Abstract
Background
Recent studies have reported conflicting data on the association between maternal intake of vitamin D during pregnancy and asthma.
Objective
Assess the influence of prenatal vitamin D status on immune function at birth.
Methods
In an inner-city birth cohort of 568 newborns, 520 of whom had at least one atopic parent, we measured umbilical cord (UC) plasma concentration of 25-hydroxy vitamin D (25(OH)D) and the cytokine responses of UC blood mononuclear cells (UCMCs) to stimuli including phytohemaglutinin (PHA), lipopolysaccharide (LPS), and peptidoglycan (PG). In a subset, UCMC expression of regulatory T-cell markers and the suppressive activity of CD4+CD25+ UCMCs was measured.
Results
The 25th, 50th, and 75th percentiles of UC plasma 25(OH)D level were 15.0, 20.2, and 25.6 ng/mL, respectively. Most cytokine responses of UCMC were not correlated with UC 25(OH)D concentration; however, IFN-γ release after LPS stimulation was weakly positively correlated with UC 25(OH)D concentration (r = 0.11, p =0.01). PHA responses were not significantly correlated with 25(OH)D concentration. The UC plasma 25(OH)D concentration was inversely related to the number of CD25+ (r= -0.20, p=0.06), CD25Bright (r= -0.21, p=0.05), and CD25+FoxP3 (r= -0.29, p=0.06) cells as a proportion of CD4+ T cells in UC blood (r = -0.26, p = 0.04) but not to the suppressive activity of CD4+CD25+ cells (r=0.17, p=0.22).
Conclusion and Clinical Relevance
UC 25(OH)D concentration was not correlated with most UCMC cytokine responses to multiple stimuli. There was a suggestion of a weakly positive correlation with IFN-γ release after LPS stimulation. The proportions of CD25+, CD25bright, and CD25+FoxP3 cells to total CD4+ T cells were inversely correlated with UC 25(OH)D concentration. Our findings suggest that higher vitamin D levels at birth may be associated with a lower number of T regulatory cells. Vitamin D status in utero may influence immune regulation in early life.
Introduction
In recent decades the prevalence of asthma and allergy has increased in many industrialized countries. [1, 2] A number of hypotheses have been proposed to explain this trend, including a decline in exposure to infection and microbial constituents (the “hygiene hypothesis”) and an increase in exposure to environmental pollutants, such as diesel exhaust particles. More recently, immunologic and epidemiologic studies have suggested a role for vitamin D in the development of asthma and allergy.[2]
The National Health and Nutrition Examination Survey in adults found a strong relationship between low serum 25-hydroxyvitamin D (25(OH)D) concentration and reduced lung function in a cross-sectional analysis of nearly 25,000 participants. [3] In children, a recently published study showed lower levels of vitamin D to be associated with increased markers of allergy and asthma severity.[4] Furthermore, higher maternal vitamin D intake during pregnancy was associated with lower risk of wheeze at 3 and 5 years of age. [5, 6] While this study did not show an association between maternal vitamin D intake and atopic dermatitis, a more recent study in obese adults showed lower vitamin D levels was associated with increased risk of atopic dermatitis. [7] These studies suggest that vitamin D status may play a role in lung and/or immune system development and in the pathogenesis of asthma and atopic diseases.
However, not all evidence supports the hypothesis that vitamin D deficiency is a risk factor for asthma.[8, 9] A study from Northern Finland indicated that children receiving vitamin D supplements in the first year of life had a higher risk of developing asthma, atopy and allergic rhinitis in adulthood. [10] Additionally, a study from the United Kingdom showed that a high maternal plasma concentration of 25(OH)D during late pregnancy was associated with a higher risk of eczema in children at 9 months of age.[11] A more recent study from Sweden showed that higher intake of vitamin D in the first year of life was associated with increased risk of developing atopic dermatitis by 6 years of age. [12]
In vitro studies have established that the vitamin D metabolites 25(OH)D and 1,25-dihydroxyvitamin D have immunoregulatory activity that may influence a number of disease processes including autoimmune diseases, predisposition to infections, and allergy. [13] While most studies agree that vitamin D can inhibit Th1 cytokine release, suggesting a role for vitamin D in the pathogenesis of autoimmune diseases and infection, the role of vitamin D on Th2 responses and regulatory T cells is less clear.[13-15] Vitamin D appears to stimulate Th2 cytokine secretion by peripheral blood mononuclear cells [16-20] but may suppress these Th2 responses by human cord blood mononuclear cells [21, 22] Additional in vitro studies have shown that vitamin D metabolites can promote regulatory T cell induction [23-25], which could dampen Th2 polarization
To date, no large cohort studies have investigated the influence of prenatal vitamin D status on immune function at birth. The purpose of our study is to examine the relationship of umbilical cord (UC) plasma 25(OH)D concentration, as an indicator of prenatal vitamin D status in late pregnancy, to the immune responses of umbilical cord blood mononuclear cells (CBMCs). We focused attention on innate immune responses because of the apparent importance of early life innate immunity in asthma and allergy pathogenesis and because of prior literature suggesting that vitamin D may influence innate immune responses [13, 14, 26]. We also included an exploratory analysis of the correlation between adaptive immune responses and 25(OH)D. In addition, in a subset of subjects we looked at the relationship of cord plasma 25(OH)D concentration to regulatory T cell phenotype and function. Our primary hypothesis was that umbilical cord plasma 25(OH)D concentration would be related to CBMC release of IFN-α, IFN-γ, and IL-10 in response to the innate stimuli lipopolysaccharide (LPS) and peptidoglycan (PG). We also hypothesized that regulatory T cell number and function would be positively associated with plasma 25(OH)D at the time of birth.
Methods
Study design and subjects
Participants were recruited as part of a prospective birth-cohort study designed to evaluate the environmental and immunologic causes of asthma in children living in low-income urban communities. [27] Pregnant women in Baltimore, Boston, New York, and St. Louis were recruited at the obstetrical practices of the participating institutions and by means of flyers and newspaper advertisements. Mothers were enrolled if they or their expected baby's biologic father had a history of asthma, allergic rhinitis or eczema, as assessed by questionnaire. Only mothers living in census tracts with at least 20% of the residents with income below the federal poverty level were eligible. Informed consent and maternal health questionnaires were obtained at the prenatal visit. Babies were enrolled only if the gestational age at birth was at least 34 weeks; an umbilical cord blood specimen could be collected; and the immediate postnatal period was free of respiratory distress syndrome, bronchopulmonary dysplasia, or pneumonias. Babies of HIV-infected mothers were excluded. Additionally, a small cohort in which neither the mother nor the father had a history of asthma or atopic disease was also recruited for this study. The same enrollment process and eligibility requirements were applied to the non-atopic cohort.
Collection of umbilical cord blood samples
At the time of birth, umbilical cord blood was collected from all participants. An attempt was made to collect 50 ml of cord blood, and at least a 5 ml sample was required for the child to be eligible to continue in the study. The cord blood samples were collected by needle and syringe, transferred to polypropylene tubes containing RPMI cell culture medium and heparin, maintained at room temperature, and transported to the laboratory within 16 hours of collection. The samples were separated by Ficoll centrifugation following standardized procedures, and the separated CBMCs were then used immediately for cytokine stimulation studies, as described below. The plasma fraction, diluted with RPMI medium, was frozen at -80 C.
Measurement of CBMC cytokine responses
The CBMCs were incubated with a number of stimuli including the innate, mitogenic, and adaptive stimuli as previously reported [28]. After incubation for 24 hrs (innate and polyclonal stimuli) or 5 days (antigens), cell supernatant fluids were collected, divided into aliquots, frozen at -80°C, and shipped to a central laboratory for cytokine analysis. Cytokine concentrations in supernatants were analyzed using a bead-based multiplex assay (Beadlyte, Upstate Biotechnology, Lake Placid, NY). The cytokines measured were selected based on their importance in specific innate and adaptive immune responses that have been related to allergic inflammation and the immune response to respiratory viruses [27].
For this analysis, we focused on the relationship of cord plasma 25(OH)D concentration to CBMC responses to innate stimuli LPS and PG. We included an analysis of cord plasma 25(OH)D and cytokine responses to the nonspecific mitogen stimuli phytohemagglutinin (PHA) for comparison. As part of an exploratory analysis we looked at the relationship of 25(OH)D to the additional innate stimuli polyinosinic-polycytidylic acid and type C CpG motifs (CpG-C), and to the adaptive stimuli respiratory syncytial virus, cockroach extract, dust mite (D. pteronyssinus) extract, and tetanus toxoid.
Regulatory T-cell analysis
In the subset of participants at the Boston site, regulatory T-cell studies were also performed.[29] To assess regulatory T-cell function of the CBMCs, the sample from each subject was divided into 2 equal aliquots, and one aliquot was depleted of CD25+ T lymphocytes using MACS columns with a positive CD25+ T-cell selection kit (Miltenyi Biotech Inc., Auburn, CA). The second aliquot was not depleted of CD25+ T cells but was subjected to the same separation process using MACS columns with anti-FITC, which is an irrelevant antibody (Miltenyi Biotech Inc., Auburn, CA). The CD25+ microbeads removed between 85-95% of CD4+CD25+ T cells as analyzed by FACS (data not shown). Undepleted or CD25+-depleted cells were cultured in triplicate in 96 well round-bottom plates containing AIM-V serum-free medium (Invitrogen Corp., Grand Island, NY) alone or with 5μg/ml PHA added. After four days in culture the cells were pulsed with [3H] thymidine (NEN™, Life Science Products, Inc., Boston, MA), and proliferation was measured using a β-scintillation counter (Wallac Microbeta Trilux, Perkin Elmer, Waltham, MA). Results were expressed as proliferation index (PI = average cpm in PHA-stimulated cells/ average cpm in non-stimulated cells). To establish the effects of CD25 depletion on proliferative activity, we calculated the suppressive index (SI), which is a ratio of PI for CD25 depleted to CD25 undepleted cell sample. To assess regulatory T-cell phenotype by surface staining, aliquots of 2 × 106 UCMCs were washed in phosphate buffered saline (PBS) then resuspended in PBS containing mouse IgG (Invitrogen Corporation, Carlsbad, CA) to serve as an Fc receptor block. Cells were then stained with fluorochrome-labeled mAbs to : CD4 (clone SK3) and CD25 (clone 2A3), (BD Bioscience, San Jose, CA). After the incubation with mAbs, red blood cells were lysed, and the cells were washed with PBS before fixing in 2% Ultrapure formaldehyde (Polysciences, Inc., Warrington, PA).
A modification of the surface staining procedure was used for intracellular Foxp3 staining. After the final PBS wash, but before formaldehyde fixation, the cells were resuspended in Foxp3 Fix/Perm buffer (BioLegend, San Diego, CA) and incubated in the dark, at room temperature for 30 minutes. The cells were then washed twice with Foxp3 Perm buffer (BioLegend, San Diego, CA) and resuspended in 50 μl of Perm buffer containing 100 μg/ml human IgG Cohn fraction II and III (Sigma-Aldrich, St. Louis, MO) for 10 minutes before adding the anti-Foxp3 or isotype control mAbs. Cells were incubated for an hour in the dark, washed once with Perm buffer, and then once with PBS before fixing in 2% formaldehyde.
Samples were analyzed using the FACSCanto cytometer (BD Bioscience, San Jose, CA) running DiVA acquisition software. Cell viability was determined by the Live/Dead fixable green stain according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA). Specimens with viabilities less than 85% were excluded from analysis.
Measurement of umbilical cord plasma 25(OH)D concentration
The cord plasma 25(OH)D concentration was determined by liquid chromatography tandem mass spectroscopy (LCMSMS). After the extraction of 25(OH)D2 and 25(OH)D3 from standards, quality controls, and cord plasma with methanol, the extract was transferred to a new 96-well plate and placed in the HPLC autosampler. Analysis was performed using an LX4 Turbo Flow system (Cohesive Technologies, Franklin, MA) and a TSQ Ultra triple quadrupole mass spectrometer (ThermoFisher, Franklin, MA).
After analytes were ionized in an atmospheric-pressure chemical-ionization source, detection was accomplished by selected reaction monitoring (SRM) of the following ion pairs: m/z 395.1/209.1, 251.1, and 179.1 for 25(OH)D2; m/z 383.1/211.1 for 25(OH)D3; m/z 389.1/211.1 for 25(OH)D2-d6; and m/z 401.1/209.1, 251.1, and 179.1 for 25(OH)D3-d6. The obtained signals of 25(OH)D2 and 25(OH)D3 in the calibrators, controls, and samples were normalized according to their respective internal standard 25(OH)D3-d6 or 25(OH)D2-d6 signals. Concentrations in the samples and controls were calculated off the normalized six-point calibration curves [0–128 ng/ml (0–320 nmol/liter)]. Samples with concentrations that exceeded the highest calibrator were diluted and run again. The total 25OHD concentrations of each control and sample were calculated by summing the measured values of 25OHD2 and 25OHD3. [28, 29].
The detection limit for the assay was 4 ng/ml and the interassay coefficient of variation was ∼10%. The LCMSMS measurements of 25(OH)D concentration were adjusted for the dilution of the cord blood in RPMI medium at the time of specimen collection such that the concentrations used for analysis represent the actual cord plasma concentrations.
Statistical Analysis
The relationship of cord plasma 25(OH)D concentration and cytokine responses to potential confounders was assessed by Pearson correlation. To assess whether demographic and other characteristics including mother's race, newborn gender, gestational age, birth weight, maternal education, family income, maternal smoking, BMI, number of children, season or study site might be confounders in analyses of the relationship between cord plasma 25(OH)D concentration and UCMC cytokine responses, we examined the relationships between these characteristics and 25(OH)D concentration. We did not have data on prenatal vitamin use or nutritional intake to include in our assessment. The relationship of cytokine responses to cord plasma 25(OH)D concentration was assessed using mixed models that incorporated all replicate measurements of cytokine levels while adjusting the variance for the non-independence of replicate measures. Cytokine responses and cord plasma 25(OH)D concentrations were standardized in all mixed models so that model estimates would be comparable to Pearson correlations. All analyses were done using SAS version 9.1.3 (SAS, Inc, Cary, NC).
Results
Mother and Newborn Characteristics
Of the total 609 umbilical cord blood samples collected, we measured the cord plasma 25(OH)D concentration on 568 samples. Of the 41 other samples, 35 were not included because the quantity of cord blood or RPMI medium mixed together upon collection were not recorded, making it impossible to adjust the measured concentration for dilution. Six other subjects did not have sufficient cord plasma sample available at the time the 25(OH)D measurements were made.
Most of the mothers were African American and had previously had one or more children (Table 1). The majority of women were in their twenties, although nearly 25% were under 20 years old. The newborns had a mean gestational age at birth of 38.8 weeks and a mean weight of 3,240 grams. These maternal and newborn characteristics of the main cohort of high-risk children were similar to those of the smaller group of children of parents with no atopy or asthma history.
Table 1. Characteristics of the mothers and newborns enrolled in the URECA Study.
| Characteristic | Main Cohort | Non-atopic Families | ||
|---|---|---|---|---|
| n | % | n | % | |
| Number of mothers | 557 | 49 | ||
| Mother's age in years at child's birth | ||||
| 13-19 | 126 | 23 | 13 | 27 |
| 20-29 | 323 | 58 | 27 | 55 |
| 30-39 | 102 | 18 | 9 | 18 |
| 40-42 | 6 | 1 | 0 | 0 |
| Race or ethnicity of mother | ||||
| Hispanic of any race | 107 | 20 | 9 | 18 |
| Black alone | 391 | 71 | 39 | 80 |
| White alone | 22 | 4 | 0 | 0 |
| More than one race and others | 30 | 6 | 1 | 2 |
| Missing | 7 | -- | 0 | -- |
| Previous Births | ||||
| 0 | 220 | 40 | 17 | 35 |
| 1-2 | 243 | 44 | 24 | 49 |
| >3 | 94 | 17 | 8 | 16 |
| Number of smokers in home | ||||
| 0 | 284 | 52 | 29 | 59 |
| 1 | 168 | 31 | 14 | 29 |
| 2 or more | 97 | 18 | 6 | 12 |
| Missing | 1 | -- | 0 | -- |
| Number of newborns | 560 | 49 | ||
| Race or ethnicity of newborn | ||||
| Hispanic of any race | 115 | 21 | 10 | 20 |
| Black alone | 387 | 70 | 37 | 76 |
| Other | 58 | 9 | 2 | 4 |
| White alone | 7 | 1 | 0 | 0 |
| More than one race and other | 44 | 8 | 2 | 4 |
| Missing | 7 | -- | 0 | -- |
| Sex of newborn | ||||
| Male | 287 | 51 | 25 | 51 |
| Female | 273 | 49 | 24 | 49 |
| Mean | SD | Mean | SD | |
| Gestational age (weeks) | 38.7 | 1.5 | 39.0 | 1.3 |
| Birth weight (grams) | 3237 | 514 | 3212 | 504 |
| Length (cm) | 50.1 | 2.5 | 49.9 | 2.7 |
Distribution of umbilical cord plasma 25(OH)D concentration
The distribution of cord plasma 25(OH)D concentration by site is shown in table 2. The values at each site had a Gaussian distribution. The mean (SD) cord plasma 25(OH)D concentration for all sites combined was 20.7 (9.7) ng/ml. The mean concentration was lower in Boston than in the other sites (Table 2), a difference that was statistically significant (p = <0.001 for overall analysis of variance comparing the four sites). This difference was not explained by race, BMI, income, smoking, gestational age, or birth weight in multivariate analysis.
Table 2. Distribution of umbilical cord plasma 25(OH)D concentration in 568 newborns enrolled in the URECA Study.
| Study site | N | Umbilical cord plasma 25(OH)D concentration (ng/mL) | |||||
|---|---|---|---|---|---|---|---|
| Mean | 10th percentile | 25th percentile | Median | 75th percentile | 90th percentile | ||
| Baltimore | 146 | 22.4 | 11.4 | 15.6 | 21.0 | 25.6 | 36.0 |
| Boston | 142 | 16.9 | 9.0 | 9.0 | 15.0 | 21.0 | 30.0 |
| New York | 112 | 22.0 | 10.8 | 15.1 | 20.7 | 27.0 | 35.2 |
| St. Louis | 168 | 21.7 | 9.3 | 15.0 | 20.9 | 27.0 | 35.2 |
| Overall | 568 | 20.7 | 9.0 | 15.0 | 20.2 | 25.6 | 33.0 |
Relationship of cord plasma 25(OH)D concentration to maternal and newborn covariates
There were no significant relationships between the cord plasma 25(OH)D concentration and mother's race, newborn gender, gestational age, birth weight, maternal education, family income, maternal smoking, or maternal BMI (p > 0.1 for all). In the main cohort of newborns of parents with atopy or asthma, the 25(OH)D concentration was significantly related to study site (lower concentration in Boston), the number of previous children (lower concentration with higher number of previous children), season (higher concentration in the summer, the season with greatest exposure to UV light from the sun, as shown in Figure 1), newborn's ethnicity, and number of smokers in the home. These five factors were also associated with some CBMC cytokine responses in the main cohort; therefore, we adjusted for these factors in the models assessing the relationship between CBMC cytokine responses and cord plasma 25(OH)D concentration.
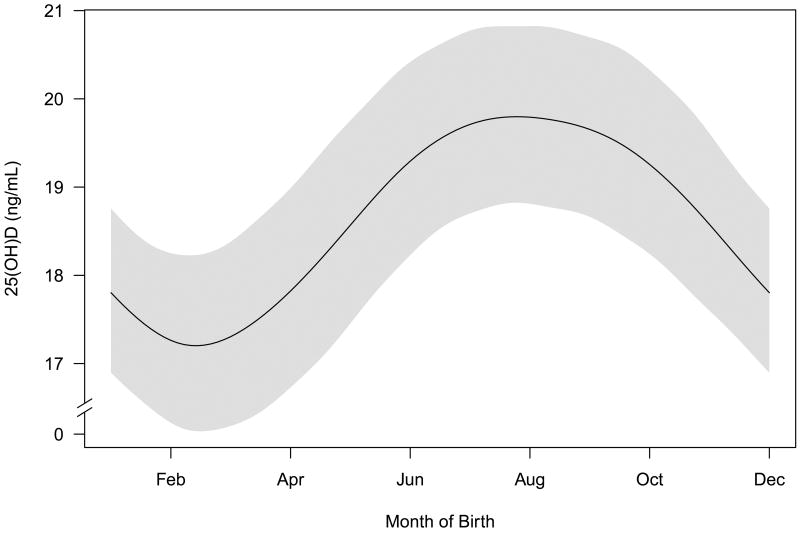
Figure 1.
Relationship between time of year and vitamin D levels. The line is a smoothed estimate of the average umbilical cord plasma concentration of 25 hydroxyvitamin D [25(OH)D] and the shaded area has a width of two standard deviations. Models are fit using a generalized additive model.
Relationship of cord plasma 25(OH)D concentration to UCMC cytokine responses
In the main cohort of high-risk children, there was a significant positive correlation between IFN-γ response to LPS and cord 25(OH)D level (r = 0.11; p = 0.01) (Table 3). There was a suggestion of a negative correlation between IFN-α response to PG and cord 25(OH)D concentration (r = -0.09; p = 0.05). The IL-10 responses to LPS and PG were not significantly correlated with cord 25(OH)D levels. None of the measured cytokine responses to PHA were significantly correlated with cord 25(OH)D concentration. In the smaller group of children of nonatopic, non-asthmatic parents, there was a similar positive correlation between IFN-γ response to LPS and cord 25(OH)D level (r = 0.31; p = 0.05), but no other significant correlations between cord 25(OH)D level and cytokine responses to LPS or PG. A positive correlation in this smaller group between cord 25(OH)D level and IFN-γ response to PHA was not seen in the larger group of high-risk newborns.
Table 3. Correlations between umbilical cord plasma 25(OH)D concentration and umbilical cord mononuclear cell cytokine responses among 568 newborns in URECA Study*.
| Stimulus | Cytokine response | Main cohort (n = 520) r (p value) |
Non-atopic families (n = 48) r (p value) |
|---|---|---|---|
| Lipopolysaccharide | IFN-α | -0.06 (0.13) | 0.00 (0.98) |
| IFN-γ | 0.11 (0.01) | 0.31 (0.05) | |
| IL-10 | 0.07 (0.14) | -0.12 (0.44) | |
| Peptidoglycan | IFN-α | -0.09 (0.05) | -0.02 (0.85) |
| IFN-γ | 0.02 (0.65) | 0.28 (0.05) | |
| IL-10 | 0.04 (0.34) | 0.05 (0.75) | |
| Phytohemagglunin | IFN-γ | 0.00 (1.99) | 0.19 (0.23) |
| IL-10 | -0.02 (0.70) | -0.28 (0.03) | |
| IL-13 | 0.00 (0.96) | -0.08 (0.63) | |
| IL-4 | 0.03 (0.47) | -0.07 (0.58) |
Correlations are adjusted for study site, child's race, parity of mother, season of birth, and number of smokers in the home, as described in text.
In exploratory analyses, we examined the correlation of cord plasma 25(OH)D concentration with other UCMC cytokine responses and with responses to other stimuli including polyinosinic-polycytidylic acid, CpG-C, respiratory syncytial virus, cockroach extract, dust mite (D. pteronyssinus) extract, and tetanus toxoid (Table 4). Although several correlations among the many examined have nominal p-values less than 0.05, there is no consistent pattern to suggest a clear influence of cord plasma 25(OH)D on these immune responses.
Table 4. Correlations between umbilical cord plasma 25(OH)D concentration and umbilical cord mononuclear cell cytokine responses among 568 newborns in URECA Study – Exploratory analyses*.
| Stimulus | Cytokine response | Main cohort (n = 520) r (p value) |
Non-atopic families (n = 48) r (p value) |
|---|---|---|---|
| LPS | IL-12-p4 | 0.07 (0.13) | 0.14 (0.31) |
| TNF-α | 0.02 (0.64) | 0.18 (0.18) | |
| Peptidoglycan | IL-12-p4 | 0.06 (0.22) | 0.28 (0.07) |
| TNF-α | -0.11 (0.01) | 0.06 (0.70) | |
| CpG-C | IL-12-p4 | 0.04 (0.30) | 0.23 (0.08) |
| TNF-α | 0.02 (0.69) | 0.14 (0.30) | |
| IFN-α | 0.05 (0.22) | 0.26 (0.08) | |
| IFN-γ | 0.03 (0.46) | 0.35 (0.02) | |
| IL-10 | 0.09 (0.04) | -0.01 (0.96) | |
| Polyinosinic-polycytidylic acid | IL-12-p4 | 0.05 (0.30) | 0.08 (0.60) |
| TNF-α | -0.04 (0.43) | 0.35 (0.01) | |
| IFN-α | 0.05 (0.24) | 0.08 (0.62) | |
| IFN-γ | 0.02 (0.59) | 0.15 (0.31) | |
| IL-10 | 0.03 (0.47) | -0.27 (0.08) | |
| Respiratory syncytial virus | IL-12-p4 | 0.04 (0.40) | 0.13 (0.41) |
| TNF-α | -0.04 (0.42) | 0.08 (0.61) | |
| IFN-α | -0.03 (0.43) | -0.01 (0.94) | |
| IFN-γ | -0.05 (0.32) | 0.12 (0.47) | |
| IL-10 | 0.05 (0.20) | -0.24 (0.15) | |
| Cockroach extract | IFN-γ | 0.09 (0.04) | -0.04 (0.63) |
| IL-13 | 0.07 (0.10) | 0.00 (0.86) | |
| IL-10 | 0.06 (0.18) | -0.26 (0.03) | |
| Dust mite extract | IFN-γ | 0.00 (0.99) | -0.18 (0.19) |
| IL-13 | 0.01 (0.89) | -0.03 (0.84) | |
| IL-10 | 0.06 (0.19) | -0.24 (0.14) | |
| Tetanus toxoid | IFN-γ | 0.02 (0.57) | 0.11 (0.40) |
| IL-13 | -0.01 (0.84) | 0.04 (0.61)-0.20 | |
| IL-10 | 0.09 (0.04) | (0.17) |
Correlations are adjusted for study site, race of newborn, parity of mother, season of birth, and number of smokers in the home, as described in text. Correlations with p values less than 0.05 are in bold font.
Relationship of cord plasma 25(OH)D concentration to UCMC regulatory T-cell markers and function
The suppressive index, i.e. the ratio of PHA-induced proliferation for CD25-depleted to CD25-undepleted UCMCs, was not significantly correlated with the cord plasma 25(OH)D concentration (Table 5). The cord plasma 25(OH)D concentration was negatively correlated with the number of CD25+ cells (r= -0.20, p=0.06), CD25Bright cells (r=,-0.21, p= 0.05) and CD25+FoxP3 cells (r= -0.29, p=0.06), expressed as a proportion of total CD4+ T cells, with borderline statistical significance.
Table 5. Correlations between umbilical cord plasma 25(OH)D concentration and umbilical cord mononuclear cell characteristics related to T-regulatory cells among URECA Study newborns in born in the Boston site.
| Chararcteristic | N | r (p value) |
|---|---|---|
| CD25+ cells as a proportion of CD4+ T cells | 105 | -0.20 (0.06) |
| CD25Bright cells as a proportion of CD4+ T cells | 106 | -0.21 (0.05) |
| CD25+FoxP3 cells as a proportion of CD4+ T cells | 55 | -0.29 (0.06) |
| Suppressive Index | 69 | 0.17 (0.22) |
Correlations are adjusted for child's race, parity of mother, season of birth, and number of smokers in the home.
Discussion
Innate immunity and regulatory T cells are postulated to play key roles in the development of adaptive immunity, allergy, and asthma in early life [26, 30]. These processes have also been hypothesized to be influenced by vitamin D [2]. Therefore, we focused our analysis on the hypotheses that umbilical cord plasma 25(OH)D concentration would be correlated with innate immune responses and regulatory T-cell numbers or function at the time of birth. We observed little correlation between cord plasma 25(OH)D concentration and the cytokine responses of CBMC to innate stimuli or to PHA. There was a weakly positive correlation between cord plasma 25(OH)D concentration and CBMC release of IFN-γ upon stimulation with LPS in children born to atopic (r = 0.11, p = 0.01) or nonatopic (r = 0.31, p = 0.05) families, suggesting that prenatal vitamin D status may influence innate immune responses at birth. These modest correlations must be interpreted cautiously, however, and could represent chance findings in light of the multiple correlation tests performed. Because IFN-γ plays a key role in the development of Th1 cells [31], this weakly positive correlation suggests that prenatal vitamin D status could secondarily influence the subsequent differentiation of naïve T cells into Th1 vs. Th2 cells upon antigen stimulation. Interestingly, it has been reported that 1,25-dihyroxy vitamin D inhibits IFN-γ expression by T cells in vitro [13]. These findings suggest that the effects of vitamin D on the development of innate IFN-γ responses in utero could be distinct from the effects on postnatal T cell responses and that the influence of vitamin D to either predispose or prevent the development of allergy could be dependent on the timing of exposure.
In addition to the association with IFN-γ responses, there was a negative correlation of borderline significance between cord plasma 25(OH)D and PG-induced IFN-α responses. These observed correlations between cord 25(OH)D level and IFN-γ and IFN-α responses at birth suggest that prenatal vitamin D status could influence patterns of immune development and vulnerability to infection in early life, both of which could in turn influence the risk of developing asthma and allergy. Because IL-10 is a key immunomodulatory cytokine related to regulatory T-cell function, we hypothesized that prenatal vitamin D status would be related to the IL-10 responses of CBMCs to innate stimuli; however, no significant relationships were observed. Cytokine responses to the nonspecific mitogen PHA, indicative of overall T-cell function, were also not significantly correlated with cord plasma 25(OH)D concentration, except for a positive correlation for IFN-γ response that was limited to the relatively small number of nonatopic families and could be a type 1 error due to chance.
In addition to analyses done to address pre-specified hypotheses, we undertook a series of exploratory hypotheses to take full advantage of the multiple immune stimuli used and cytokine responses measured for the URECA Study. No consistent pattern was seen among the correlations between cord plasma 25(OH)D concentration and the cytokine responses examined. While the cord plasma vitamin D level was not correlated with IFN-γ production in response to specific antigens, as it was for LPS-induced IFN-γ production, the IFN-γ responses of UCMCs to antigenic stimuli were much smaller than the responses to LPS, reflecting the relatively undeveloped state of adaptive immunity at the time of birth. [32]
For the subset of newborns whose umbilical cord blood was subjected to regulatory T-cell studies, we observed borderline significant negative correlations between cord plasma 25(OH)D concentration and the proportion of CD-4+ T cells expressing CD25+, CD25+bright and FoxP3, i.e. relationships opposite to that predicted by our hypothesis that higher prenatal vitamin D levels enhance regulatory T-cell numbers or function. Prior reports of both in vitro and in vivo studies indicate that vitamin D enhances the development or suppressive activity of T-regulatory cells.[33] Our data suggest that there may be distinct effects of vitamin D on the prenatal differentiation of cells expressing CD25+, CD25+bright and FoxP3, which could result in fewer regulatory T cells and thus potentially predispose to allergy. The biologic significance of these findings, however, remains uncertain. Follow-up of these children will allow us to determine whether the effects of prenatal vitamin D status on immunologic function persist during postnatal development.
The distribution of umbilical cord plasma 25(OH)D concentration in our cohort must be interpreted with recognition that UC and neonatal plasma 25(OH)D concentrations are approximately 33 percent lower than maternal concentrations [34, 35]. Thus, the observed 25th percentile value of 15.0 ng/mL in our cohort would correspond to a maternal concentration of approximately 22.5 ng/mL, while our median value of 20.2 ng/mL would correspond to a maternal level of approximately 30.3 ng/mL. Although the optimal concentration of plasma 25(OH)D remains controversial and may vary for different physiologic functions, it has been proposed [36] that a level less than 30 ng/mL should be considered vitamin D insufficiency, rather than the usually cited threshold of 20 ng/mL for defining vitamin D deficiency. The observed distribution of cord plasma 25(OH)D concentration suggests that a substantial portion of the mothers of the newborns in our study had plasma 25(OH)D levels near or below the lower limit of the sufficient range. This is consistent with previous studies which have also demonstrated that a substantial portion of urban mothers and newborns in the US have 25(OH)D levels in the insufficient range. [35]
We observed, as have others [34], that cord plasma 25(OH)D concentration varied with season, higher levels observed in the summer when sun exposure is highest. Because season was related to cord plasma 25(OH)D concentration and to some of the cytokine responses measured, we adjusted for season in our analyses of the main cohort.
Our study does have several limitations. We do not have data on maternal intake of vitamin D, nor do we have data on maternal plasma 25(OH)D concentration during pregnancy, both of which would help give a more complete estimate of prenatal vitamin D status during the entire pregnancy. We also do not have data on other dietary factors which could be correlated with vitamin D and potentially confound the relationships examined if such micronutrients were also related to immune function at birth. We were unable to find a clear explanation for lower mean UCMC 25(OH)D levels observed in Boston compared to other sites. We adjusted for study site in our analysis of the main cohort so that these differences would not affect our results. Finally, our sample size for newborns from non-atopic families is small.
In summary, our results suggest that innate immune responsiveness at birth, along with the proportion of T cells expressing CD25+, CD25Bright and FoxP3, are correlated with prenatal vitamin D status, as assessed by cord plasma 25(OH)D concentration. The influence of innate immunity in early life on the development of allergy, along with the high prevalence of vitamin D insufficiency in northern urban communities, suggests that the immune effects of prenatal vitamin D insufficiency have the potential to affect the subsequent risk of developing childhood allergy, asthma, and perhaps other disorders. Follow-up of our cohort will permit evaluation of whether cord plasma 25(OH)D concentration predicts future allergy and asthma outcomes.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contracts number NO1-AI-25496 and NO1-AI-25482, and from the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9(3):202–7. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 4.Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 6.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oren E, Banerji A, Camargo CA., Jr Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121(2):533–4. doi: 10.1016/j.jaci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Wjst M. The vitamin D slant on allergy. Pediatr Allergy Immunol. 2006 doi: 10.1111/j.1399-3038.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 9.Wjst M. Introduction of oral vitamin D supplementation and the rise of the allergy pandemic. Allergy Asthma Clin Immunol. 2009;5(1):8. doi: 10.1186/1710-1492-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 11.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Back O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89(1):28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 13.Van Etten E, Gysemans C, Verstuyf A, Bouillon R, Mathieu C. Immunomodulatory properties of a 1,25(OH)(2) vitamin D(3) analog combined with IFNbeta in an animal model of syngeneic islet transplantation. Transplant Proc. 2001;33(3):2319. doi: 10.1016/s0041-1345(01)02007-3. [DOI] [PubMed] [Google Scholar]
- 14.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 Suppl):1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 15.May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3(4):377–93. doi: 10.2174/1568010042634596. [DOI] [PubMed] [Google Scholar]
- 16.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112(3):585–92. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 17.Iho S, Kura F, Sugiyama H, Takahashi T, Hoshino T. The role of monocytes in the suppression of PHA-induced proliferation and IL 2 production of human mononuclear cells by 1,25-dihydroxyvitamin D3. Immunol Lett. 1985;11(5-6):331–6. doi: 10.1016/0165-2478(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 18.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84(10):3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 20.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160(11):5314–9. [PubMed] [Google Scholar]
- 21.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52(1):12–8. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Annesi-Maesano I. Perinatal events, vitamin D, and the development of allergy. Pediatr Res. 2002;52(1):3–5. doi: 10.1203/00006450-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167(4):1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 24.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 25.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116(1):146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroder NW. The role of innate immunity in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2009;9(1):38–43. doi: 10.1097/ACI.0b013e32831d0f99. [DOI] [PubMed] [Google Scholar]
- 27.Gern JE, Visness CM, Gergen PJ, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9(1):17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shreffler WG, Visness CM, Burger M, et al. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multicenter study. BMC Immunol. 2006;7:29. doi: 10.1186/1471-2172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ly NP, Ruiz-Perez B, McLoughlin RM, et al. Characterization of regulatory T cells in urban newborns. Clin Mol Allergy. 2009;7:8. doi: 10.1186/1476-7961-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finotto S. T-cell regulation in asthmatic diseases. Chem Immunol Allergy. 2008;94:83–92. doi: 10.1159/000154869. [DOI] [PubMed] [Google Scholar]
- 31.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 32.Gold DR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, O'Connor GT, Wood RA, Burger M, Wright RJ, Witter FR, Lee-Parritz A, Sperling R, Sadovsky Y, Togias A, Gern J. Parental Characteristics, Somatic Fetal Growth, and Season of Birth Influence Innate and Adaptive Cord Blood Cytokine Responses. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2009.08.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verity CM, Burman D, Beadle PC, Holton JB, Morris A. Seasonal changes in perinatal vitamin D metabolism: maternal and cord blood biochemistry in normal pregnancies. Arch Dis Child. 1981;56(12):943–8. doi: 10.1136/adc.56.12.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46(1):42–4. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]