with equivalent cancer control, laparoscopy emerged as an alternative standard of care for patients undergoing radical nephrectomy for RCC.1–4 However, despite potential advantages relative to open surgery, urologists have slowly adopted LRN and—at least through the early 21st century—ORN remained the predominant surgical therapy for patients with localized kidney cancer.5–7
In an effort to explain the protracted dissemination of LRN, we have demonstrated previously that, for many patients with kidney cancer, the surgery performed depends more on the treating urologist than on characteristics of the patient or tumor.8 Accordingly, further characterization of which urologists are performing this technique may inform efforts to increase utilization of laparoscopy in the treatment of kidney cancer patients. In particular, it remains unknown whether, and to what extent, use of laparoscopy depends on surgeon characteristics (e.g., time since medical school graduation) and/or the environment in which he or she practices (e.g., rural versus urban setting).
We hypothesized that use of LRN (vs ORN) is more frequent among patients treated by more recently-trained surgeons. At the same time, however, we posit that the effect of surgeon characteristics (including era of training) varies across practice environments. To test these hypotheses, we used SEER-Medicare data to describe longitudinal trends in surgeons’ adoption of LRN and to assess whether use of this technique is associated with specific surgeon and/or practice setting characteristics. Once available, these data may clarify barriers and facilitators to surgeons’ adoption of LRN and potentially other innovative surgical therapies.
Methods
Data Source
We used data from the NCI’s SEER Program and the Centers for Medicare and Medicaid Services (Medicare) to identify patients diagnosed with incident kidney cancer from 1995 through 2005. SEER is a population-based registry that collects data regarding incidence, treatment, and mortality. The demographics, cancer incidence, and mortality trends in the SEER registries are representative of the entire United States population.9 The Medicare Program provides primary health insurance for 97% of the United States population aged ≥ 65 years, and linkage to Medicare claims is achieved for >90% of SEER cases over age 65.10
Cohort identification and assignment of surgical procedures
We identified 15,744 patients diagnosed with non-urothelial, non-metastatic kidney cancer from 1995 through 2005. For this group of patients, we searched inpatient and physician claims to identify kidney cancer-specific diagnosis and procedure codes (list of codes available from authors upon request). We applied a validated claims-based algorithm to assign each patient to one of four procedures: LRN, ORN, open partial nephrectomy, or laparoscopic partial nephrectomy.11 We limited our analytic cohort to patients who underwent unilateral LRN or ORN as primary treatment for early-stage kidney cancer (n=10,917).
Patient-level Covariates
For each patient in the analytic cohort, we used SEER data to determine demographic and cancer-specific information. Based on patient-level zip-codes, we assigned patients to one of three socioeconomic strata.12 We measured pre-existing comorbidity by using a modified Charlson Index based on claims submitted during the 12 months prior to surgery.13, 14
Surgeon-level Covariates
To identify the treating surgeon for each patient, we used Unique Physician Identifier Numbers (UPIN) available in the Medicare database. We linked the list of surgeon UPINs to the American Medical Association (AMA) Physician Masterfile, which contains demographic, educational, and certification information for over one million residents and physicians in the United States. We then determined surgeon age, gender, year of medical school graduation, and practice size. We assigned each surgeon a rural-urban designation based on an established classification scheme using the zip code of the primary office address.15 We determined academic affiliation (major, minor, or none) based on the methods described by Shahinian et al.16 Finally, we determined each surgeon’s association with a National Cancer Institute (NCI) Cancer Center based on whether they performed at least one radical nephrectomy at a hospital carrying this designation.
Statistical Analysis
The primary outcome for this study was patient receipt of LRN. We used chi-square tests to evaluate the association between surgery received and patient- and surgeon-level covariates. We used Mantel-Haenszel chi-square tests to evaluate longitudinal trends in the patient receipt of LRN. In order to characterize longitudinal trends in surgeon adoption of LRN, we determined the proportion of surgeons in the SEER-Medicare dataset who performed at least one LRN in each calendar year. We defined “new adopters” as surgeons who performed their first LRN in a given calendar year.
Next, we fit multilevel logistic regression models to estimate the association between surgeon or practice setting characteristics and patient receipt of LRN. The analyses were stratified by year of medical school graduation (i.e., before vs after 1991), because we felt urologists graduating after 1991 would be completing residency as laparoscopy was introduced into training programs. Our model included a surgeon-specific random-effect and surgeon and patient level covariates as fixed effects. Because our primary research question focused on adoption of laparoscopy by urologists, we excluded from multilevel analyses those patients treated by non-urologists (n=806, 7% of cohort). Surgeon-level fixed-effects included gender, nephrectomy case volume, and practice setting measures (i.e., practice type, rural/urban status, NCI cancer center affiliation). We collapsed practice type into three levels: solo/two-person practices, group (>2 person) practices, and HMO/hospital/medical school/other practices. We collapsed rural-urban designation into two categories: rural/micropolitan and urban settings. We selected patient-level fixed effects (i.e., age, year of surgery, comorbidity, and tumor size) a priori based on clinical experience and prior empirical work. Statistical testing was 2-sided, and carried out at 5% significance, using SAS version 9.1. We obtained an exemption for this study from our Institutional Review Board.
Results
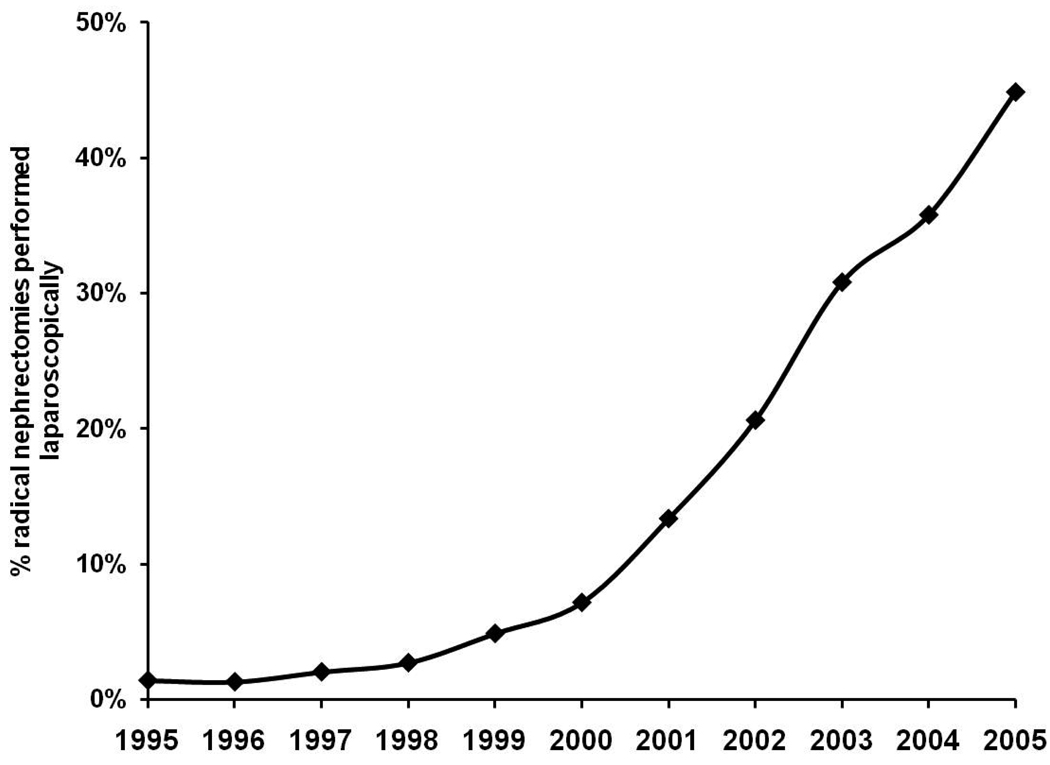
We identified 2,229 surgeons who performed 10,917 radical nephrectomies (median 3 cases, range 1–84) from 1995–2005. We noted significant differences in receipt of LRN according to race/ethnicity (12% Hispanic vs 20% Caucasian, p<0.001), socioeconomic status (18% low vs 24% high, p<0.001), tumor size (12% tumors > 7cm vs 24% tumors ≤ 4cm, P<0.001) and tumor histology (35% chromophobe vs 31% papillary vs 19% clear cell, p<0.001). The annual proportion of patients treated with LRN increased significantly from 1.4% in 1995 to 44.9% in 2005 (Figure 1, p<0.001).
Figure 1.
Longitudinal trends in patient receipt of LRN
Within our analytic cohort, 749 surgeons performed 2,188 LRNs (median 2 cases, range 1–55) and 2,039 surgeons performed 8,729 ORNs (median 3 cases, range 1–31). Laparoscopy was used more frequently for patients treated by recent medical school graduates (49% graduating after 1991 vs 23% graduating between 1981–1990, p<0.001), surgeons in larger practices (30% medical school vs 23% HMO vs 10% solo/two-person, p<0.001), urban settings (21% urban vs 13% rural, p<0.001), and those with major academic (26% vs 17% no afilliation, p<0.001) and cancer center affiliations (28% vs 19% no affiliation, p<0.001).
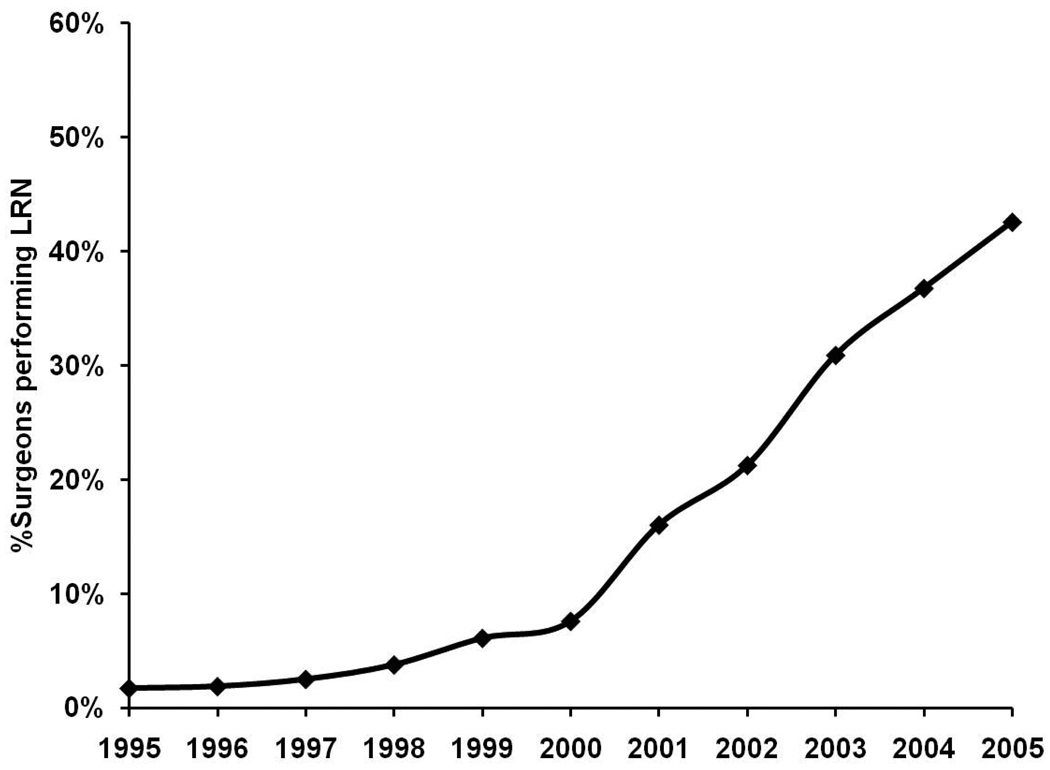
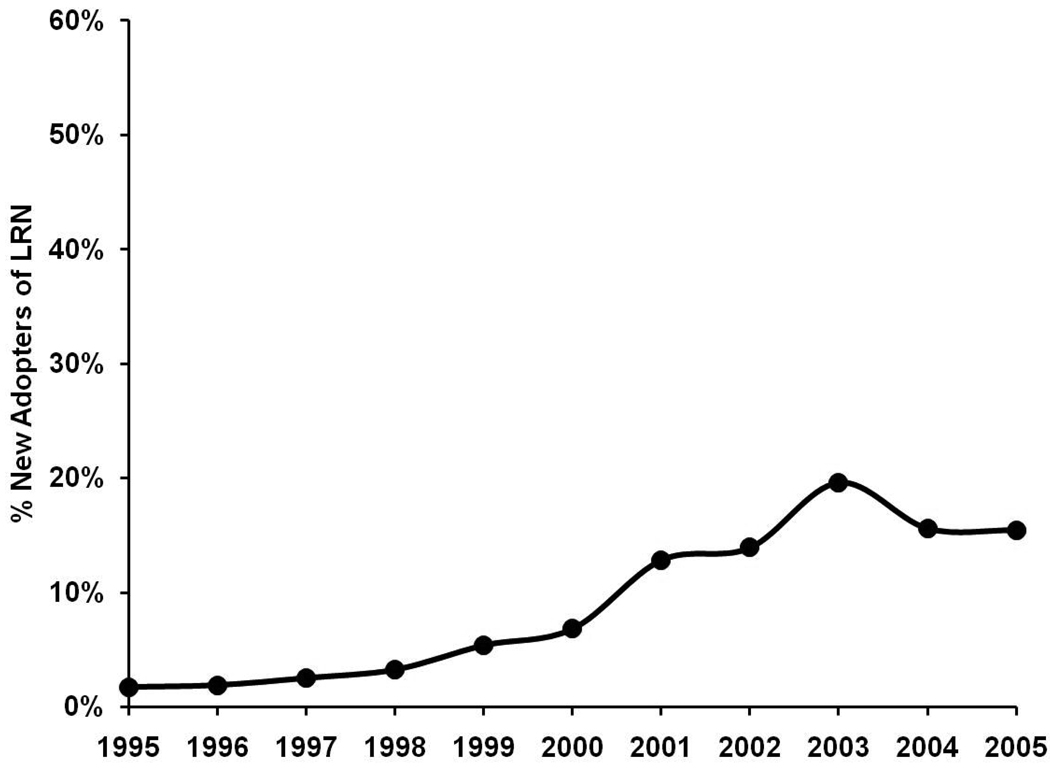
Figures 2 and 3 present trends in surgeon-level adoption of LRN from 1995–2005. The proportion of surgeons performing at least one LRN in a given year increased gradually from 1.7% in 1995 to 7.6% in 2000. After 2000, this proportion of surgeons increased more rapidly to its peak of 42.6% in 2005 (Figure 2). The proportion of surgeons that were “new adopters” of LRN increased from 1.7% to 6.8% from 1995 through 2000. After 2000, the annual proportion of new adopters increased to 12.8% in 2001 and peaked at 19.6% in 2003 (Figure 3).
Figure 2.
Longitudinal trend in performance of LRN by surgeons.
Figure 3.
Longitudinal trend in new adoption of LRN by surgeons.
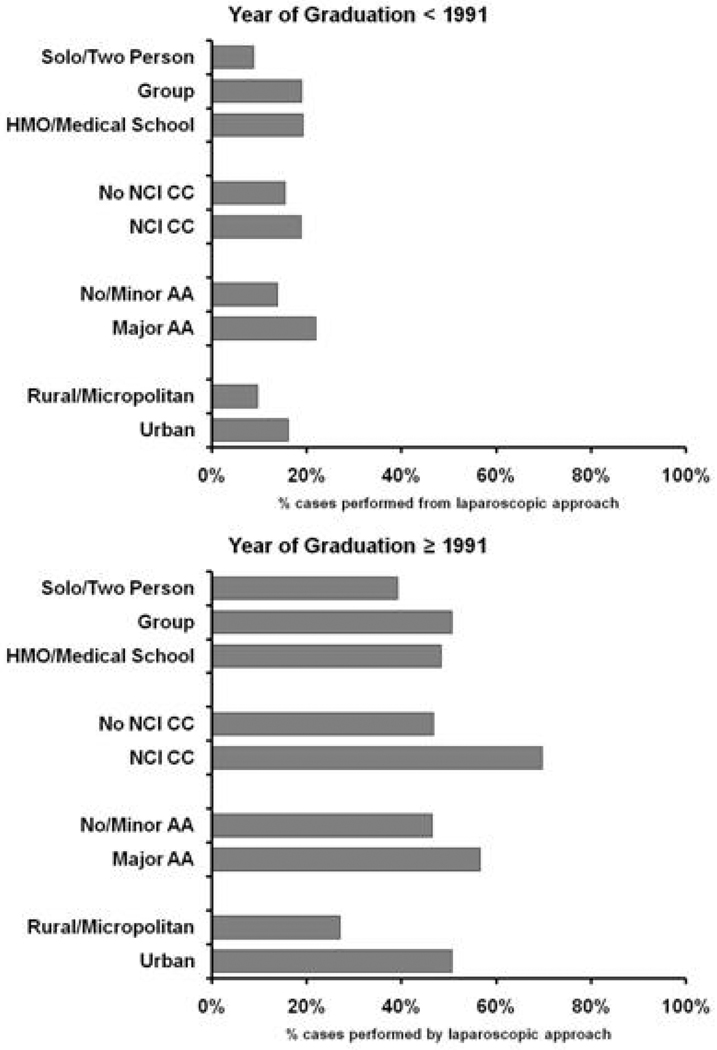
Figure 4 presents unadjusted proportions of patients who received LRN in each practice environment, stratified by surgeon year of medical school graduation. Overall, the use of laparoscopy was substantively greater for patients treated by more recent medical school graduates. Among patients treated by remote medical school graduates, similar proportions received LRN whether or not they were treated in a cancer center (18% vs 15%, p=0.08); however, a greater proportion of those whose surgeons had a major academic affiliation, urban practice setting, or larger practice size were treated with LRN (all p<0.001). Among those treated by recent graduates (i.e., ≥1991), a greater proportion of patients treated by surgeons affiliated with NCI cancer centers (70% vs 47%, p<0.001), urban settings (51% vs 27%, p<0.001) and major academic centers (57% vs 46%, p<0.001) received LRN; however, among this group, there were no significant differences in patient receipt of LRN based on surgeon practice size (p=0.08).
Figure 4.
Surgeon and practice setting characteristics and use of LRN.
According to our multilevel analyses, patients treated by recently trained urologists were significantly more likely to receive LRN if the urologist worked in an urban environment (OR 2.92, 95% CI 1.10–7.75) or in affiliation with an NCI Cancer Center (OR 2.37, 95% CI 1.11–5.06). We observed no difference in use of LRN across practice types (i.e. solo or two-person versus group practice versus HMO/hospital/medical school based practice) or degrees of academic affiliation (all p>0.05). Conversely, patients treated by more remote medical school graduates (i.e. graduated before 1991) were more likely to be treated with a minimally invasive approach if the urologist had a major academic affiliation (OR 1.78, 95% CI 1.34–2.38). Moreover, those whose urologists were working in a group (OR 1.99, 95% CI 1.51–2.63) or HMO/hospital/medical school based practice (OR 1.91, 95% CI 1.28–2.84) were more likely to be treated with LRN, compared to those with urologists in solo/two-person practices.
Discussion
Among a population-based cohort of Medicare beneficiaries with early-stage kidney cancer, the proportion of patients treated with LRN increased significantly from 1995 through 2005. While utilization of this minimally-invasive technique increased gradually during the earlier years studied, the pace of adoption accelerated after 2000, such that nearly 45% of patients treated in 2005 underwent a laparoscopic approach to radical nephrectomy. This trend reflects that, by 2001, between 10 and 20% of urologists had performed at least one LRN; this threshold is generally considered a “tipping point” after which innovative technology is rapidly and widely adopted.17,18 While these data are encouraging, estimates from referral centers suggest that 70–80% of patients undergoing radical nephrectomy may be treated safely with a laparoscopic approach,19,20 suggesting that—at a population-level—there remain opportunities for greater application of less-invasive surgical techniques. Consistent with the literature for other innovative surgical therapies, our results also suggest that disparities between population-level utilization of laparoscopic kidney cancer surgery and that reported at certain tertiary centers may be explained, at least partially, by characteristics of both the treating surgeons and their practice environments.21, 22
Notably, our findings counter the notion that uniform and equitable adoption of LRN will occur spontaneously as training in this minimally-invasive technique becomes more commonplace. Although patients treated by more recent medical school (and therefore residency) graduates were significantly more likely to undergo LRN, we observed that—even among those treated by more recent trainees—use of laparoscopy varies substantially based on a surgeon’s affiliation with academic hospitals and/or NCI-designated Cancer Centers. Similar findings have been described for the treatment of other urological cancers, including the utilization of continent reconstruction among patients undergoing radical cystectomy for bladder cancer and use of androgen deprivation therapy among patients with localized prostate cancer.16,23 For patients with kidney cancer, this finding may reflect unique resources available in these practice settings including, for instance, higher kidney cancer case volumes, fellowship-trained surgeons, and/or multiple urologists with an interest and/or training in laparoscopic surgical techniques.
We also observed that patients treated by urologists in small or rural practice settings were less likely to be treated with LRN. This finding most likely reflects differential access to ‘informational externalities’ that help promote adoption of new technologies. For instance, the greater social isolation and lower case volumes associated with a rural practice environment may limit urologist’s ability to observe and or ‘trial’ laparoscopy, two fundamental steps in the adoption of new surgical technologies.18, 24 Social isolation may also restrict exposure to more advanced aspects of this technology (e.g., camera systems) that facilitate use and adoption of laparoscopy. Furthermore, initial studies that compared LRN to ORN demonstrated longer operative times with a minimally invasive approach. This would likely decrease overall case volume for practice groups who initially adopting LRN.4 Rural and smaller practices may have less institutional resources to absorb the costs associated with this.
In addition to the implications for patients with kidney cancer, our findings are illustrative with respect to the adoption of surgical technology more generally. Barkun et al. recently proposed a conceptual framework (i.e., the IDEAL paradigm) for adoption of surgical technology that includes four stages, starting with innovation and development and proceeding to critical assessment and long-term monitoring.25 Likewise, others have described contextual factors that influence technology adoption, including patient demand, professional impact (i.e., financial and social costs), commercial promotion, and magnitude of perceived clinical benefit.18 Based on the diffusion curves presented herein, urologists’ adoption of LRN appears to have entered the late assessment stage, with a near-majority of surgeons performing the procedure. Accordingly, there is a need for further research aimed at assessing once again the comparative effectiveness (e.g., length-of-stay), long-term survival outcomes, and safety (e.g., complications) of ORN versus LRN at a population-level.25 Moreover, while it is assumed that patients with kidney cancer are requesting minimally-invasive surgery and that urologists recognize its clinical benefits, empirical support for these assumptions requires further study of surgeon and patient attitudes and preferences related to surgical treatment of patients with kidney cancer.25
This study has several limitations. First, our results may not be generalizable to a non-Medicare eligible population. Second, claims data cannot be used to appraise several important determinants of optimal application of LRN including, for instance, attitudes and preferences related to laparoscopy, clinical contraindications and relative patient benefit from a nephron-sparing surgical technique in lieu of a minimally-invasive radical nephrectomy. Third, this analysis focuses on radical nephrectomy; therefore our findings do not necessarily reflect the influence of a surgeon’s practice environment on the adoption of other new technologies or techniques (e.g., robotic-assisted surgery and partial nephrectomy). Fourth, as we used Medicare claims, we may be underestimating the operative volume of individual surgeons treating patients younger than 65 years old, and the proportion of “new adopters” among the surgeons we identified in our results. Fifth, we did not have data to assess actual training in laparoscopy, and we assumed that urologists who graduated after 1991 were more likely to be exposed to such techniques in residency. Finally, we could measure only a limited set of surgeon and practice environment characteristics (most of which are structural in nature); as such, there is a need for future studies that assess the degree to which difficult-to-measure barriers such as technical complexity and/or an absence of adopters in their local communities influence urologists’ uptake of this and other new surgical therapies. These limitations notwithstanding, we believe that our findings represent an important step towards better understanding how surgeon and practice setting characteristics influence the longitudinal adoption of LRN.
Conclusions
Among Medicare beneficiaries with early-stage kidney cancer, the proportion of patients treated with LRN (vs ORN) increased significantly from 1995 through 2005. Adoption of LRN has been influenced by characteristics of both the treating surgeons and their practice environments, including proximity to training, academic affiliation, and urban/rural status. Taken together, these data confirm that a urologist’s propensity to perform LRN depends not only on when he or she completed training, but also where he or she establishes practice. Lessons learned through this work may inform future efforts to predict and address potential barriers and facilitators to urologists’ adoption yet-to-be discovered surgical innovations.
Table 1.
Patient and tumor characteristics by type of surgical therapy
| Total | LRN | ORN | p* | |
|---|---|---|---|---|
| n | n (%) | n (%) | ||
| Overall | 10,917 | 2188 (20) | 8729 (80) | |
| PATIENT CHARACTERISTICS | ||||
| Age (years) | 0.129+ | |||
| 65–69 | 2806 | 570 (20) | 2236 (80) | |
| 70–74 | 3109 | 556 (18) | 2553 (82) | |
| 75–79 | 2766 | 608 (22) | 2158 (78) | |
| 80–84 | 1614 | 312 (14) | 1302 (86) | |
| >84 | 622 | 142 (23) | 480 (77) | |
| Race/ethnicity | <0.001 | |||
| Caucasian | 9014 | 1850 (20) | 7164 (80) | |
| African-American | 796 | 159 (20) | 637 (80) | |
| Hispanic | 691 | 85 (12) | 606 (88) | |
| Other | 416 | 94 (23) | 322 (77) | |
| Gender | 0.006 | |||
| Male | 6264 | 1198 (19) | 5066 (81) | |
| Female | 4653 | 990 (21) | 3663 (79) | |
| Married | 0.227 | |||
| Yes | 6823 | 1343 (20) | 5480 (80) | |
| No | 4094 | 845 (21) | 3249 (79) | |
| Socioeconomic status (tertiles)a | <0.001+ | |||
| High | 3725 | 879 (24) | 2846 (76) | |
| Intermediate | 3643 | 668 (18) | 2975 (82) | |
| Low | 3535 | 641 (18) | 2894 (82) | |
| Charlson comorbidity indexb | 0.131+ | |||
| 0 | 6252 | 1240 (20) | 5012 (80) | |
| 1 | 3598 | 537 (15) | 3061 (85) | |
| ≥2 | 1710 | 366 (21) | 1344 (79) | |
| TUMOR CHARACTERISTICS | ||||
| Tumor sizec | <0.001+ | |||
| ≤ 4 cm | 4099 | 1001 (24) | 3098 (76) | |
| 4–7 cm | 3914 | 840 (21) | 3074 (78) | |
| > 7 cm | 2645 | 314 (12) | 2331 (88) | |
| Tumor histology | <0.001 | |||
| Clear cell | 9352 | 1772 (19) | 7580 (81) | |
| Papillary | 681 | 211 (31) | 470 (69) | |
| Chromophobe | 310 | 110 (35) | 200 (65) | |
| Other | 564 | 95 (17) | 479 (83) | |
| Tumor graded | 0.558 | |||
| Well-differentiated | 1372 | 300 (22) | 1072 (78) | |
| Moderately-differentiated | 3924 | 846 (22) | 3078 (78) | |
| Poorly-differentiated | 1827 | 412 (23) | 1415 (77) | |
| Undifferentiated | 448 | 82 (18) | 366 (82) | |
| Tumor lateralitye | 0.167 | |||
| Left | 5412 | 1114 (21) | 4298 (79) | |
| Right | 5501 | 1074 (19) | 4427 (81) |
p-value for association between patient or tumor characteristics and type of surgical therapy;
Mantel-Haenszel Chi-Square test used for ordinal data;
data missing in 14 cases;
data missing in 349 cases;
data missing in 259 cases;
data missing in 3346 cases;
data missing in 4 cases
Table 2.
Surgeon and practice environment characteristics by type of surgical therapy
| Total | LRN | ORN | p* | |
|---|---|---|---|---|
| n | n (%) | n (%) | ||
| Surgeon agea | <0.001+ | |||
| 30–39 | 2194 | 791 (36) | 1403 (64) | |
| 40–49 | 3569 | 754 (21) | 2815 (79) | |
| 50–59 | 3265 | 430 (13) | 2835 (87) | |
| ≥60 | 1434 | 155 (11) | 1279 (89) | |
| Surgeon gendera | 0.730 | |||
| Male | 10246 | 2084 (20) | 8162 (80) | |
| Female | 216 | 46 (21) | 170 (79) | |
| Surgeon specialty | <0.001 | |||
| Urology | 10111 | 2082 (21) | 8029 (79) | |
| Other surgery | 254 | 23 (9) | 231 (91) | |
| Other/unspecified | 552 | 83 (15) | 469 (85) | |
| Year of medical school graduationa | <0.001+ | |||
| <1970 | 2525 | 187 (7) | 2338 (93) | |
| 1971–1980 | 3190 | 440 (14) | 2750 (86) | |
| 1981–1990 | 3228 | 760 (23) | 2468 (77) | |
| 1991–2005 | 1519 | 743 (49) | 776 (51) | |
| Practice sizea | <0.001 | |||
| Solo/Two-person | 2984 | 294 (10) | 2690 (90) | |
| Group | 5784 | 1385 (24) | 4399 (76) | |
| HMO/Hospital | 505 | 115 (23) | 390 (77) | |
| Medical School | 331 | 98 (30) | 233 (70) | |
| Other/unclassified | 858 | 238 (28) | 620 (72) | |
| Rural/urban statusb | <0.001 | |||
| Urban | 9680 | 2034 (21) | 7646 (79) | |
| Micropolitan | 652 | 78 (12) | 574 (88) | |
| Rural | 129 | 17 (13) | 112 (87) | |
| Academic Affiliationc | <0.001 | |||
| Major | 2565 | 679 (26) | 1886 (74) | |
| Minor | 4032 | 773 (19) | 3259 (81) | |
| None | 3896 | 677 (17) | 3219 (83) | |
| NCI Cancer Center Affiliationd | <0.001 | |||
| Yes | 831 | 230 (28) | 601 (72) | |
| No | 10059 | 1956 (19) | 8103 (81) |
p-value for association between provider characteristics and kidney cancer surgery type;
data missing in 455 cases;
Mantel-Haenszel Chi-Square test used for ordinal data;
data missing in 456 cases;
data missing in 424 cases;
data missing from 27 cases
Table 3.
Surgeon and practice setting factors associated with LRN#
| Adjusted OR | 95%CI | |
|---|---|---|
| Year of medical school graduation <1991 | ||
| Academic affiliation | ||
| Minor or no affiliation* | 1.00 | |
| Major affiliation | 1.78 | 1.34–2.38 |
| Practice type | ||
| Solo/two-person* | 1.00 | |
| Group | 1.99 | 1.51–2.63 |
| HMO/hospital/Medical School/Others | 1.91 | 1.28–2.84 |
| Rural/urban status | ||
| Rural/Micropolitan* | 1.00 | |
| Urban | 1.18 | 0.75–1.85 |
| NCI Cancer Center Status | ||
| No* | 1.00 | |
| Yes | 0.72 | 0.45–1.17 |
| Year of medical school graduation ≥ 1991 | ||
| Academic affiliation | ||
| Minor or no affiliation* | 1.00 | |
| Major affiliation | 1.05 | 0.61–1.79 |
| Practice type | ||
| Solo/two-person* | 1.00 | |
| Group | 1.01 | 0.44–2.31 |
| HMO/hospital/Medical School/Others | 0.97 | 0.40–2.32 |
| Rural/urban status | ||
| Rural/Micropolitan* | 1.00 | |
| Urban | 2.92 | 1.10–7.75 |
| NCI Cancer Center | ||
| No* | 1.00 | |
| Yes | 2.37 | 1.11–5.06 |
Model adjusted for patient age, year of surgery, patient comorbidity, tumor size, provider gender, and provider case volume, with statistically significant interactions of year of medical school graduation with academic affiliation and NCI cancer center;
Odds ratios and 95% CI for covariate effect on receipt of LRN versus ORN;
Denotes reference group
Acknowledgments
This research was supported by the National Institutes of Health Intramural Research Program (NIH-N02-CP-11004) and Training in Clinical Investigation in Urology grant (NIH-T32-DK007782); and the Edwin Beer Research Fellowship in Urology and Urology-Related Fields from the New York Academy of Medicine to D.C.M.
Footnotes
Detailed tables displaying results are available at http://www.med.umich.edu/urology/research/ManuscriptAppendices/index.html
REFERENCES
- 1.Wolf JS, Jr, Merion RM, Leichtman AB, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation. 2001;72:284. doi: 10.1097/00007890-200107270-00021. [DOI] [PubMed] [Google Scholar]
- 2.Simforoosh N, Basiri A, Tabibi A, et al. Comparison of laparoscopic and open donor nephrectomy: a randomized controlled trial. BJU Int. 2005;95:851. doi: 10.1111/j.1464-410X.2005.05415.x. [DOI] [PubMed] [Google Scholar]
- 3.Oyen O, Andersen M, Mathisen L, et al. Laparoscopic versus open living-donor nephrectomy: experiences from a prospective, randomized, single-center study focusing on donor safety. Transplantation. 2005;79:1236. doi: 10.1097/01.tp.0000161669.49416.ba. [DOI] [PubMed] [Google Scholar]
- 4.Dunn MD, Portis AJ, Shalhav AL, et al. Laparoscopic versus open radical nephrectomy: a 9-year experience. J Urol. 2000;164:1153. [PubMed] [Google Scholar]
- 5.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 6.Miller DC, Taub DA, Dunn RL, et al. Laparoscopy for renal cell carcinoma: diffusion versus regionalization? J Urol. 2006;176:1102. doi: 10.1016/j.juro.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 7.Hollenbeck BK, Taub DA, Miller DC, et al. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Miller DC, Saigal CS, Banerjee M, et al. Diffusion of surgical innovation among patients with kidney cancer. Cancer. 2008;112:1708. doi: 10.1002/cncr.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117. [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:3. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 11.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9:92. doi: 10.1186/1472-6963-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Morrill R, Cromartie J, Hart G. Metropolitan, urban, and rural commuting areas: toward a better depiction of the United States settlement system. Urban Geography. 1999;20:727. [Google Scholar]
- 16.Shahinian VB, Kuo YF, Freeman JL, et al. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol. 2007;25:5359. doi: 10.1200/JCO.2006.09.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladwell M. The tipping point: how little things can make a big difference. 1st Ed. New York: Little, Brown, and Company; 2000. [Google Scholar]
- 18.Wilson CB. Adoption of new surgical technology. BMJ. 2006;332:112. doi: 10.1136/bmj.332.7533.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richstone L, Kavoussi L. Barriers to the diffusion of advanced surgical techniques. Cancer. 2008;112:1646. doi: 10.1002/cncr.23369. [DOI] [PubMed] [Google Scholar]
- 20.Bhayani SB, Clayman RV, Sundaram CP, et al. Surgical treatment of renal neoplasia: evolving toward a laparoscopic standard of care. Urology. 2003;62:821. doi: 10.1016/s0090-4295(03)00670-8. [DOI] [PubMed] [Google Scholar]
- 21.Hawley ST, Hofer TP, Janz NK, et al. Correlates of between-surgeon variation in breast cancer treatments. Med Care. 2006;44:609. doi: 10.1097/01.mlr.0000215893.01968.f1. [DOI] [PubMed] [Google Scholar]
- 22.Vanderveen KA, Paterniti DA, Kravitz RL, et al. Diffusion of surgical techniques in early stage breast cancer: variables related to adoption and implementation of sentinel lymph node biopsy. Ann Surg Oncol. 2007;14:1662. doi: 10.1245/s10434-006-9336-x. [DOI] [PubMed] [Google Scholar]
- 23.Gore JL, Saigal CS, Hanley JM, et al. Variations in reconstruction after radical cystectomy. Cancer. 2006;107:729. doi: 10.1002/cncr.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers EM. Diffusion of innovations. 4th Ed. New York: Free Press; 1995. Chapter 1. [Google Scholar]
- 25.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]