Abstract
Background
The calcium binding protein S100A12 is highly upregulated in the serum and sputum of patients with allergic asthma and is suggested to be a biomarker and pathologic mediator of asthma.
Objective
To test the role of S100A12 in mediating airway inflammation in a mouse model of allergic lung inflammation.
Methods
Transgenic mice that express human S100A12 and wild type littermates were sensitized and challenged with ovalbumin and assessed for inflammation, lung structure and function.
Results
Following ovalbumin sensitization and challenge, S100A12 transgenic mice showed reduced peribronchial and perivascular inflammation, mucus production and eosinophilia as well as attenuated airway responsiveness to contractile agonist compared to wild type sensitized and challenged animals. This is explained, at least in part, by remodeled airways in S100A12 transgenic mice with thinning of the airway smooth muscle. S100A12 exposure induced Fas expression and activation of caspase 3 in cultured airway smooth muscle cells, suggesting that airway smooth muscle abnormalities observed in S100A12 transgenic mice may be mediated through myocyte apoptosis.
Conclusion and Clinical Relevance
S100A12 is one of the most abundant proteins found in the airways of human asthmatics, and it was postulated that S100A12 could mediate the inflammatory process. Our study shows for the first time that transgenic expression of S100A12 in the lung of mice does not exacerbate lung inflammation in a model of ovalbumin-induced allergic inflammation. We speculate that the high levels of S100/calgranulins found in BALF of asthmatics and of ovalbumin-treated transgenic S100A12 mice do not significantly mediate pulmonary inflammation.
Keywords: animal models, calgranulins, airway remodeling, inflammation, RAGE, apoptosis, smooth muscle
Introduction
Asthma is a chronic lung disease characterized by airway inflammation, excessive mucus production, airway hyperresponsiveness, and lung remodeling. Differential proteomic analysis of bronchoalveolar lavage fluid (BALF) identified S100A12 as one of the most upregulated proteins in response to allergen stimulation in patients with allergic asthma, but not in healthy controls [1]. Members of the S100/calgranulins family such as S100A8 (myeloid related protein-8, calgranulin A), S100A9 (myeloid related protein-14, calgranulin B) and S100A12 (EN-RAGE, calgranulin C) are Ca2+-binding proteins implicated in the regulation of a variety of cellular activities [2]. S100/calgranulins proteins are endogenously expressed in myeloid cells and found at increased concentration in the sera of subjects with chronic inflammatory diseases such as rheumatoid arthritis, chronic inflammatory bowel disease and vascular disease [3] [4] [5] , as well as in the sputum of patients with asthma [6], and other acute and chronic lung diseases [7]. Yang and colleagues reported that recombinant S100A12 provokes activation of human and murine mast cells leading to the release of histamine and cytokines, and therefore suggest that S100A12 could amplify inflammatory pathways in asthma [6]. However, direct studies examining the role of S100A12 in mediating allergic lung inflammation in vivo have not been performed.
We previously identified RAGE (Receptor for Advanced Glycation Endproducts) as a receptor for S100A12 and reported that injection of recombinant bovine S100A12 into mice leads to enhanced inflammation, which was attenuated by co treatment with soluble RAGE, the ligand binding domain of RAGE [8]. Others have shown that toll like receptor 4 is also activated by S100/calgranulins [9]. RAGE was first isolated from lung tissue [10] and is expressed abundantly in alveolar type I cells [11]. While RAGE mediates vascular inflammation and atherosclerosis [12] [13], the physiological role of RAGE in the lung is incompletely understood. Mice lacking RAGE develop spontaneously pulmonary fibrosis [14] and interestingly, have an attenuated inflammatory response to intranasally inoculated Streptococcus pneumonia [15]. This suggests that RAGE is important for the inflammatory response and tissue remodeling in the lung.
Because S100A12 activates RAGE and is abundantly expressed in asthma, we examined whether expression of S100A12 further augments lung inflammation in an ovalbumin (OVA) model of lung inflammation. We exploited the fact that S100A12 is not present in mice [16] and used transgenic (TG) mice that express human S100A12 under the control of smooth muscle-specific promoter SM22α. To our surprise, we found that S100A12 TG mice subjected to allergen sensitization and challenge exhibited less airway inflammation, reduced airway responsiveness, and attenuated lung inflammation compared to wild type (WT) littermate animals.
Methods
S100A12 Transgenic Animals
C57BL/6J mice hemizygous for human S100A12 expressed in vascular smooth muscle cells driven by the SM22α promoter [17] were previously described [18]. Hemizygous TG and WT littermates were used for all experiments. All procedures were carried out with the approval of the Institutional Animal Care and Use Committee of The University of Chicago.
RNA isolation and Quantitative RT-PCR
Was performed as previously described [18] with the following primer pairs: S100A12: 5’AGACACCGAAGCTACTCTCCTTCA and 5’ATCCCTACTCTTTGTGGGTGTGGT; GAPDH: 5’TCAACAGCAACTCCCACTCTTCCA and 5’ACCCTGTTGCTGTAGCCGTATTCA; Caspase 10: 5’AGGGCAGCTGTGTACAGGATGAAT and 5’TGCAGGACCATCTCCATTTCCACT; Caspase 3: 5’GCACGATGTGCCAGTCATTCCTTT and 5’AGGCTGCATCCACCTCAGTTATGT; Fas: 5’GAACACTGTGACCCTTGCACCAAA and 5’CTCTTTGCACTTGGTGTTGCTGGT; CXCL10: 5’GCTGATATGGGGATATAGGTTC and 5’GCAGGTACAGCGTACAGTTC; CCL9: 5’ATGAAGCCTTTTCATACTGCCC and 5’TTATTGTTTGTAGGTCCGTGGTT
Total RNA was prepared from three pooled HASMC cultures (all stimulated with cytokines in the presence or absence of S100A12) and after transcribing into cDNA subjected to an RT2 Profiler PCR Array for Chemokines (SA Bioscience). Findings were confirmed by qRT-PCR in independent derived cDNA samples for CXCL10 and CCL9.
Western analysis
Was performed as previously described [18] with the following antibodies: mS100A8 (Abcam), S100A12 (Abcam), caspase-3 (active) (Millipore), β-actin (Millipore), and GAPDH (Sigma). Bands were quantified by densitometry and normalized to GAPDH expression.
OVA-Sensitization and OVA-Airway Challenge
Mice (8 weeks old) were sensitized with 10 µg OVA (grade V, Sigma) emulsified in 2 mg alum (AlumImuject, Pierce) i.p, or with PBS in alum, on days 0, 7, and 14. Mice were challenged with nebulized OVA (1% in PBS) for 20 min twice daily on days 19 through 23, and studied on day 25.
Histological Analyses
Was performed as previously described [19]. Briefly, the airway was installed with 4% paraformaldehyde under physiologic pressure of 25 cm H2O and the lungs fixed in formalin for 24 hours. After histological processing, three slides from each lung lobe were stained with H&E, Masson trichrome, Verhoeff van Giessen, or periodic acid Schiff (PAS), and examined in a blinded fashion. A score of peribronchiolar and perivascular inflammation was determined in H&E slides as follow: 0, no cells; 1, few isolated cells; 2, a ring of inflammatory cells one-cell deep; 3, a ring of inflammatory cells two- to four-cells deep; and 4, a ring of inflammatory cells of more than four-cells deep. Scoring for mucus production within goblet cells was performed in slides stained with PAS by examining five consecutive fields from each lobe. Numerical scores were determined as follows: 0, < 1% goblet cells; 1, 1–5% goblet -cells; 2, 5–25% goblet cells; and 3, >25% goblet cells [19].
Bronchoalveolar Lavage (BAL)
Was performed as previously reported and cell count and differentials in BALF was obtained by flow cytometry for eosinophils (CCR3+), T cells (CD3+) and macrophages (size) using a FACS LSR-II (BD)[19].
ELISA
Serum specific OVA IgG1 was measured using a commercially available ELISA kit. S100A12 quantities were determined in serum and BALF by double-sandwich ELISA using hS100A12 IgG (1 µg/ml Abcam) followed by incubation with biotinylated anti human EN-RAGE antibody (R&D). Recombinant S100A12 was used to prepare the standard curve (1 to 500 ng/ml). The lower limit of detection of S100A12 was 30 ng/ml and the intra-assay and inter-assay coefficients of variation were 12% and 15%, respectively. CXCL10 was measured in cell culture supernatant of HASMC by ELISA according to the manufacturer’s instruction (R&D System).
Determination of Respiratory System Resistance
Anesthetized and tracheostomized mice were mechanically ventilated at a rate of 160 breath/min, tidal volume of 150µl with a positive end-expiratory pressure of 3 cm water, using a computer controlled ventilator (Flexivent, SCIREQ). Methacholine (MCh) was administered via a catheter placed into the jugular vein at increasing doses from 0 to 160 µg. For each i.v. MCh dose, data were continuously collected for 5 min and the maximum value of respiratory system resistance was recorded.
Hydroxyproline (HOP) Assay
HOP content was determined as previously described [20].
Cell Culture
Primary cultures of human airway smooth muscle cells (HASMC) were established by the explant method from the trachealis muscle of donors unsuitable for lung transplantation, and with approval form the University of Chicago Institutional Review Board. SMC were characterized by morphology and immunofluorescence staining using smooth muscle-α-actin antibody (Sigma). Cells from passage 6–7 were used for all experiments. When indicated, cells were stimulated with recombinant TNF-α (0.1 ng/ml) and IFN-γ (10 ng/ml, Pierce), or with activating Fas monoclonal antibody (clone CH11, 50 ng/ml, Millipore). HASMC were transiently transfected with a plasmid expression vector in which S100A12 is driven by the CMV promoter (in pcDNA3.1, Invitrogen) or vector only (control) using the Neon Transfection System (Invitrogen). Results of representative experiments are shown.
Statistics
All continuous data are reported as mean ± SEM. Experiments were performed in at least 7 mice per group; S100A8 expression in the lung was measured in triplicate; cell culture experiments were performed in triplicate and repeated three times. Signal intensity was measured by densitometry. Independent sample t-test and one-way analysis of variance were used for mean comparison between two or multiple groups, respectively. The Bonferroni correction was used to adjust for multiple comparisons. Two tailed p values less than 0.01 were considered statistically significant for each test to ensure an overall study significance level of p less than 0.05.
Results
S100A12 is released into BALF of S100A12 TG mice during lung inflammation
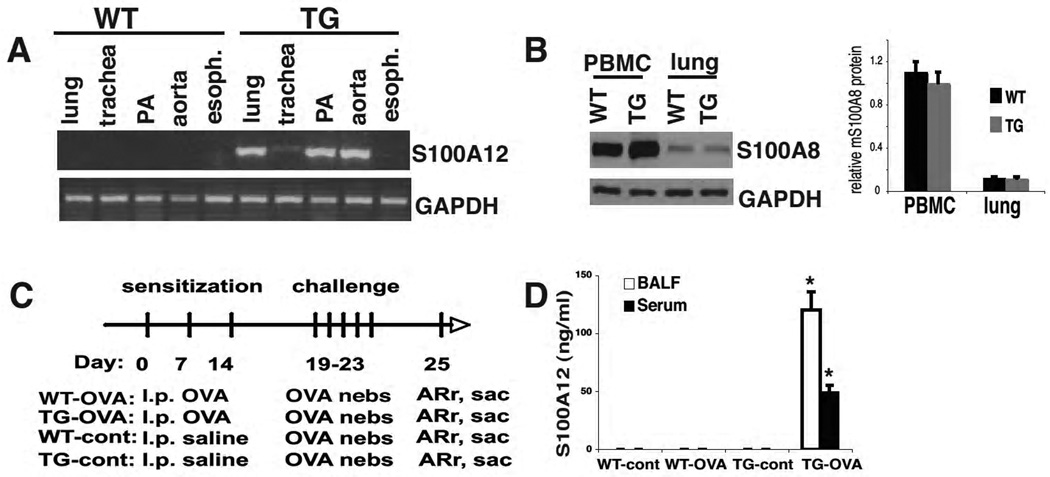
S100A12 TG mice developed normally, were fertile and as previously reported, developed aneurysms of the thoracic aorta [18]. RT-PCR analysis of different organs rich in smooth muscle myocytes revealed S100A12 mRNA in the lung, trachea, pulmonary artery, and aorta of TG mice but not of WT animals (Figure 1A). S100A12 mRNA was not detected in the esophagus and other tissues. Importantly, endogenous levels of murine S100A8 in myeloid cells and in lung tissue were not altered by expression of human S100A12 in TG animals (Figure 1B).
Figure 1.
Characterization of S100A12 TG mice. (A) RT-PCR detects S100A12 in the lung, trachea, pulmonary artery, and aorta of TG mice but not WT mice. (B) Expression of endogenous murine S100A8 protein is unaltered in peripheral blood mononuclear cells (PBMC) and lung of TG and WT mice. (C) OVA sensitization and challenge protocol to induce lung inflammation. (D) In response to OVA aerosol challenge, S100A12 is present in the BALF and serum of OVA-sensitized S100A12 TG mice and not in TG-control or WT mice.
Because S100A12 is increased in the BALF of asthmatic patients and directly provokes mast cell degranulation and cytokine release [6], we tested the hypothesis that TG mice that express human S100A12 in the lung will exhibit marked augmentation of pulmonary inflammation compared to WT animals in a model of allergic lung inflammation. To test this hypothesis, TG and WT mice were sensitized with OVA/alum (TG-OVA and WT-OVA) or with PBS/alum (TG-control and WT-control) followed by allergen challenges with nebulized OVA. All measurements were made two days after the last challenge (Figure 1 C). Because S100A12 in this mouse model is expressed mostly in the vascular SMC [18], we first determined whether S100A12 is secreted into the BALF as seen in patients with allergic asthma or COPD. As expected, S100A12 was not detected in the BALF or sera of WT-OVA or WT-control animals but it was present at high levels in the BALF and in the sera of TG-OVA mice (120 ±35 ng/ml and 50±35 ng/ml, respectively) but not in TG-control animals (Figure 1D). These data show that an inflammatory milieu facilitates the release of S100A12 into BALF and sera in TG mice, in a similar manner to what is observed in patients with asthma [1].
S100A12 TG mice show attenuated airway inflammation and epithelial remodeling in response to allergic OVA sensitization and challenge
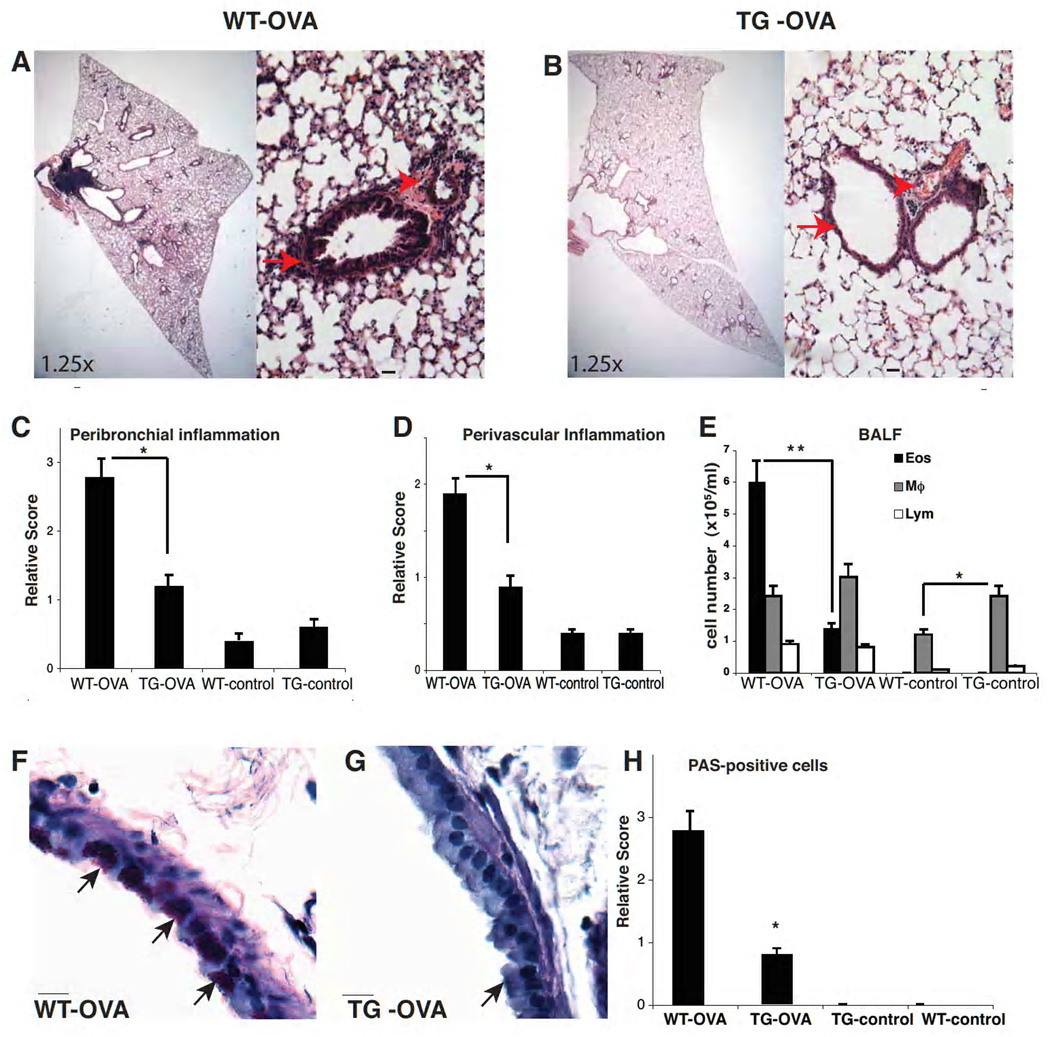
Histological evaluation of TG-OVA mice revealed strikingly attenuated inflammation in their lungs compared to WT-OVA mice (Figure 2 A and B). Quantification of peribronchial and perivascular inflammation shows pronounced infiltration of inflammatory cells in WT-OVA animals, which was significantly reduced in TG-OVA mice (Figure 2 C, D). WT-control and TG-control mice had no or only minimal peribronchial and perivascular inflammation. Furthermore, eosinophils in BALF were less abundant in TG-OVA mice compared to WT-OVA mice (1.4×105 vs. 6×105 cells/ml, p<0.001). Although there was no significant difference in T-cell counts or macrophage counts between TG-control and TG-OVA animals, TG-control mice had more macrophages in the BALF at baseline than WT mice (2.4 ×105 vs. 1.2 ×105 cells /ml, p<0.05, Figure 2 E).
Figure 2.
Attenuated lung inflammation in TG-OVA mice. (A and B) H&E stained lung section in 1.25× magnification and 20× magnification; scale bar 10 µm. Arrows and arrowheads show peribronchial and perivascular and inflammation, respectively. (C) Quantification of the peribronchial and (D) of perivascular inflammation (* p<0.05). (E) Analysis of inflammatory cells in BALF. TG-control mice have increased macrophage counts compared to WT-control mice (*p<0.05), and TG-OVA mice have less eosinophila than WT-OVA mice (**p<0.01). (F and G) Representative lung sections stained with PAS (arrow marks PAS positive cells; 40× magnification; scale bar, 10µ). (H) Quantification of PAS positive cells (*p<0.01).
Differences in airway epithelium remodeling between TG-OVA and WT-OVA mice were also evident. Goblet cell metaplasia and mucus production were determined in PAS-stained histological sections. PAS positive cells were more abundant in the bronchi of WT-OVA mice than in TG-OVA animals (Figure 2 F–H). At baseline, WT-control and TG-control mice had a few PAS positive cells. Taken together, these findings demonstrate that compared to WT animals, S100A12TG mice have a reduced inflammatory response to OVA sensitization and challenge, suggesting that S100A12 is a negative regulator that attenuates rather than exacerbates allergen-induced airway inflammation.
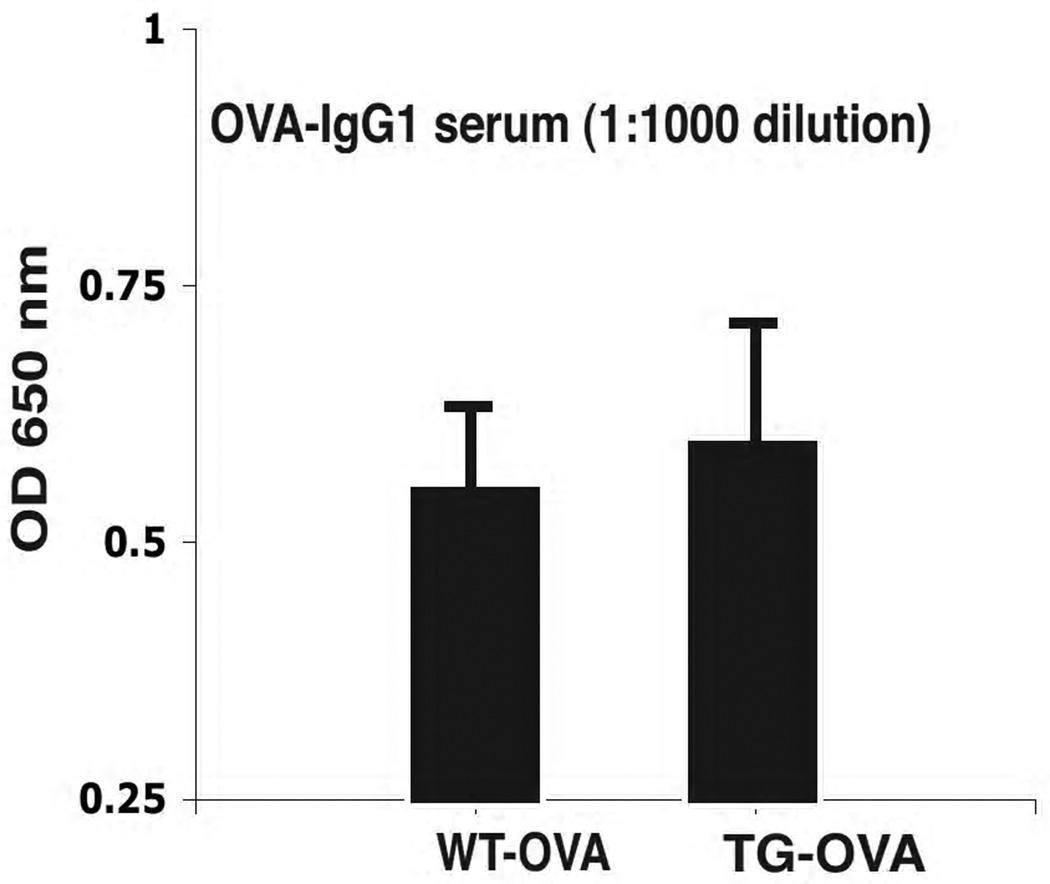
S100A12 TG mice have undistinguishable humoral responses to WT mice
The reduced airway inflammation seen in OVA-sensitized S100A12 TG mice after exposure to nebulized OVA could potentially result from a deficient control of the humoral immune response during OVA sensitization. Thus, to examine the effects of S100A12 on antibody production, we measured OVA specific IgG1 in the serum of all groups of mice. OVA specific serum IgG1 was not detectable in TG and WT mice at 0 or 12 days after sensitization, but similar levels were found in both strains 24 days after sensitization (Figure 3). These findings indicate that S100A12 TG and WT mice have equivalent capacity of immunoglobulin production after immunization with OVA.
Figure 3.
S100A12-TG mice produce comparable levels of OVA-specific serum IgG1.
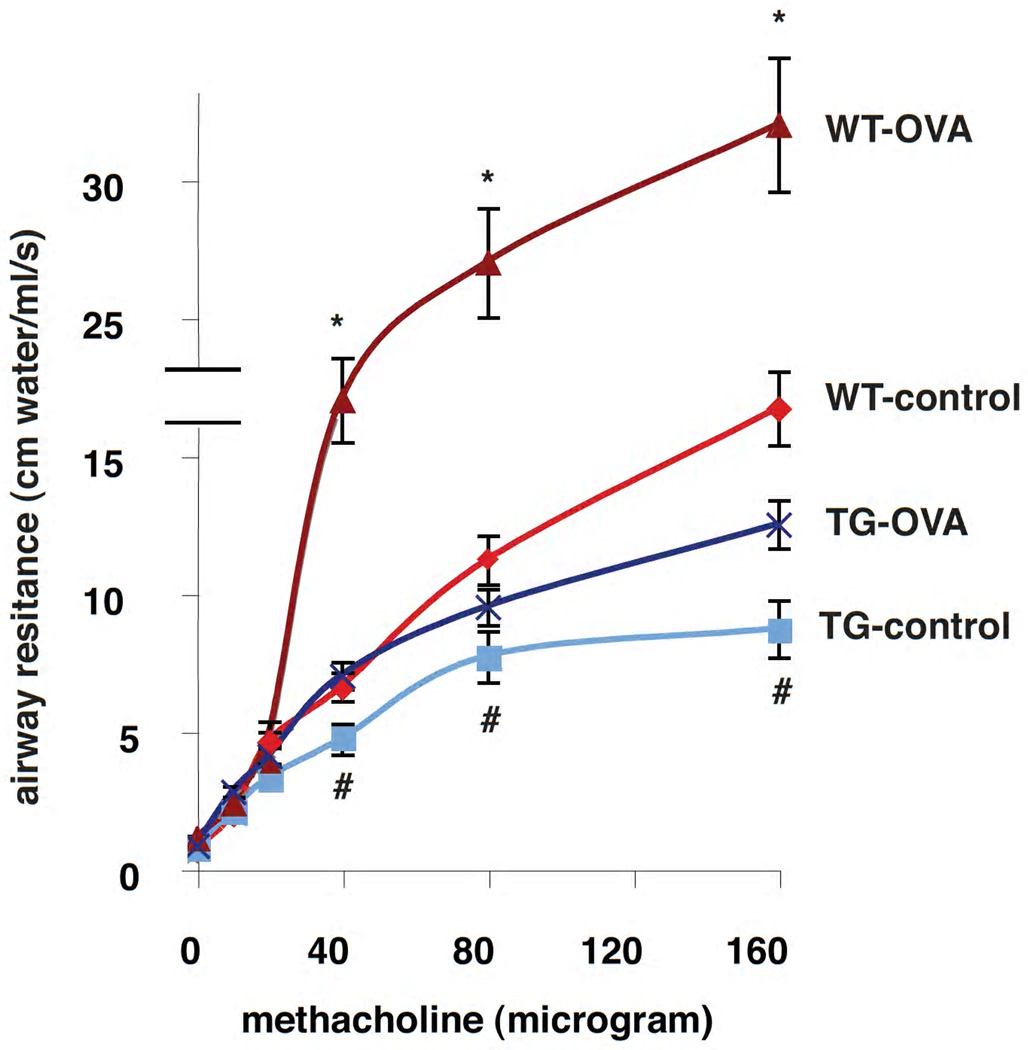
S100A12 TG mice exhibit reduced airway reactivity to bronchoconstriction stimulation
Airway hyperresponsiveness (AHR), the exaggerated response to a bronchoconstrictor agent, is a cardinal feature of asthmatic patients. Airway inflammation, lung remodeling, and excess abundance of airway smooth muscle are thought to play a key role in the pathogenesis of AHR. Because we found diminished inflammation and remodeling of the airway epithelium in TG-OVA mice compared to WT-OVA mice, we decided to measure lung function in our four animal groups. Total airway resistance was measured at baseline and in response to intravenous methacholine (MCh), a cholinergic drug that induces smooth muscle cell contraction and bronchoconstriction. Figure 4 shows that at baseline, there was no significant difference in airway resistance between WT-control and TG-control mice, or between WT-OVA and TG-OVA. As expected, with Mch administration, WT-OVA and TG-OVA mice exhibited higher airway resistance compared to their control groups. Although at low doses (10µg and 20µg) of MCh there was no significant difference in airway resistance between TG-OVA and WT-OVA, TG-OVA mice exhibited significantly lower airway resistance than WT-OVA mice in response to higher doses of MCh. Furthermore, respiratory resistance at 40 and 80 µg MCh was also significantly lower in TG-control mice compared to WT-control animals. Taken together, these findings indicate that S100A12 TG mice exhibit innate hypo-responsiveness to bronchoconstrictor agent MCh, and S100A12 TG animals undergoing allergic lung inflammation are unable to fully respond to stimulation with a contractile agonist. This is suggestive of structural defects in the airways and/or in the properties of the smooth muscle myocytes in S100A12 TG mice that could render these animals hypo-responsive to MCh.
Figure 4.
Bronchoconstrictor responsiveness is attenuated in S100A12 TG mice. Respiratory system resistance was measured after bolus i.v. injections of methacholine (* p<0.001 WT-OVA vs.TG-OVA, and # p<0.05 TG-control vs. WT-control).
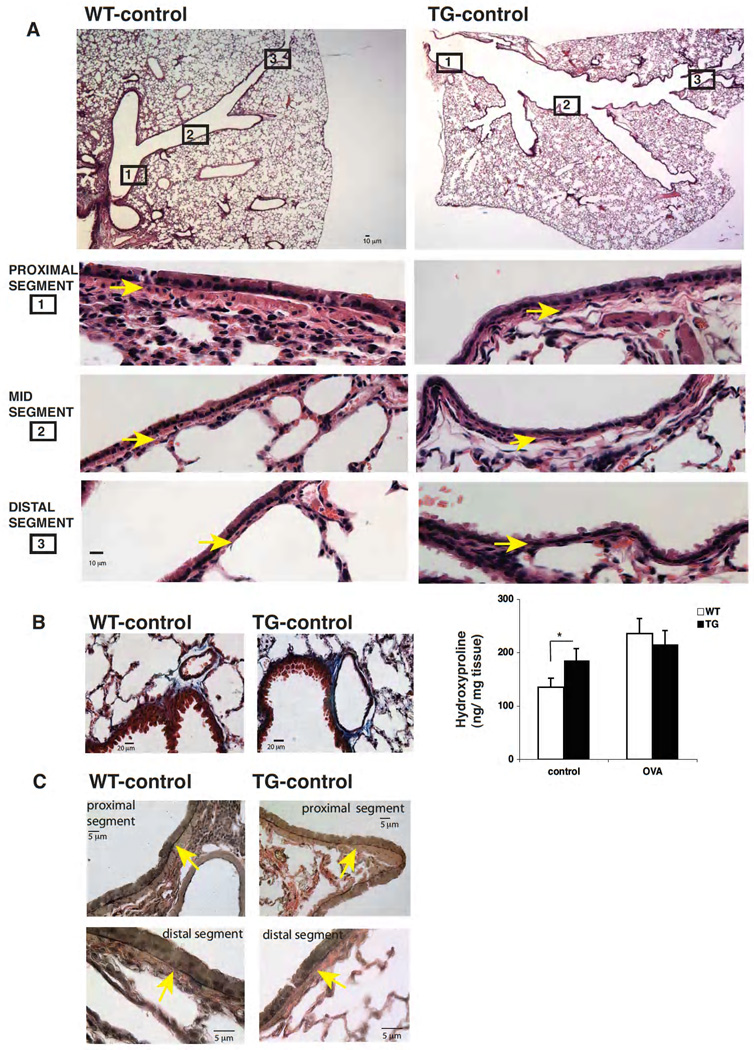
S100A12 TG mice have remodeled airways
We found remodeling of the lung with enlargement of the central airways and irregular airway structure in S100A12 TG control mice. A representative picture is shown in Figure 5A. We speculate that the limited response of TG mice to contractile stimulation might stem from the presence of less abundance of smooth muscle in their airways, and/or might relate to the central airway enlargement. We also noticed increased fibrosis in S100A12 TG lungs in Masson trichrome stained sections, particularly in the perivascular region (Figure 5B). Quantification of hydroxyproline (HOP) content in lung lysates, a measure of collagen deposition and fibrosis, revealed significantly increased HOP in TG-control lungs compared to WT-control lungs. Whether there is a relationship between the appearance of fibrosis, altered airway smooth muscle layer, and the dilated airways remains unknown.
Figure 5.
S100A12-TG mice exhibit lung structural abnormalities. (A) Reduction of airway smooth muscle and dilated and irregularly shaped large airways in S100A12-TG mice is evident compared to WT mice. Upper panel shows H&E stains at 2.5× magnification and the six lower panels inserts show representative images of the proximal (1), mid (2) and distal (3) airway segments at 40× magnification; scale bar, 10 µm. Arrows indicate the airway smooth muscle. (B) Masson trichrome stain showed enhanced peri-vascular fibrosis in S100A12 TG lungs. Hydroxyproline content in the lungs is shown on the right (* p<0.05). (C) Verhoeff van Giessan stain revealed thinning and degradation of elastic fibers in S100A12 TG airway (arrows indicate elastic fibers). Original magnification 40× for the proximal airway, and 100× for the distal airway.
Interestingly, these abnormalities are key features of the Mounier-Kuhn syndrome, a rare disease of tracheobronchomegaly of unkown etiology [21], and which is also notably accompanied by atrophy of the elastic fibers in the airways. Because we reported degradation of elastic fibers in the aorta of S100A12 TG mice [18], we examined elastic fibers morphology and found qualitative changes of the elastic fibers in the lungs of S100A12 mice compared to WT mice. A representative histology image (Figure 5C) shows thinner elastic fibers in the proximal airway and indication of early degradation of elastic fibers in the distal airway of S100A12TG mice.
S100A12 modulates chemokine expression and apoptosis in cultured airway smooth muscle cells
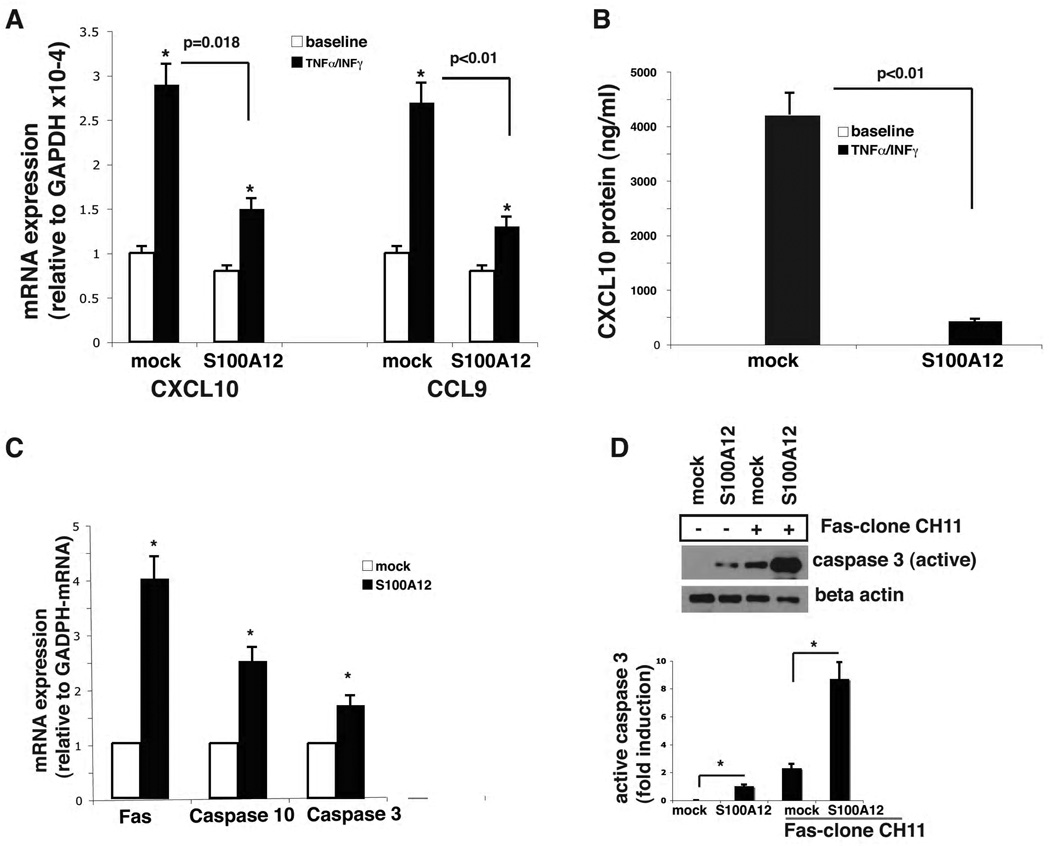
We explored whether the reduction of infiltrating inflammatory cells into the airway of TG-OVA mice might stem from immunomodulatory effects of airway smooth muscle myocytes expressing S100A12. Toward this goal, we analyzed global expression of cytokine and chemokines in HASMC that were transiently transfected with a plasmid encoding S100A12 (or vector alone) and stimulated with TNF-α and INF-γ. Co-exposure to TNF-α and INF-γ is known to activate HASMC to produce important cytokines and chemokines such as CXCL10 (also known as interferon inducible protein-10), CXCL9, and others [22, 23], and thereby may orchestrate the homing of infiltrating cells to the lung in asthma [24, 25]. We analyzed changes in mRNA expression using a chemokine RT2 Profiler polymerase chain reaction array, and found many genes differentially expressed (data not shown). We validated the results for CXCL10, and CCL9. Levels of CXCL10 mRNA were increased 2.9 -fold in vector alone transfected HASMC in response to TNF-α and INF-γ stimulation. This increase was attenuated by 78% in cells that expressed S100A12 (Figure 6A). Similarly, TNF-α and INF-γ exposure enhanced CCL9 mRNA levels, which were reduced by 82% in HSMC expressing S100A12 (Figure 6A). This effect was also observed at the protein level. At baseline, there were no detectable amounts of CXCL10 in either S100A12 or control HASM- transfected cells. However, in response to TNF-α and INF-γ stimulation, secretion of CXCL10 increased to 430 pg/ml ± 47 and 4230 pg/ml ± 402 in S100A12 and control HASMC, respectively (Figure 6B). This data indicate that S100A12 attenuates chemokine secretion in stimulated HASMC, and may thereby have anti-inflammatory activity in certain conditions.
Figure 6.
S100A12 modulates chemokine secretion in activated HASMC and is pro-apoptotic. (A) quantitative RT-PCR shows increased mRNA for CXCL10 and CCL-9 in cytokine stimulated HASMC (0.1ng/ml TNF-α and 10ng/ml INF-γ for 6 hours, * p=0.001 vs baseline), that is attenuated in cells expressing S100A12 (p=0.018 and p<0.01 vs mock transfected). Data are expressed as mean ±SEM. (B) ELISA for CXCL10 protein measured in cell culture supernatant of HASMC stimulated as indicated. Data are expressed as mean ±SEM. (C) qRT-PCR shows increased mRNA of Fas, caspase 10 and caspase 3 in HASMC transfected with S100A12, but not mock transfected cells (*p<0.05). (D) Western blot revealed activation of caspase 3 in S100A12 transfected cells compared to mock transfected control cells. Active caspase 3 abundance was further increased by co-stimulation with Fas-clone CH11 antibody (50 ng/ml for 48 hrs, *p< 0.01).
Next, we investigated the effect of S100A12 on apoptosis of HASMC as a potential cellular mechanism for the airway remodeling and reduced respiratory function we found in S100A12 TG mice. Previously it was shown that S100/calgranulins induced cell death in a variety of mammalian cell lines [26, 27]. Moreover, recently it was demonstrated that S100B mediates myocyte apoptosis in vivo by interacting with its receptor RAGE [28]. We therefore exposed HASMC to S100A12 and measured the expression of genes relevant to apoptosis pathways using first the RT2-PCR profiler assay (data not shown). This screening assay revealed increased mRNA for Fas, caspase 10 and caspase 3. Quantitative RT-PCR confirmed a 4-fold, 2.5-fold, and 1.7-fold increase, respectively, of Fas, caspase10 and caspase 3 mRNA expression in S100A12-transfected cells compared to mock transfected control cells (Figure 6C). We next examined the protein levels of active caspase 3, an enzyme known to play a central role in the execution-phase of cell apoptosis. Figure 6D shows that control treated smooth muscle myocytes express no active caspase 3. However, expression of S100A12 induces active caspase 3 in smooth muscle airway cells. Importantly, ligation of Fas with Fas antibody markedly augmented the expression of active caspase 3 in control cells and even further in S100A12 expressing airway myocytes. These findings indicated that S100A12 is pro-apoptotic for airway smooth muscle because it induces Fas and active caspase 3. The apoptotic effect of S100A12 was not limited to smooth muscle cells because treatment of airway epithelial cells with S100A12 also induced Fas and caspase 3 expression (data not shown).
Discussion
In this study we show for the first time that transgenic expression of human S100A12 results in multiple pulmonary abnormalities, including dilated airways, abnormal airway smooth muscle, and increased fibrosis in mice. These pathological features resemble those described in patients with Mounier-Kuhn syndrome, a rare disease of profound remodeled airway leading to tracheobrochomegaly, and for which no animal model exists. Altered expression of S100A12 in different cell types such as bronchiolar epithelia, reactive pneumocytes, macrophages, and polymorphonuclear granulocytes has been described in common non-neoplastic lung disorders ranging from smoke-induced bronchial damage to granulomatous disease. This suggests that S100A12 could be relevant for sustained inflammation and lung remodeling [29]. Moreover, S100A12 is increased in sputum from patients with asthma [6] and is among the most up regulated proteins in the BAL fluid of asthmatics following segmental antigen challenge [1]. Interestingly, the S100 gene cluster on chromosome 1q21 has been linked to atopic dermatitis [30] and to increased IgE serum levels in patients with atopic asthma [31]. However, it is not clear how S100 polymorphisms affect S100 protein levels and/or activity. Despite an established role of S100A12 in activating inflammatory pathways in endothelial cells, macrophages [8], and mast cells [6], definite evidence that S100A12 directly mediates allergic inflammation in the lung is missing. Since S100A12 constitutes up to 5% of neutrophil cytosolic proteins and is released upon activation of protein kinase C [32], it is difficult to establish whether augmented levels of S100A12 in subjects with asthma are simply reflecting an increased cellular inflammatory milieu, or whether S100A12 is directly involved in the exacerbation process and therefore, could represent a candidate for anti-inflammatory intervention as previously suggested [1, 33, 34].
We were surprised to find decreased airway inflammation and blunted augmentation of constrictor responsiveness in S100A12 TG mice in response to allergic immunization and challenge. Moreover, decreased native airway responsiveness (i.e., in the absence of airway inflammation) was also observed. The interrelationship of these observations is difficult to dissect. We can speculate that the decreased airway responsiveness might stem from the dilation of the airway itself - as narrowed bigger airways still have less resistance than narrowed smaller airways – and/or from abnormal airway smooth muscle with reduced contractile function. In OVA-sensitized and OVA-challenged mice, a diminished respiratory response might stem in addition, from the lesser inflammation that they develop. The mechanism by which S100A12 dampens OVA-induced lung inflammation and airway resistance is unknown, but could involve at last in part, modulation of the immune response from airway smooth muscle that results in reduced production of CXCL10, as well as augmented apoptosis of airway SM myocytes. We speculate that deficient abundance of airway smooth muscle could further dampen the inflammatory response in S100A12 TG mice because it is increasingly recognized that airway SMC are active participants in the pathophysiology of asthma. Exaggerated airway smooth muscle mass present in asthmatics [35, 36] is not only a central player of the abnormal narrowing of the airways but is also a source of a plethora of biologically active agents such as pro- and anti-inflammatory mediators, cell-adhesion molecules, lipid mediators, chemokines, and cytokines [37]. For these reasons, there is growing interest in manipulating airway smooth muscle abundance in order to reduce bronchoconstriction and lung inflammation [38]. For example, mice lacking pro-inflammatory mast cell protease (MCP) 4 exhibit, paradoxically, enhanced lung inflammation because of impaired proteolytic cleavage of SMC growth factors leading to exaggerated SMC expansion with thickened and inflamed airway SMC layers [39]. Thus, it is intriguing that our S100A12 TG mice have reduced airway smooth muscle (presumably via apoptosis) accompanied with reduced pulmonary inflammation. Consistent with previous findings showing a pro-apoptotic role of the homolog S100A8/9 in a variety of cell lines, we found that S100A12 mediates apoptosis in vitro. Moreover, cultured epithelial cells also underwent increased expression of Fas and caspase 3 after exposure to S100A12. Since we observed only minor abnormalities in the airway epithelium of S100A12-TG lung, we speculate that the regenerative/ proliferative capacity of epithelial cells might compensate, at least in part, for this pro-apoptotic effect of S100A12. In addition, we can not exclude the possibility of apoptosis of selective subpopulations of inflammatory cells, which could have resulted in attenuation of lung inflammation. However, IgG1 production in response to OVA was not impaired in S100A12 TG mice.
By virtue of its ability to activate RAGE and to induce reactive oxygen species and expression of cytokines such as IL-6 and TNF-α, S100A12 is widely accepted to be a “pro-inflammatory” mediator. Our unexpected finding of attenuated allergic lung inflammation in mice expressing S100A12 underscores a complex and pleiotropic role of S100A12 in modulating inflammation. We suggest that the elevated levels of S100/calgranulins seen in human asthma are likely a consequence of increased inflammatory cells in the lung, and that S100A12 could rather oppose lung inflammation. In support of this hypothesis, S100A8/9, the murine homolog of human S100A12, was found elevated in the BALF in mouse models of allergic [40] and LPS [41] triggered inflammation. However, treatment with neutralizing antibodies to S100A8/9 had no effect on airway hyperresponsiveness (and induced a slight reduction of eosinolphils in BALF) in the allergic model, and had minimal effect on LPS triggered inflammation. There are conflicting reports about the other members of the S100 family. While S100A8, S100A11 and S100A6 were shown to be elevated in experimental models of allergic lung inflammation [42] [43], suppressed levels of S100A8, S100A9 and S100A11 were found by Wong et al [44]. A possible explanation for this discrepancy could be related to the chronic nature of lung inflammation in the Wong study. These findings support a complex role for S100 proteins in orchestrating acute and chronic inflammation, affecting both pro-inflammatory and anti-inflammatory pathways.
Limitations of our study include the fact that mouse models of allergic pulmonary inflammation do not exactly replicate human asthma [45] and that the C57BL/6 mouse strain poorly develops airway smooth muscle remodeling in experimental asthma models [46] and might condition the airway inflammation and smooth muscle remodeling outputs. Nevertheless, it was shown that mice of C57Bl6/J background exhibited increased S100A9 in the BALF after allergen challenged [40], and we reported that S100A12 is functionally active in S100A12-TG animals of C57Bl6 background [18, 47]. Furthermore, although forced expression of S100A12 in smooth muscle is not the “physiologic” source of elevated S100A12 seen in humans asthmatics, S100A12 is induced in cells that are ultimately linked to lung disease such as the inflammatory cells, bronchiolar epithelia and reactive pneumocytes [29]. Most importantly, the S100A12 levels present in the BALF of our TG-OVA mice are comparable to those present in patients with asthma. The fact that S100A12 is functionally active in mice [6, 18] is not surprising given that exists over 90% homology between mouse and human splice variants of RAGE [48], a cell surface receptor that mediates some responses of S100A12 [8]. An alternative approach of using intranasal administration of recombinant S100A12 is less optimal since S100A12 biological function is variable and is greatly affected by intracellular zinc and calcium concentration, as well as by the oxidation state [49]. Recent studies utilizing recombinant S100A12 failed to induce cytokine production in human peripheral blood mononuclear cells [50], although the same authors observed mast cell-dependent edema when recombinant S100A12 was injected into the food pad of mice [6].
Clinical relevance: We showed that transgenic expression of human S100A12 in smooth muscle of mice resulted in remodeling of the large airways and blunted lung inflammation after OVA-sensitization and challenge. We suggest that S100A12 may play a pleiotropic role in human asthma and further studies are needed to understand the precise role of elevated S100A12 found in asthmatics.
Acknowledgement
Source of Funding: GlaxoSmithKline Research & Education Foundation for Cardiovascular Diseases (MAH), and the NIH (MAH, K08-HL090917; BCM, K01HL092588, CTSA UL1 RR024999)
We thank Julian Solway for providing the SM22α promoter and for advice in manuscript preparation. We thank Elizabeth McNally for her comments on the manuscript and continued support. We thank Judy Earle and Jason Churchill for technical assistance.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- LPS
lipopolysaccaride
- MCh
methacholine
- OVA
ovalbumine
- RAGE
receptor for advanced glycation end products
- HASMC
human airway smooth muscle cells
- TG
transgenic
- VSMC
vascular smooth muscle cells
- WT
wild type
Footnotes
The authors have no financial conflict of interest. Disclosure: MA. Hofmann Bowman, None; A. Heydeman, None; J. Gawdzik, None; R. Shilling, None; B. Camoretti-Mercado, None.
Contributor Information
Marion A. Hofmann Bowman, Department of Medicine, Sections of Cardiology, The University of Chicago.
Ahlke Heydemann, Department of Medicine, Sections of Cardiology, The University of Chicago.
Joe Gawdzik, Department of Medicine, Sections of Cardiology, The University of Chicago.
Rebecca A. Shilling, Department of Medicine, Section of Pulmonary and Critical Care, The University of Chicago
Blanca Camoretti-Mercado, Department of Medicine, Section of Pulmonary and Critical Care, The University of Chicago.
References
- 1.Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ. Differential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challenge. Mol Cell Proteomics. 2005;4:1251–1264. doi: 10.1074/mcp.M500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Heizmann CW, Ackermann GE, Galichet A. Pathologies involving the S100 proteins and RAGE. Subcell Biochem. 2007;45:93–138. doi: 10.1007/978-1-4020-6191-2_5. [DOI] [PubMed] [Google Scholar]
- 3.Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, Fitzgerald O, Roth J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42:1383–1389. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
- 4.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, Sorg C, Roth J. S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet. 2003;361:1270–1272. doi: 10.1016/S0140-6736(03)12986-8. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG, Hunt J, Hart P, Hughes JM, Perry MA, Alewood PF, Geczy CL. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119:106–114. doi: 10.1016/j.jaci.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz E, Muhlebach MS, Tessier PA, Alexis NE, Duncan Hite R, Seeds MC, Peden DB, Meredith W. Different expression ratio of S100A8/A9 and S100A12 in acute and chronic lung diseases. Respir Med. 2008;102:567–573. doi: 10.1016/j.rmed.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 9.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 10.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 11.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 12.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE™/™ mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, Kasper M, Bierhaus A, Oury TD. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, Bierhaus A, van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182:4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 16.Fuellen G, Nacken W, Sorg C, Kerkhoff C. Computational searches for missing orthologs: the case of S100A12 in mice. OMICS. 2004;8:334–340. doi: 10.1089/omi.2004.8.334. [DOI] [PubMed] [Google Scholar]
- 17.Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS. Structure and expression of a smooth muscle cell-specific gene, SM22 alpha. J Biol Chem. 1995;270:13460–13469. doi: 10.1074/jbc.270.22.13460. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res. 2010;106:145–154. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD, Chen B, Weinstock JV, Solway J, Hamann KJ, Sperling AI. Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med. 2006;203:1173–1184. doi: 10.1084/jem.20051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heydemann A, Huber JM, Demonbreun A, Hadhazy M, McNally EM. Genetic background influences muscular dystrophy. Neuromuscul Disord. 2005;15:601–609. doi: 10.1016/j.nmd.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Noori F, Abduljawad S, Suffin DM, Riar S, Pi J, Bennett-Venner A, Alsumrain M, Klukowicz AJ, Miller RA. Mounier-Kuhn Syndrome: A Case Report. Lung. doi: 10.1007/s00408-009-9220-0. [DOI] [PubMed] [Google Scholar]
- 22.Hardaker EL, Bacon AM, Carlson K, Roshak AK, Foley JJ, Schmidt DB, Buckley PT, Comegys M, Panettieri RA, Jr, Sarau HM, Belmonte KE. Regulation of TNF-alpha- and IFN-gamma-induced CXCL10 expression: participation of the airway smooth muscle in the pulmonary inflammatory response in chronic obstructive pulmonary disease. FASEB J. 2004;18:191–193. doi: 10.1096/fj.03-0170fje. [DOI] [PubMed] [Google Scholar]
- 23.Clarke DL, Clifford RL, Jindarat S, Proud D, Pang L, Belvisi M, Knox AJ. TNF-alpha and IFN-gamma synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-kappaB, and the transcriptional coactivator CREB-binding protein. J Biol Chem. 285:29101–29110. doi: 10.1074/jbc.M109.0999952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, Hansen JB, Dissing S, Malling HJ, Skov PS, Poulsen LK. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. J Immunol. 2000;165:1548–1556. doi: 10.4049/jimmunol.165.3.1548. [DOI] [PubMed] [Google Scholar]
- 25.Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, Dufour JH, Luster AD. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002;168:5278–5286. doi: 10.4049/jimmunol.168.10.5278. [DOI] [PubMed] [Google Scholar]
- 26.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–760. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]
- 27.Ghavami S, Kerkhoff C, Chazin WJ, Kadkhoda K, Xiao W, Zuse A, Hashemi M, Eshraghi M, Schulze-Osthoff K, Klonisch T, Los M. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/HtrA2. Biochim Biophys Acta. 2008;1783:297–311. doi: 10.1016/j.bbamcr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res. 2010;106:93–101. doi: 10.1161/CIRCRESAHA.109.195834. [DOI] [PubMed] [Google Scholar]
- 29.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19:1437–1445. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 30.Cookson WO, Ubhi B, Lawrence R, Abecasis GR, Walley AJ, Cox HE, Coleman R, Leaves NI, Trembath RC, Moffatt MF, Harper JI. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet. 2001;27:372–373. doi: 10.1038/86867. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M, Mehla K, Batra J, Ghosh B. Association of a chromosome 1q21 locus in close proximity to a late cornified envelope-like proline-rich 1 (LELP1) gene with total serum IgE levels. J Hum Genet. 2007;52:378–383. doi: 10.1007/s10038-007-0118-5. [DOI] [PubMed] [Google Scholar]
- 32.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 33.Kheradmand F, Corry DB. Discovery of novel markers in allergic lung inflammation through proteomic-based technologies. Expert Rev Proteomics. 2008;5:9–12. doi: 10.1586/14789450.5.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Halayko AJ, Ghavami S. S100A8/A9: a mediator of severe asthma pathogenesis and morbidity? Can J Physiol Pharmacol. 2009;87:743–755. doi: 10.1139/Y09-054. [DOI] [PubMed] [Google Scholar]
- 35.Hershenson MB, Brown M, Camoretti-Mercado B, Solway J. Airway smooth muscle in asthma. Annu Rev Pathol. 2008;3:523–555. doi: 10.1146/annurev.pathmechdis.1.110304.100213. [DOI] [PubMed] [Google Scholar]
- 36.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 37.Panettieri RA, Jr, Kotlikoff MI, Gerthoffer WT, Hershenson MB, Woodruff PG, Hall IP, Banks-Schlegel S. Airway smooth muscle in bronchial tone, inflammation, and remodeling: basic knowledge to clinical relevance. Am J Respir Crit Care Med. 2008;177:248–252. doi: 10.1164/rccm.200708-1217PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camoretti-Mercado B. Targeting the airway smooth muscle for asthma treatment. Transl Res. 2009;154:165–174. doi: 10.1016/j.trsl.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, Wernersson S. Mouse Mast Cell Protease 4 Is the Major Chymase in Murine Airways and Has a Protective Role in Allergic Airway Inflammation. J Immunol. 2009 doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 40.Greenlee KJ, Corry DB, Engler DA, Matsunami RK, Tessier P, Cook RG, Werb Z, Kheradmand F. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177:7312–7321. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozinovski S, Cross M, Vlahos R, Jones JE, Hsuu K, Tessier PA, Reynolds EC, Hume DA, Hamilton JA, Geczy CL, Anderson GP. S100A8 chemotactic protein is abundantly increased, but only a minor contributor to LPS-induced, steroid resistant neutrophilic lung inflammation in vivo. J Proteome Res. 2005;4:136–145. doi: 10.1021/pr049829t. [DOI] [PubMed] [Google Scholar]
- 42.Calvo FQ, Fillet M, de Seny D, Meuwis MA, Maree R, Crahay C, Paulissen G, Rocks N, Gueders M, Wehenkel L, Merville MP, Louis R, Foidart JM, Noel A, Cataldo D. Biomarker discovery in asthma-related inflammation and remodeling. Proteomics. 2009;9:2163–2170. doi: 10.1002/pmic.200800643. [DOI] [PubMed] [Google Scholar]
- 43.Xu YD, Cui JM, Wang Y, Yin LM, Gao CK, Liu YY, Yang YQ. The early asthmatic response is associated with glycolysis, calcium binding and mitochondria activity as revealed by proteomic analysis in rats. Respir Res. 11:107. doi: 10.1186/1465-9921-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong WS, Zhao J. Proteome analysis of chronically inflamed lungs in a mouse chronic asthma model. Int Arch Allergy Immunol. 2008;147:179–189. doi: 10.1159/000142040. [DOI] [PubMed] [Google Scholar]
- 45.Kumar RK, Foster PS. Murine model of chronic human asthma. Immunol Cell Biol. 2001;79:141–144. doi: 10.1046/j.1440-1711.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- 46.Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003;168:959–967. doi: 10.1164/rccm.200210-1188OC. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in Vascular Smooth Muscle Accelerates Vascular Calcification in Apolipoprotein E-Null Mice by Activating an Osteogenic Gene Regulatory Program. [October 11, 2010];Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.217745. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22:1572–1580. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- 49.Ma W, Lee SE, Guo J, Qu W, Hudson BI, Schmidt AM, Barile GR. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007;48:1355–1361. doi: 10.1167/iovs.06-0738. [DOI] [PubMed] [Google Scholar]
- 50.Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, Rahimi F, Di Girolamo N, Song C, Jessup W, Kockx M, Bobryshev YV, Freedman SB, Geczy CL. Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009;183:593–603. doi: 10.4049/jimmunol.0900373. [DOI] [PubMed] [Google Scholar]