Abstract
Gout is an ancient disease that still plagues us. Its pathogenic culprit, uric acid crystal deposition in tissues, is a strong inflammatory stimulant. In recent years, the mechanisms through which uric acid crystals promote inflammation have been a subject of increasing interest among rheumatologists and immunologists. Uric acid has been identified as an endogenous adjuvant that drives immune responses in the absence of microbial stimulation. Because uric acid is a ubiquitous metabolite that is produced in high quantities upon cellular injury, the ramifications of its effects may be considerable in health and in disease. Uric acid crystals also have been shown to trigger interleukin-1β–mediated inflammation via activation of the NOD-like receptor protein (NLRP)3 inflammasome, a multimolecular complex whose activation appears to be central to many pathological inflammatory conditions. In this article, we review the possible mechanisms of uric acid–mediated inflammation and offer some historical perspectives on what has been learned about the complex effects of a relatively simple substance.
Keywords: Uric acid, Monosodium urate, Inflammation, Adjuvanticity, NLRP3, IL-1β, TLR, Lipids
Introduction
It has been more than a century since Garrod demonstrated that uric acid is the cause of gout. Uric acid in the form of monosodium urate (MSU) crystals precipitates in synovial cavities and other anatomic locations, provoking strong inflammation and debilitating pain. The basic parameters of gout pathogenesis and treatment are well-established. Nonetheless, gout remains a clinical concern that affects about 1% of our population, and interest in uric acid as a regulator of inflammation and immune responses has risen steadily during the past several years. Renewed interest in uric acid and gout has been driven by recent observations indicating that uric acid 1) is an endogenous danger signal and 2) triggers NOD-like receptor protein (NLPR)3-dependent inflammation, two effects with important implications for systemic inflammatory responses. Thus, the relationship between uric acid and organismal biology touches on issues of evolution, biophysics, and immune regulation itself.
Why Do We Have Gout Anyway?
In some past eras, the question of why humans develop gout has been treated satirically, as gout has often been associated with copious dietary consumption and higher social classes [1]. High uric acid levels have been implicated in many pathological conditions, including heart disease and diabetes. Uric acid also drives a canonical inflammatory response and may act as an endogenous adjuvant that directs immune activities. Clinically, gout incidence is on the rise due to a variety of lifestyle and demographic factors [2••].
Uric acid is a small, organic, heterocyclic compound found in lower and higher organisms. It is a catabolite of purines (adenine and guanine) derived from RNA and DNA. In most species, uric acid can be processed to highly soluble allantoin, even to ammonia [3]. Gout as a condition emerged in humans and other primates after the evolutionary loss of uricase, the uric acid catabolic enzyme (mostly confined to the liver) that converts uric acid to allantoic acid. In addition, the kidney filters uric acid but then reclaims most of the filtered load through reuptake [4]. Such a continuous effort to block the removal of metabolic “waste” may root in several advantages associated with high uric acid.
Uric acid is a strong peroxynitrite scavenger and antioxidant. One clinical observation that may speak to uric acid’s antioxidative effect is the near absence of multiple sclerosis (MS) in gout patients [5]. It is believed that the peroxynitrite is responsible for myelin degradation in MS, and peroxynitrite production can be blocked by higher uric acid levels. Conversely, there is a strong association of low serum uric acid levels with increased incidence of MS. In both human patients and a murine MS model (experimental autoimmune encephalomyelitis), high serum uric acid levels can reverse the disease progress. It also has been suggested that modest uric acid levels are protective in ischemic stroke [6]. Despite these potential advantages, it is questionable whether avoidance of MS and other late-life pathological conditions could have created enough evolutionary pressure to promote the loss of uricase, as these diseases do not prevent the carriers from entering the gene pool. One alternative theory suggests that the retention of uric acid can offset hyponatremic states to maintain a sufficiently high blood pressure, and that this was advantageous during past eras in which dietary salt availability was limited [7]. Another theory, first proposed in the 1950s, suggests that uric acid is structural homolog of caffeine (which in turn is a structural homolog of adenosine), and that high uric acid levels promoted mental alertness for primates and contributed to the development of human intelligence [8]. This hypothesis has been increasingly supported by experimental observations, although its role in evolution remains to be confirmed.
Monosodium Urate Precipitation and Crystal Formation
In all likelihood, uric acid’s inflammatory effects depend on its precipitation into MSU crystals, and the formation of MSU crystals is an obligatory step in the development of gout. However, the biology of MSU crystal formation is not fully understood. Typically, a serum uric acid level higher than 120 µg/mL (6.8 mg/dL) is defined as hyperuricemia. However, crystallization at this concentration is very difficult to reproduce in vitro in standard buffers [9]. Also, gout tends to have a rapid onset, whereas the crystallization process in vitro is quite slow. These observations suggest that mechanisms may exist to promote crystal formation in vitro. It is noted that uric acid levels inside a cell can be very high [10]. However, there have been no reports of intracellular MSU crystal formation. One possible defining factor is the availability of sodium, which is substantially higher in serum than in cytosol.
Hyperuricemia is associated with excessive food intake, particularly certain dietary items such as red meat and alcohol. In addition, large-scale cell death often induces robust MSU precipitation. Consequently, treatment with anticancer therapies (eg, chemotherapy and radiation therapy) is a situation in which uric acid levels need to be actively managed to avoid gouty attack [11]. Nonetheless, high serum uric acid levels do not uniformly lead to gout, as only about 10% of hyperuricemic patients experience gouty episodes [12]. However, gout can occasionally occur in individuals with normal uric acid levels [13]. Clearly, additional factors must be involved in MSU precipitation. MSU crystals rarely appear in central organs or deep cavities but are usually found in extremities, a phenomenon that has been attributed mainly to the subtle decrease in temperature in the distal joints. During the past several decades, mathematical models have been proposed to delineate the rate of crystallization in relation to environmental factors such as the temperature, pH, salt, vibration, and even the materials of the container in which experiments are conducted [14–17].
One area of recent progress in the understanding of MSU precipitation relates to a possible role for antibodies. The involvement of natural antibodies in MSU precipitation was first reported by Kam et al. [18, 19] about 18 years ago. In two papers, they described a then-unexpected phenomenon—that serum from gout patients could precipitate uric acid solutions, whereas control serum could not. To follow up, they immunized rabbits with MSU. The resulting immune serum precipitated soluble uric acid into crystals but spared other salt solutions from the crystallization process. On the other hand, sera from rabbits immunized with crystals made up of two different control salts could precipitate those specific crystals instead. No cross-precipitation was observed. The active factor was traced to serum IgG. This led them to hypothesize that serum antibodies can specifically recognize crystal surfaces and can serve to stabilize the initial crystal nuclei, a rate-limiting step in crystallization. In other words, these antibodies appeared to push the soluble/solid equilibrium toward solidification. We reported in 2009 that in mice, there were also antibodies that precipitated uric acid [9]. These antibodies appeared to be naturally occurring without any immune induction. In contrast to the Kam et al. group, we found that nearly all MSU-binding antibodies were IgMs that existed in both MSU immunized and pre-immune mice. The purified IgM antibodies facilitated MSU precipitation from uric acid solution in phosphate-buffered saline. Injection of these antibodies into recipient mice reduced their soluble serum uric acid levels, suggesting MSU crystal formation in the host. Most importantly, mice with B-cell deficiency (ie, mice that could not make anti-MSU antibodies) could not sense uric acid as a danger signal (ie, they did not appear to have an MSU crystal–dependent response), whereas when the same mice were reconstituted with the purified antibodies, they were capable of detecting the presence of uric acid in an immunization regimen and mounting a CD8 T-cell response against a co-injected antigen. In all, it seems that in addition to other factors, antibodies against MSU crystals are likely to play an important role in the phase transition, and hence the biological functions of MSU crystals.
Inflammatory Pathways
MSU crystals can be recognized by innate phagocytes, including dendritic cells (DCs), macrophages, and neutrophils. It has been shown that antigen-presenting cells (APCs) can sense uric acid as one of the proinflammatory endogenous signals released by damaged cells/tissues. These damage-associated molecular patterns can trigger a systemic inflammatory response similar to pathogen-associated molecular patterns [10]. The list of inflammatory pathways reportedly used by MSU crystals is long. There is no indication that a dominant mechanism will emerge soon. Nonetheless, there have been two major research focuses in the area of crystal responses. One is classical immunology oriented, focusing on processes such as antibody opsonization and complement fixation. The other (more current) area of investigation has focused on the role of immune pattern recognition of MSU structure. This includes responses by Toll-like receptors (TLRs) and leucine-rich repeat motifs (LRRs), which are nucleotide-binding sensor domains on NLRPs, including NLRPs that activate inflammasomes (multimolecular complexes that serve as platforms for interleukin [IL]-1 activation). One of the earliest hypotheses—that crystal recognition occurs mainly through membrane lipid binding—has also recently been undergoing re-examination.
Inflammatory Phagocytosis
Early studies suggest that MSU crystals isolated from sites of gouty inflammation are covered with immunoglobulins, mainly IgG [20–22]. Whether this antibody coating is the same phenomenon or a continuation of the antibody-mediated MSU precipitation discussed above is not known. The configurations of these antibodies with respect to the crystal surface have been worked out both in humans (IgG, by electron microscope (EM)) and mice (IgM, by monoclonal antibody fragmentation analysis). Fab portions are used to bind the crystal, while the Fc portion is pointed away [23]. MSU crystals that have been engulfed by macrophages are often antibody coated as well, suggesting a role for Fc receptor–mediated uptake. In phagocytes, MSU coated with IgG promotes the production of super-oxide anion [21]. Some data suggest that phagocytic responses to crystals may also be FcR dependent but Fc or IgG independent. In two interesting papers, Naccache et al. [24, 25] reported that CD16 (FcγRIII) on neutrophils can bind directly to the MSU surface, triggering intracellular tyrosine phosphorylation that is dependent on CD11b. They also reported that the same recognition mechanism can be triggered by a structurally different crystal, calcium pyrophosphate dehydrate. These observations introduce a phagocytic receptor (CD16) into the mix, one that is often linked to strong inflammation. How CD16 might recognize at least two different crystals without the benefit of antibody coating of their surfaces remains to be determined.
Complement
Both the classical and alternative complement activation pathways are involved in MSU-mediated inflammation. In early work, polypeptides on the surface of MSU crystals incubated with serum were analyzed by electrophoresis, and C1q, C1r, and C1s were identified, with C1q in abundance [26]. Because control (non-MSU) crystals did not show similar enrichment, these data suggested that the surface of MSU may convert C1 and adsorb the resulting fragments, leading to activation of the classical pathway. Although these processes are likely to depend on the presence of antibody Fc for C1q conversion, it actually has been questioned whether antibody coating of MSU is absolutely required for classical pathway activation, as MSU crystals can trigger C3 activation in the absence of immunoglobulin [27]. Regarding the alternative pathway, it was found that MSU surface does not induce the conversion of factor B or C3 individually. However, in the presence of all members of this pathway, both of these factors can be cleaved without the participation of the classical pathway (ie, in C2-deficient serum) [28]. In this cascade, C5 fragments (particularly C5a, a potent mediator of chemotaxis) are produced. It is important to note that we still do not know how a crystal surface can induce complement fixation. Regarding the consequences of complement activation in response to MSU, membrane attack complex formation appears to be important. In C6 (required for the membrane attack complex)-deficient mice, both gouty inflammation and associated IL-8 production are significantly decreased [29].
Toll-like Receptors
TLRs are critical sensors of microbial presence inside and outside a cell. However, several reports have suggested that they may also recognize substances from endogenous sources. For instance, both heat shock proteins and high-mobility group protein B1 trigger inflammatory responses via TLR4. MSU was reported to interact with two TLRs (TLR2 and TLR4), along with the associated receptor, CD14. Using antibody-blocking and transfection approaches, it was reported that MSU interacted with TLR2 on chondrocytes to induce nitric oxide [30]. Macrophages were similarly found to rely on TLR2/TLR4 for MSU recognition, leading to IL-1β production [31]. In a later report, the same research group further suggested that CD14 could also bind MSU crystals [32]. This binding led to CXCL1 production and IL-1β release. It is unclear whether all three structures are required to interact with MSU simultaneously, or whether each is used by specific cell types under different circumstances. Moreover, not all studies have supported this model. For instance, a report by Chen et al. [33] using TLR knockouts and gene transfectants failed to document a requirement for receptors in a peritoneal gout model, and in our own laboratory (unpublished observations), the activation of DCs and macrophages by MSU crystals did not appear to require TLRs [34•]. The dichotomy between these two sets of findings may relate to issues of sensor redundancy or to unique experimental settings.
Inflammasomes and Interleukin-1β
In 2006, Martinon et al. [35] reported that IL-1β production in response to MSU stimulation is lost in macrophages deficient in NLRP3 (formerly NALP3, also called cryopyrin) [35]. NLRP3 regulates caspase-1 cleavage of pro–IL-1β into its secreted/active form. It is currently established that NLRP3, Apoptosis-Associated Speck-Like Protein Containing a CARD (ASC), and caspase-1 form a unique inflammasome (designated the NLRP3 inflammasome) that is responsible for MSU-driven IL-1β as well as IL-18 production. Hoffman et al. [36•] recently showed that deletion of an LRR domain (previously characterized as a sensor domain for inflammasome activation by pathogens) in NLRP3’s C-terminus resulted in blunted IL-1β production in MSU-stimulated macrophages. These studies confirm the role of NLRP3 in MSU-driven IL-1β release and suggest that the LRR is the sensor for MSU. It also was reported that NLRP3 is required for the ability of alum to act as an adjuvant in antibody induction [37], and that the presence of alum in vaccine preparations induced the production of uric acid, which subsequently triggers APC activation [38]. This reported chain of events suggests a role for NLRP3 in alum’s adjuvanticity, as the NLRP3 is necessary for MSU-mediated IL-1β production. However, the role of inflammasome in vaccination remains a topic of debate, as several other groups also using alum as adjuvant did not observe a loss of antibody production in NLRP3-deficient mice [37, 39].
One unexplained aspect of the MSU/inflammasome model concerns the question of how MSU crystals that appear to be mainly extracellular can access the inflammasome intracellularly. Hornung et al. [40••] reported that phagolysosomes formed in macrophages upon uptaking MSU (as well as alum) were unstable, which might permit the intracellular release of phagocytosed crystals. The release of these solids into the cytosol was accompanied by the release of cathepsin B, which itself can lead to NLRP3 activation via a yet-undefined mechanism [40••]. It is important to recognize that such a scenario suggests the release of phagolysosomal content, which has long been considered a death signal to the cell [41]. Although this method of activation may be involved in acute inflammation, it may be less applicable to immune response generation, as APCs containing ruptured phagolysosomes would need to survive long enough to migrate to draining lymph nodes and exert a delayed adjuvant effect.
Lipid Binding
Our group recently revisited an old paradigm in crystal-mediated cellular activation in order to update some details as they apply to signaling mechanisms. Mandel suggested nearly 50 years ago that MSU crystal surfaces can directly determine their binding to plasma membrane via crystal–lipid interactions. He predicted that the ruggedness of the surface—hence the degree of electrostatic interactions—was responsible for the cellular response [42]. That notion was supported a series of papers but was largely lost as investigators turned to protein-based receptors as the primary mediators of cellular responses.
Our interest in crystal–lipid interactions was based on our inability—despite significant efforts—to identify a protein-based, MSU-specific receptor on DCs. Although such receptors may exist (and candidates such as TLR2/4, CD14, and CD16 have been identified) on macrophages and neutrophils, they do not appear to be functional in DCs. In contrast, we discovered that cholesterol depletion from the plasma membrane completely blocked DC response to MSU [34•]. Using atomic force microscopy and synthetic chemistry, we determined the following chains of events on DCs: 1) MSU forms spatially tight intermolecular bonding (possibly hydrogen bonding) with cell surface cholesterol within the first 30 s after contacting a DC, and this interaction leads to lipid sorting due to the fluidity of the membrane; 2) lipid sorting aggregates immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors, which are preferentially segregated into cholesterol/sphingolipid-rich regions of lipid rafts; 3) on DCs, Syk kinase is recruited by this accumulation and subsequently activates phosphatidylinositol 3-kinase (PI3K); and 4) this activation triggers activities mediated by PI3Ks linked to actin/microfilament movement, permitting further accumulation of signaling molecules at the plasma membrane for self-amplification.
Our work provides an explanation of how particulate materials, even without any specific protein receptor, may trigger strong DC activation in cases independent of opsonization and antibody binding. Confirmation of our model awaits validation by other investigators, and it is possible that this particular mechanism operates in parallel with traditional protein/receptor-based recognition. However, our theory makes two simple and testable predictions: 1) membrane lipid disruption must inhibit crystal-mediated activation; cholesterol reduction, either at its synthesis or its quantity in the plasma membrane, must reduce/blunt MSU-mediated inflammation in vivo; and 2) Syk/PI3K deficiency will lead to the abolishment of MSU-mediated cellular responses. We anticipate that these predictions will be put to experimental verification in the near future.
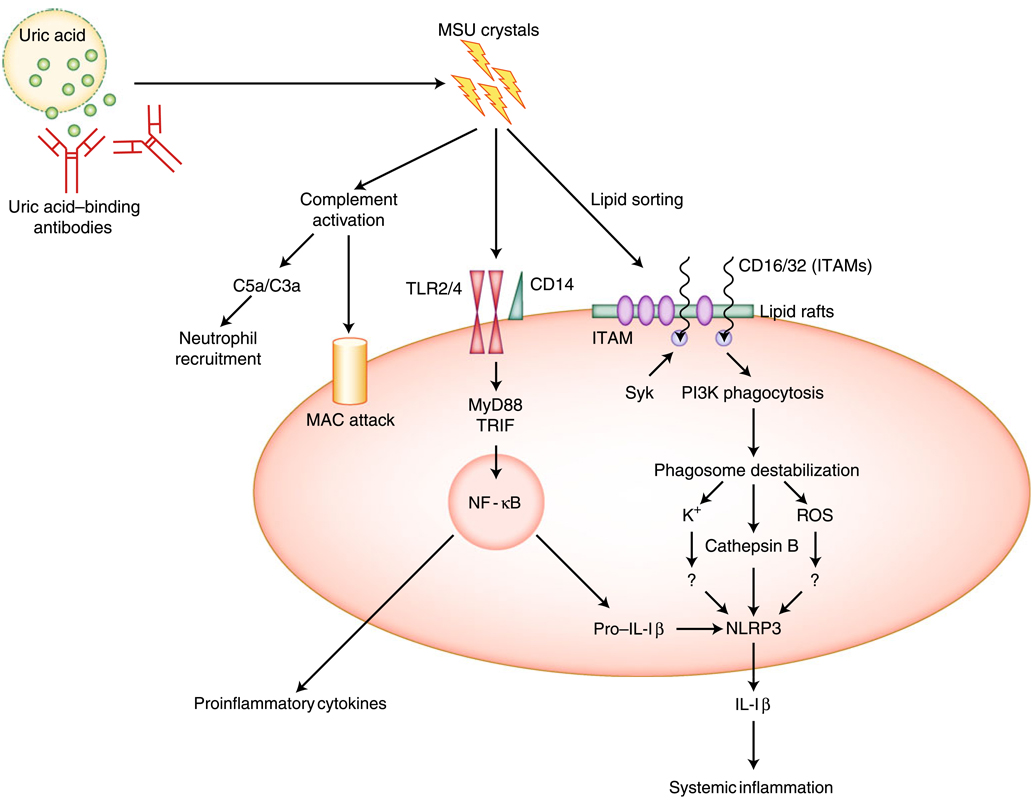
The current proposed MSU activation pathways are illustrated in Fig. 1.
Fig. 1.
Proposed pathways of monosodium urate (MSU)-mediated cellular activation. Uric acid released from injured cells forms MSU crystals upon binding by uric acid–binding antibodies. The crystals convert the complement components via both the classical and the alternative pathways to produce C3a and C5a, as well as membrane attack complexes (MACs). Upon engaging the plasma membrane, MSU interacts with protein receptors Toll-like receptor (TLR)2/4 and CD14, and likely triggers the MyD88/TRIF pathway that leads to nuclear factor-κB (NF-κB) activation. This chain of events may control the activation of antigen-presenting cells (APCs), as well as other proinflammatory cytokine production, with the exception of interleukin (IL)-1β and IL-18. MSU crystals may also bind to cholesterol and trigger lipid sorting, which leads to Syk recruitment by immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors (eg, CD16 and CD32) enriched in the lipid rafts. Syk then turns on phosphatidylinositol 3-kinase (PI3K)-mediated inflammatory phagocytosis. The interaction by MSU with CD16 may be independent of the lipid sorting process. The phagocytosed MSU crystals cause phagolysosomal membrane damage, and cathepsin B released from these vesicles triggers NOD-like receptor protein (NLRP)3 inflammasome activation via a yet-unidentified pathway. This pathway controls the IL-1β production and may be responsible for the systemic inflammation. Question marks indicate suspected links. ROS—reactive oxygen species
Adjuvanticity Versus Inflammation
As a matter of usage, inflammasome activation and IL-1β production have become surrogate readouts for inflammation and, by extension, immune cell response to stimulants. Although this simplistic approach draws the focus onto inflammasome activities, it is important to recognize that uric acid–mediated responses are multifaceted. For instance, MSU-driven IL-1β production (and hence MSU-driven inflammation) is likely distinct from uric acid–mediated adjuvanticity. In several recent papers, Chen et al. [43] confirmed that uric acid is a major trigger of liver inflammation following drug-induced cell death and, in a murine peritoneal gout model, that neutrophil infiltration is greatly reduced in Myd88-deficient (ie, IL-1 signaling–impaired) mice. However, a set of sophisticated bone marrow adoptive transfer experiments revealed that IL-1 receptors on nonhematopoietic cells, rather than neutrophils, were critical for the inflammation. Wild-type recipients of IL-1R–deficient bone marrow (ie, IL-1R–deficient myeloid cell) grafts showed an unaltered response to MSU, whereas the reciprocal transfer (IL-1R–functional marrow into Myd88-deficient mice) did not result in inflammatory responses against MSU crystals. This subtle detail has significant implications—that hematopoietic cells do not make IL-1β for themselves, and their activation is achieved without IL-1R signaling. In other words, IL-1β is an agent for systemic inflammation, not the activation of APCs themselves. Subsequent work by the same group further revealed that response to dead cells (containing uric acid) also depended on the IL-1R /MyD88 loop in a similar manner [43]. In contrast to neutrophils, monocyte responses (reflecting antigenicity more than acute inflammation) were much less IL-1β dependent. The work presented by Chen et al. thus helps define the boundaries between localized “danger” sensing, APC activation, and the full-fledged inflammatory response, and possibly suggests how uric acid works as an immune adjuvant.
Conclusions
The incentive must have been strong for primates to silence their uricase gene, ensuring a state of high uric acid in humans. The consequences for human health—both positive and negative—have been profound. The antioxidative effect of uric acid may be prominent in its protection against demyelination in MS. However, from a modern medicine vantage point, high uric acid is undesirable. A preponderance of medical literature suggests a link between uric acid elevation and increased cardiovascular disease [44]. Hypertension is also positively associated with high serum uric acid levels. More recently, it has been shown that serum uric acid levels influence the onset of insulin-insensitive diabetes independent of other risk factors. These conditions are antithetical to its antioxidative effect. One way of reconciliation is to ascertain that high serum uric acid at least at times results in systemic or localized MSU crystallization, as crystalline MSU is injurious to many tissues and triggers strong inflammation. This observation has not been made in human or animal models.
The inflammatory effect of MSU crystals is mediated in large part by the NLRP3 inflammasome that drives IL-1β and IL-18 production. IL-1β produced is likely the main agent to trigger systemic inflammation, promoting extensive neutrophil infiltration and tissue restructuring, such as is observed in gout. The means through which MSU triggers the inflammasome remain a matter of debate, with evidence for direct MSU contact with NLRP3, action through an intermediate downstream of phagosomal rapture, or via a signaling event emanating from the plasma membrane. Each of these models awaits further validation and a fuller explanation of its mechanisms. The separation of APC activation by MSU from crystal effects on systemic inflammation represents an important intellectual advance and suggests that APC activation by MSU is achievable without triggering painful inflammatory reactions. Such knowledge may prove a valuable lead in solid particle–based vaccine development.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Faranak Ghaemi-Oskouie, Department of Microbiology and Infectious Disease and Immunology Research Group, University of Calgary, 4A18 HRIC, 3330 Hospital Drive NW, Calgary, Alberta T2N 4N1, Canada.
Yan Shi, Email: yshi@ucalgary.ca, Department of Microbiology and Infectious Disease and Immunology Research Group, University of Calgary, 4A18 HRIC, 3330 Hospital Drive NW, Calgary, Alberta T2N 4N1, Canada.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Dunn JP, Brooks GW, Mausner J, Rodnan GP, Cobb S. Social class gradient of serum uric acid levels in males. JAMA. 1963;185:431–436. [Google Scholar]
- 2. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–38. doi: 10.1038/nrrheum.2009.236. This is a comprehensive and contemporary review of gout.
- 3.Smyth CJ, Holers VM. Gout, Hyperuricemia, and Other Crystal-Associated Arthropathies. New York: Marcel Dekker; 1998. [Google Scholar]
- 4.Enomoto A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 5.Hooper DC, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seet RCS, et al. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis. 2010;209:215–219. doi: 10.1016/j.atherosclerosis.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe S, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 8.Sofaer JA, Emery AE. Genes for super-intelligence? J Med Genet. 1981;18:410–413. doi: 10.1136/jmg.18.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanevets U, Sharma K, Dresser K, Shi Y. A role of IgM antibodies in monosodium urate crystal formation and associated adjuvanticity. J Immunol. 2009;182:1912–1918. doi: 10.4049/jimmunol.0803777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 11.Steele TH. Hyperuricemic nephropathies. Nephron. 1999;81 Suppl 1:45–49. doi: 10.1159/000046298. [DOI] [PubMed] [Google Scholar]
- 12.Vitart V, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger N, Norquist JM, Watson DJ. Serum urate during acute gout. J Rheumatol. 2009;36:1287–1289. doi: 10.3899/jrheum.080938. [DOI] [PubMed] [Google Scholar]
- 14.Fiddis RW, Vlachos N, Calvert PD. Studies of urate crystallisation in relation to gout. Ann Rheum Dis. 1983;42 Suppl 1:12–15. doi: 10.1136/ard.42.suppl_1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata H, Nishio S, Yokoyama M, Matsumoto A, Takeuchi M. Solubility of uric acid and supersaturation of monosodium urate: why is uric acid so highly soluble in urine? J Urol. 1989;142:1095–1098. doi: 10.1016/s0022-5347(17)39003-1. [DOI] [PubMed] [Google Scholar]
- 16.Kippen I, Klinenberg JR, Weinberger A, Wilcox WR. Factors affecting urate solubility in vitro. Ann Rheum Dis. 1974;33:313–317. doi: 10.1136/ard.33.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tak HK, Cooper SM, Wilcox WR. Studies on the nucleation of monosodium urate at 37 degrees c. Arthritis Rheum. 1980;23:574–580. doi: 10.1002/art.1780230509. [DOI] [PubMed] [Google Scholar]
- 18.Kam M, Perl-Treves D, Caspi D, Addadi L. Antibodies against crystals. FASEB J. 1992;6:2608–2613. doi: 10.1096/fasebj.6.8.1592211. [DOI] [PubMed] [Google Scholar]
- 19.Kam M, Perl-Treves D, Sfez R, Addadi L. Specificity in the recognition of crystals by antibodies. J Mol Recognit. 1994;7:257–264. doi: 10.1002/jmr.300070404. [DOI] [PubMed] [Google Scholar]
- 20.Landis RC, Haskard DO. Pathogenesis of crystal-induced inflammation. Curr Rheumatol Rep. 2001;3:36–41. doi: 10.1007/s11926-001-0049-7. [DOI] [PubMed] [Google Scholar]
- 21.Nagase M, Baker DG, Schumacher HR., Jr Immunoglobulin G coating on crystals and ceramics enhances polymorphonuclear cell superoxide production: correlation with immunoglobulin G adsorbed. J Rheumatol. 1989;16:971–976. [PubMed] [Google Scholar]
- 22.Ortiz-Bravo E, Sieck MS, Schumacher HR., Jr Changes in the proteins coating monosodium urate crystals during active and subsiding inflammation. Immunogold studies of synovial fluid from patients with gout and of fluid obtained using the rat subcutaneous air pouch model. Arthritis Rheum. 1993;36:1274–1285. doi: 10.1002/art.1780360912. [DOI] [PubMed] [Google Scholar]
- 23.Kozin F, McCarty DJ. Molecular orientation of immunoglobulin G adsorbed to microcrystalline monosodium urate monohydrate. J Lab Clin Med. 1980;95:49–58. [PubMed] [Google Scholar]
- 24.Barabe F, Gilbert C, Liao N, Bourgoin SG, Naccache PH. Crystal-induced neutrophil activation VI. Involvment of FcgammaRIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J. 1998;12:209–220. doi: 10.1096/fasebj.12.2.209. [DOI] [PubMed] [Google Scholar]
- 25.Desaulniers P, Fernandes M, Gilbert C, Bourgoin SG, Naccache PH. Crystal-induced neutrophil activation. VII. Involvement of Syk in the responses to monosodium urate crystals. J Leukoc Biol. 2001;70:659–668. [PubMed] [Google Scholar]
- 26.Terkeltaub R, Tenner AJ, Kozin F, Ginsberg MH. Plasma protein binding by monosodium urate crystals. Analysis by two-dimensional gel electrophoresis. Arthritis Rheum. 1983;26:775–783. doi: 10.1002/art.1780260612. [DOI] [PubMed] [Google Scholar]
- 27.Naff GB, Byers PH. Complement as a mediator of inflammation in acute gouty arthritis. I. Studies on the reaction between human serum complement and sodium urate crystals. J Lab Clin Med. 1973;81:747–760. [PubMed] [Google Scholar]
- 28.Fields TR, Abramson SB, Weissmann G, Kaplan AP, Ghebrehiwet B. Activation of the alternative pathway of complement by monosodium urate crystals. Clin Immunol Immunopathol. 1983;26:249–257. doi: 10.1016/0090-1229(83)90143-5. [DOI] [PubMed] [Google Scholar]
- 29.Tramontini N, Huber C, Liu-Bryan R, Terkeltaub RA, Kilgore KS. Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis. Arthritis Rheum. 2004;50:2633–2639. doi: 10.1002/art.20386. [DOI] [PubMed] [Google Scholar]
- 30.Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. 2005;174:5016–5023. doi: 10.4049/jimmunol.174.8.5016. [DOI] [PubMed] [Google Scholar]
- 31.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 32.Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–6378. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- 33.Chen CJ, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ng G, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. This report suggests lipid sorting may be a mechanism for MSU-mediated activation.
- 35.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 36. Hoffman HM, et al. Role of the leucine-rich repeat domain of cryopyrin/NALP3 in monosodium urate crystal-induced inflammation in mice. Arthritis Rheum. 2010;62:2170–2179. doi: 10.1002/art.27456. This article presents structural insight into how NLRP3 may handle MSU-initiated activation.
- 37.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kool M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 40. Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. This article discusses a potential pathway for MSU-mediated IL-1β production.
- 41.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 42.Mandel NS. The structural basis of crystal-induced membranolysis. Arthritis Rheum. 1976;19 Suppl 3:439–445. doi: 10.1002/1529-0131(197605/06)19:3+<439::aid-art1780190719>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 44.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]