Abstract
Electroporation (EP) is a simple in vivo method to deliver normally impermeable molecules, such as plasmid DNA, to a variety of tissues. Delivery of plasmid DNA by EP to a large surface area is not practical because the distance between the electrode pairs, and therefore the applied voltage, must be increased to effectively permeabilize the cell membrane. The design of the MultiElectrode Array (MEA) incorporates multiple electrode pairs at a fixed distance to allow for delivery of plasmid DNA to the skin potentially reducing the sensation associated with in vivo electroporation. In this report, we evaluate the effects of field strength and pulse width on transgene expression and duration using a plasmid encoding the luciferase reporter gene delivered by intradermal injection in a guinea pig model followed by EP with the MEA. As expected, the level of luciferase expression increased with the magnitude and duration of the voltage applied. In addition to adjusting transgene expression levels by altering fielding strength, levels could also be controlled by adjusting the plasmid dose. Our results indicate that the design of the MEA is a viable option for cutaneous plasmid DNA delivery by in vivo EP to a large surface area.
Keywords: In vivo electroporation, electrotransfer, gene therapy, electrode design
Introduction
Direct injection of plasmid DNA has been intensely investigated as a gene delivery approach for the treatment of a variety of diseases. One drawback to this approach is inefficient uptake of the plasmid DNA by cells, which results in low levels of gene expression 1. In vivo EP utilizes the application of a controlled pulsed external electric field that slightly surpasses the capacitance of the cell membrane to transiently increase cell membrane permeability and allow for an increase in the uptake of plasmid DNA by the cell.
The use of an electrical field to increase plasmid uptake and expression (in vivo electroporation or electrotransfer) is more efficient when the electric field is applied in more than one direction 2–5. This approach permeabilizes a greater area of the cell surface and a larger number of cells in the target tissue 4. Prior to the development of the four plate electrode (4PE) 5–7, electrotransfer of plasmid DNA to skin was typically facilitated with a two-plate caliper electrode. One drawback to this approach is the requirement of the electrode plates to be manipulated around the treatment site to administer multiple sets of pulses in different orientations. Removal and replacement of the caliper electrode from the delivery site resulted in variation in the distance between the electrode plates and thus variation in the applied voltage. The design of the multielectrode array (MEA) 8, 9 expands on the configuration of the 4PE. The MEA is composed of an array of 16 small blunt electrodes in a 4 by 4 configuration with a 2-mm gap width between electrodes. Similar to the 4PE, the design of the MEA allows for multiple sets of electric pulses to be applied in two electric field orientations at a 90° angle without removing the electrode from the skin. Another drawback to other electrode designs is that to deliver plasmid to a large surface area the gap between the electrode pairs must be increased, and thus, the applied voltage must also be increased for cell permeabilization to be effective. The configuration of the MEA allows for its dimensions to be easily expanded, enabling a larger surface area to be treated without the need to increase the distance between electrodes. This larger treatment area may be necessary for some cutaneous indications, such as dermatological disorders. In addition, if higher transgene expression is necessary for a particular condition, treatment of a larger tissue area may result in proportionally increased expression.
In our initial report describing the MEA we compared reporter gene expression after delivering plasmid DNA with the 4PE and MEA in different rodent models 8. While rodent models are often used in the design of delivery protocols, guinea pig skin is of similar thickness and vascularity to human skin and is well established as a suitable model for human skin for drug delivery and toxicity studies 10–12. The focus of the work presented here was to further evaluate and optimize the delivery parameters for the MEA in the guinea pig model. This refinement of the MEA delivery conditions will facilitate the translation of this delivery method to clinical trials. Specifically, we evaluated the effects of varying field strength and pulse width on transgene expression, examined the duration of transgene expression, evaluated if there is a dose response relationship between amount of plasmid delivered and resulting expression levels and determined if expression could be increased by expanding the treatment area.
Results
Effect of field strength and pulse width on transgene expression
Previous work determined delivery of pLuc at field strength of 300 V/cm and 150 ms pulse width applied with the MEA resulted in the highest and most reproducible luciferase protein expression 8. To evaluate the effects of field strength and pulse width for plasmid delivery with the MEA in this cutaneous model, three field strengths (300 V/cm, 250 V/cm and 225 V/cm) were examined with varying pulse widths, ranging from 50 ms to 350 ms (Figure 1). In these initial experiments, luciferase expression was evaluated utilizing an in vitro assay. To minimize the number of animals used, expression was analyzed 48 hours after delivery. In previous work, high expression was observed at this time point5,8. Additional time points were evaluated when utilizing in vivo analysis as described below in kinetics or expression section. Two days after pLuc delivery, the delivery sites were excised and luciferase activity determined by a standard in vitro luciferase assay. For all three field strengths and pulse widths with exception of 250 V/cm with 50 ms pulses, a significant increase in luciferase expression compared to injection of plasmid injection without EP was observed (p< 0.001). In general, a pulse duration of longer than 150 ms resulted in slightly higher levels of luciferase expression, but 150 ms provided the highest level of expression with the least variation and minimal visual damage to the delivery area. Damage was assessed based on the presence of redness, burning and/or thickening of the skin immediately following and during the first 48 hours after administration of electric pulses. None of the delivery conditions used here resulted in burning, but 300 V/cm did result in increased redness starting immediately after application of pulses and thickening of the skin at the time the tissue was sampled (48 hours).
Figure 1. Effect of field strength and pulse width luciferase expression.
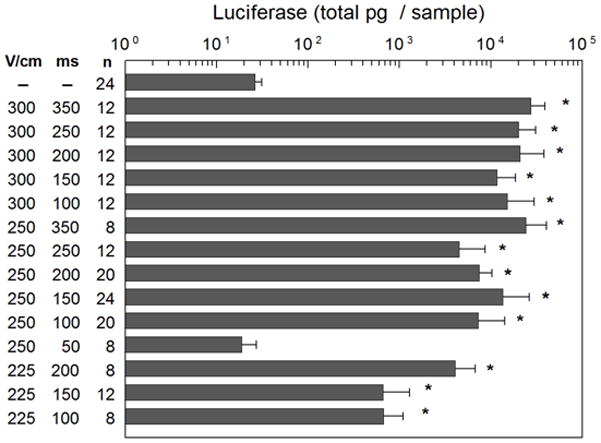
Luciferase expression was determined 48 hours after delivery using an in vitro assay for the indicated field strengths (V/cm) and pulse widths (ms). * p< 0.001 compared to plasmid injection alone (pLucE−).
Kinetics of expression
The results presented in Figure 1 utilized in vitro luciferase assays to evaluate transgene expression. The luciferase expression in subsequent figures was determined by in vivo bioluminescence imaging, which allowed for the use of fewer experimental animals and to have the same treatment site followed over time. The in vitro and in vivo assays highly correlated in a small study (r2= 0.94, n=8) and thus the in vivo assay was used for the remainder of this study.
Because a pulse width of 150 ms for each of the field strengths examined (300 V/cm, 250 V/cm and 225 V/cm) consistently resulted in the highest level of transgene expression, we next determined the duration of transgene expression after pLuc delivery at 300 V/cm, 250 V/cm and 225 V/cm with 150 ms pulses. A significant increase in luciferase expression was observed at all three field strengths at 24 hours after delivery (Figure 2). For delivery at both 300 V/cm and 250 V/cm, luciferase expression remained significantly higher through day 17 (p< 0.001) and decreased to non-significance 21 days after delivery (data not shown). At 225 V/cm, luciferase expression remained significantly higher through day 10 (p < 0.001), lost significance on day 14 (p=0.103), but increased at day 17 (p< 0.001) before returning to background levels on day 21 (data not shown). Luciferase expression for all field strengths did not significantly decrease from the initial peak at 24 hours until day 10 (p<0.001). In contrast, luciferase expression after pLuc injection without pulse application was significantly lower by 96 hours after delivery than the initial peak at 24 hours (p<0.001). Delivery using 300 V/cm 150 ms pulses resulted in slightly higher expression. However, some visible damage to the treated area was observed, although the damage level was lower than what was observed previously with longer pulse widths. Therefore, since 250 V/cm 150 ms pulses resulted in high expression levels and no visible skin damage, these EP conditions were used for the remaining experiments.
Figure 2. Luciferase expression kinetics.
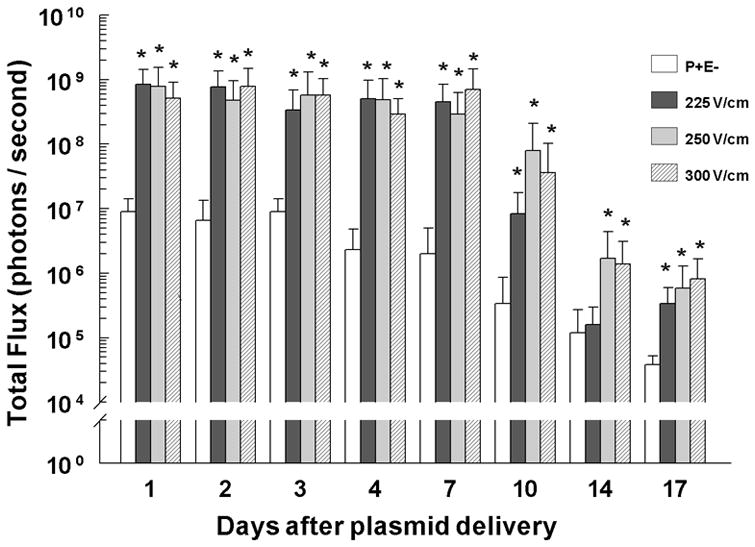
Luciferase expression was determined at the indicated time points using an in vivo assay for the indicated field strengths (V/cm) with 150 ms pulse widths. pLucE− and 225 V/cm, n=16 per group per time point; 250 V/cm, n=15 per group per time point; 300 V/cm, n=14 per group per time point. *p< 0.001 compared to pLucE− for that time point.
Dose response
To examine the relationship between plasmid dose and the resulting luciferase expression we delivered 20 μg, 50 μg, 100 μg, 150 μg and 200 μg of pLuc at 250 V/cm and 150 ms pulses (Figure 3, top panel). Two days after delivery, luciferase expression was determined using in vivo bioluminescence imaging (Caliper Life Sciences, Hopkinton, MA). Statistically discernable differences in expression were noted between 20, 100, and 200 μg (p<0.01). Since the plasmid concentration was maintained, each increased dosage required an increased injection volume. This increase in volume did not significantly affect transgene expression (Figure 3, bottom panel).
Figure 3. Control of luciferase expression by plasmid dose.
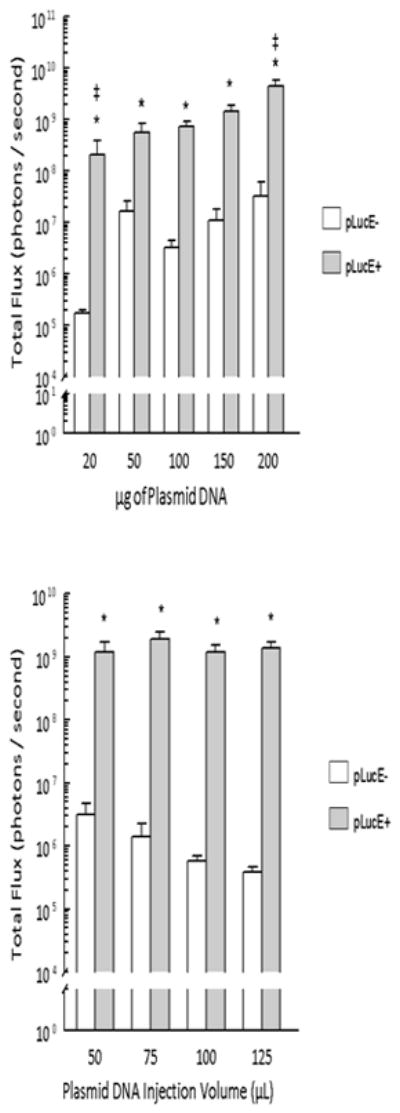
Luciferase expression was determined 48 hours after pLuc delivery using an in vivo assay. pLucE+ = 250 V/cm 150 ms.
Top panel. For 20 μg, 150 μg and 200 μg, n=6 per group for both pLucE− and pLucE+; 100 μg, n=8 per group for both pLucE− and pLucE+; 50 μg pLucE−, n=6; 50 μg pLucE+, n=5. *p< 0.05 for that plasmid dose, ‡ p< 0.05 compared to expression with pLucE+ delivering 100 μg. Bottom panel. Luciferase expression was determined for 100 μg pLuc in the indicated injection volume. n=8 per group. *p< 0.001 compared to pLucE− for that injection volume.
3.4 Expanded treatment area
The MEA is designed to be easily expanded by the addition of electrode rows while maintaining the same distance between electrodes. This allows delivery to a larger area without an increase in the applied voltage. To test this concept, larger injection areas with and without pulse delivery were evaluated. Three types of injections at a concentration of 2 μg/μl pLuc were utilized: a single 50 μl injection (100 μg); a single 200 μl injection (400 μg); and four 50 μl injections (400 μg) adjacent to each other forming approximately a 12×12 mm area. Several pulse protocols were tested (Figure 4). For the single injections, pulses were applied in a single 6×6 mm area application directly to the injection area. Four individual pulse applications were applied in a 12×12 mm area containing a single 200 μl injection or four 50 μl injections. Two days after delivery, luciferase expression was determined by in vivo bioluminescence imaging. Delivery of pLuc (100 μg or 400μg dose) with electroporation (single or four pulse applications) resulted in significantly increased (p<0.05) expression. When compared to a single injection of 100 μg pLuc followed by a single pulse application, luciferase expression following delivery of 400 μg pLuc was significantly increased with a single injection with four pulse applications (p<0.05), or with four injections followed by four pulse applications (p<0.05). The increase in expression between 100 and 400 μg, no matter the pulsing protocol, was approximately 4–7 fold. These results provide evidence that the expansion of the MEA could enhance expression levels.
Figure 4. Expansion of treated area.
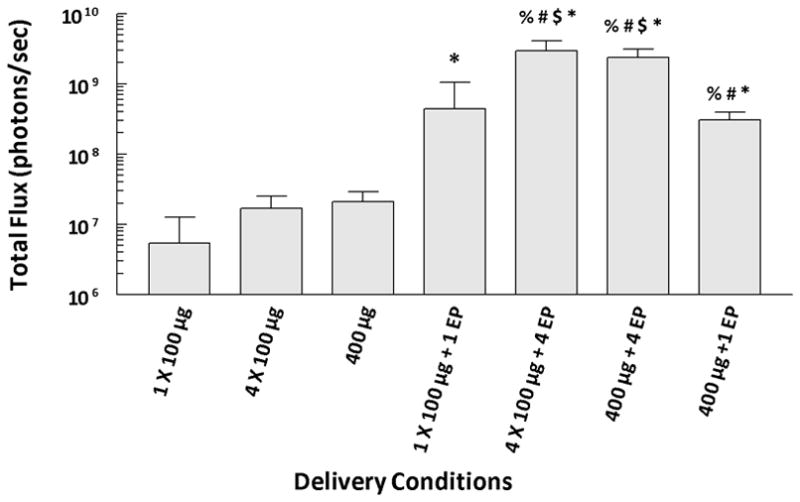
Luciferase expression was determined 48 hours after pLuc delivery using an in vivo assay. Pulses were applied at a field strength of 250 V/cm and a pulse length of 150 ms. 1EP = placing the MEA over the center of the injection area and applying one application of pulses; 4EP = placing the MEA over each of 4 quadrants and applying one application of pulses at each site for a single 400 μg injection or placing the MEA over each of 4 injection sites and applying application of pulses at each site for the four 100 μg injections. % = p<0.02 compared to four 100 μg injections without pulse application; # = p<0.02 compared to 400 μg injection; $ = p<0.05 compared to one injection of 100 μg with application of pulses; and * = p<0.05 compared to one 100 μg without pulse application.
Discussion
The first reports of successful delivery of plasmid DNA with electrotransfer were conducted in rat liver 13, mouse brain tumors 14, and mouse skeletal muscle 15. Since these initial experiments, this technique has also been used for plasmid delivery to a wide variety of tissues 16, 17 including skin 18. Plasmid electrotransfer to the skin has been performed in several preclinical models producing both systemic and tissue-specific expression 5–9, 19–38. This approach has shown promise in several fields including vaccines for infectious disease20, 23, 25, 27, 30, 33, 34 and cancer 26, 36, wound healing 7, 24, 31, and protein replacement 38. Cutaneous gene therapy could potentially be used to treat a variety of diseases. The skin is an attractive target because it is easily accessible. For example, systemic transgene expression levels can potentially be controlled by controlling the treatment area or the number of treatments.
The electrodes used for skin delivery range from penetrating or needle electrodes 19, 20, 24, 26, 30, 31, 33, 36, 37, 39 to electrodes placed on the skin surface 5–9, 20–23, 25, 28, 29, 32, 35, 38. Several descriptions of the fabrication of microneedle electrodes have been published; two have been tested in vivo for plasmid delivery 27, 39. Delivery with the MEA differs in that the blunt electrodes in the array do not penetrate the skin.
As mentioned previously, electrotransfer with the MEA at 300 V/cm 150 ms pulses resulted in high, reproducible luciferase protein expression in rodent models 8. In those previous studies, equivalent expression could be obtained with the 4PE using 100 V/cm 150 ms pulses. Although it was necessary to increase the field strength (applied voltage remained the same) to achieve comparable expression patterns with the MEA, muscle stimulation during pulse administration was greatly reduced or eliminated when compared to the pronounced muscle contractions seen with the 4PE and caliper electrodes. It was necessary to increase the field strength due to the shorter distance between the electrodes which would decrease the depth of electric field penetration. This could also explain the decreased nerve stimulation and muscle twitching. The lack of a significant increase in luciferase expression with 50 ms pulses delivered at 250 V/cm (Figure 1) indicates that within the pulse widths tested, 100 ms may be the minimum pulse duration required to reach the necessary level of cell membrane permeabilization for increased plasmid uptake at a field strength of 250 V/cm or less. In the guinea pig model, delivery of pLuc at 300 V/cm resulted in similar luciferase expression at all pulse widths examined when compared to lower field strength pulses (Figure 1), but tissue damage after delivery was frequently observed. The visually detectable damage may be the underlying cause of the increased variability in luciferase expression with this field strength. Compared to 225 V/cm, luciferase expression was consistently higher with 250 V/cm at comparable pulse widths.
It was previously reported that transgene expression decreases 3 days after plasmid DNA delivery by intradermal injection 40. Consistent with these findings, in this study luciferase expression after plasmid injection without pulses significantly decreased between days 3 and 4 after delivery compared to the initial peak expression at 24 hours (Figure 2). For all electrotransfer conditions tested, a significant decrease in luciferase expression occurred 10 days after delivery when compared to the initial peak at 24 hours. Expression after delivery with 300 V/cm and 250 V/cm remained greater than injection alone for 17 days. This decrease in expression is probably due to the limited lifespan of the skin cell. Longer expression would indicate delivery to the underlying panniculus carnosus muscle 29, 36. The significant difference in expression with 225 V/cm at day 17 (Figure 2) is most likely due to the decrease in luciferase expression in the plasmid injection alone group. In addition, the group receiving 225 V/cm pulses produced lower overall luciferase expression than the higher field strength groups beginning with day 10 after delivery. These results indicate that if a lower level of expression is desired, it could be achieved by decreasing the field strength.
Since a linear correlation between the plasmid dosage and the resulting transgene expression (r2=0.88) was observed up to the highest plasmid dose tested (Figure 3a), the maximum expression levels were not reached. Further experiments will be needed to determine the dose required to achieve the maximum level of expression.
The MEA is designed to be expandable to cover a larger surface area to increase transgene expression. This concept was evaluated by performing the delivery to an area approximately four times the original size. Electrotransfer over a 12×12 mm area following a single 200 μg injection or four 50 μg injections significantly increased expression when compared to electrotransfer after a single 50 μg injection. This supports the concept that expansion of the MEA to cover a larger surface area would enhance expression.
In summary, the level of luciferase expression increased with the magnitude of the voltage applied. For each of the field strengths examined, a pulse duration of 150 ms achieved the highest level of expression with the least variation and visible tissue damage. For all three field strengths examined, luciferase expression did not decrease from initial peak expression at 24 hours until 10 days (225 V/cm) to 17 (300 V/cm and 250 V/cm) days after delivery. In addition to adjusting transgene expression levels by altering field strengths, expression levels can also be controlled by adjusting the plasmid dose or the skin area treated. These results indicated that the design of the MEA is a viable option for delivery of plasmid DNA to a larger area of skin without the need to increase the voltage applied and could potentially be used to deliver plasmids with therapeutic potential for the treatment of a variety of diseases.
Materials and Methods
Animals
All procedures were approved by the Animal Use and Care Committee of the University of South Florida College of Medicine. Delivery was performed to female 175–200 gram Hartley guinea pigs (Elm Hill Laboratories, Chelmsford, MA or Charles River Laboratories, Wilmington, MA). During plasmid delivery and in vivo quantification of luciferase expression animals were anesthetized in an induction chamber charged with 3% isoflurane in O2 then fitted with a standard rodent mask and kept under general anesthesia during the procedure.
Plasmid delivery
The luciferase plasmid gWizLuc (pLuc) was commercially prepared (Aldevron, Fargo, ND). Endotoxin levels were <0.1 EU/μg plasmid. Electrotransfer was carried out as previously described 8. Briefly, 50 μl gWizLuc in sterile injectable saline (2 μg/μl) was injected intradermally on the guinea pig flank at four sites using a 25 gauge, 5/8-inch length needle. The MEA was then placed over the injection bubble and an electrical field applied at the indicated field strength and pulse width. Multiple conditions were tested on each animal, and on site on each animal was an injection-only control. For sites receiving electric pulses, 72 pulses were applied over the 16 electrodes, resulting in approximately 8 pulses per 2×2 section. Delivery sites were marked with a surgical marker if they were to be excised later for quantification of luciferase expression.
In Vitro Luciferase Assays
Luciferase activity was quantified as previously described 41. Briefly, at the indicated time points animals were humanely euthanized and delivery sites were excised and snap frozen on dry ice. Tissue samples were homogenized in 1 to 2 mL of homogenization buffer (25 mM Tris, pH 7.8, 2 mM EDTA, 1 mM DTT and 10% glycerol). Homogenates were centrifuged at 4°C for 2 minutes and the supernatants were removed and assayed for luciferase activity in Luciferase Assay Buffer (25 mM glycylglycine, pH 7.8, 15 mM KPO4, pH 7.8, 15 mM MgSO4, 4 mM EGTA, 2 mM ATP, 1 mM DTT and 100 μM Luciferin). Luciferase activity was quantitated using a MLX microtiter plate luminometer (Dynex Technologies, Chantilly, VA). Using a standard preparation of recombinant luciferase (Promega, Madison, WI) relative light unit values were converted to pg of luciferase and luciferase activity is reported as total pg luciferase per tissue sample.
In Vivo Bioluminescence Imaging
In vivo bioluminescence imaging was performed using the IVIS Spectrum system (Caliper Life Sciences, Hopkinton, MA) in conjunction with the Living Image acquisition and analysis software. Luciferin (Caliper Life Sciences) was dissolved to 7.5 mg/ml in PBS, filter-sterilized, and stored at −20°C. Guinea pigs were anesthetized with isofluorane and received a 50 μL intradermal injection of the luciferin solution at the delivery site. Images were acquired 2 minutes after luciferin injection. Quantitation of luciferase activity was based on total flux (photons/sec) of emitted light from the selected area around the delivery site 42
Statistical Analysis
All values are reported as the mean ± SEM unless otherwise noted. Analysis of luciferase activity was completed using a 2-tailed Student’s t-test when comparing two groups. Analysis three or more groups was completed by ANOVA with a post-hoc Fisher’s Least Significant Difference test to adjust for multiple comparisons. Statistical significance was assumed at p< 0.05. All statistical analysis was completed using the Statistical Package for the Social Sciences (SPSS).
Acknowledgments
This research was supported in part by a research grant from the National Institutes of Health R01 EB005441 and by the Frank Reidy Research Center for Bioelectrics at Old Dominion University. The authors wish to thank Dr. Mark Jaroszeski (University of South Florida) for construction of the multielectrode array.
Footnotes
Conflict of Interest
With respect to duality of interest and financial disclosures, Dr. R. Heller is an inventor on patents which cover the technology that was used in the work reported in this manuscript. One or more of the patents have been licensed to Inovio Pharmaceutical Corporation. In addition, Dr. R. Heller owns stock and stock options in Inovio and has an ownership interest in RMR Technologies.
References
- 1.Vogel JC. Nonviral skin gene therapy. Hum Gene Ther. 2000 Nov 1;11(16):2253–2259. doi: 10.1089/104303400750035780. [DOI] [PubMed] [Google Scholar]
- 2.Sersa G, Cemazar M, Semrov D, Miklavcic D. Changing electrode orientation improves the efficacy of electrochemotherapy of solid tumors in mice. Bioelectrochem Bioenerg. 1996;39:61–66. [Google Scholar]
- 3.Gilbert RA, Jaroszeski MJ, Heller R. Novel electrode designs for electrochemotherapy. Biochim Biophys Acta. 1997;1334(1):9–14. doi: 10.1016/s0304-4165(96)00119-5. [DOI] [PubMed] [Google Scholar]
- 4.Faurie C, Phez E, Golzio M, Vossen C, Lesbordes JC, Delteil C, et al. Effect of electric field vectoriality on electrically mediated gene delivery in mammalian cells. Biochim Biophys Acta. 2004 Oct 11;1665(1–2):92–100. doi: 10.1016/j.bbamem.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Heller LC, Jaroszeski MJ, Coppola D, Mccray AN, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007 Feb;14(3):275–280. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller LC, Jaroszeski MJ, Coppola D, Heller R. Comparison of electrically mediated and liposome-complexed plasmid DNA delivery to the skin. Genet Vaccines Ther. 2008 Dec 4;6(1):16–23. doi: 10.1186/1479-0556-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther. 2009 Apr;17(4):651–657. doi: 10.1038/mt.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller R, Cruz Y, Heller LC, Gilbert RA, Jaroszeski MJ. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum Gene Ther. 2010 Mar;21(3):357–362. doi: 10.1089/hum.2009.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraro B, Cruz YL, Baldwin M, Coppola D, Heller R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010 Jun;17(6):763–769. doi: 10.1038/gt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mershon MM, Mitcheltree LW, Petrali JP, Braue EH, Wade JV. Hairless guinea pig bioassay model for vesicant vapor exposures. Fundam Appl Toxicol. 1990 Oct;15(3):622–630. doi: 10.1016/0272-0590(90)90046-m. [DOI] [PubMed] [Google Scholar]
- 11.Sueki H, Gammal C, Kudoh K, Kligman AM. Hairless guinea pig skin: anatomical basis for studies of cutaneous biology. Eur J Dermatol. 2000 Jul;10(5):357–364. [PubMed] [Google Scholar]
- 12.Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL, et al. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux) technology. Pharm Res. 2001 Dec;18(12):1789–1793. doi: 10.1023/a:1013395102049. [DOI] [PubMed] [Google Scholar]
- 13.Heller R, Jaroszeski M, Atkin A, Moradpour D, Gilbert R, Wands J, et al. In vivo gene electroinjection and expression in rat liver. Febs Letters. 1996 Jul 8;389(3):225–228. doi: 10.1016/0014-5793(96)00590-x. [DOI] [PubMed] [Google Scholar]
- 14.Nishi T, Yoshizato K, Yamashiro S, Takeshima H, Sato K, Hamada K, et al. High-efficiency in vivo gene transfer using intraarterial plasmid DNA injection following in vivo electroporation. Cancer Res. 1996;56(5):1050–5. [PubMed] [Google Scholar]
- 15.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16(9):867–70. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 16.Heller LC, Heller R. In vivo electroporation for gene therapy. Hum Gene Ther. 2006 Sep;17(9):890–897. doi: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- 17.Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009 Apr;17(4):585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gothelf A, Gehl J. Gene Electrotransfer to Skin; Review of Existing Literature and Clinical Perspectives. Curr Gene Ther. 2010 Jun 16; doi: 10.2174/156652310791823443. [DOI] [PubMed] [Google Scholar]
- 19.Glasspool-Malone J, Somiari S, Drabick JJ, Malone RW. Efficient nonviral cutaneous transfection. Mol Ther. 2000;2(2):140–146. doi: 10.1006/mthe.2000.0107. [DOI] [PubMed] [Google Scholar]
- 20.Drabick JJ, Glasspool-Malone J, King A, Malone RW. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther. 2001 Feb;3(2):249–255. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- 21.Heller R, Schultz J, Lucas ML, Jaroszeski MJ, Heller LC, Gilbert RA, et al. Intradermal delivery of interleukin-12 plasmid DNA by in vivo electroporation. DNA Cell Biol. 2001 Jun;20(6):381. doi: 10.1089/10445490150504666. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Nolan E, Kreitschitz S, Rabussay DP. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochimica et Biophysica Acta-General Subjects. 2002 Aug 15;1572(1):1–9. doi: 10.1016/s0304-4165(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 23.Babiuk S, Baca-Estrada ME, Foldvari M, Baizer L, Stout R, Storms M, et al. Needle-free topical electroporation improves gene expression from plasmids administered in porcine skin. Mol Ther. 2003 Dec;8(6):992–998. doi: 10.1016/j.ymthe.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Marti G, Ferguson M, Wang J, Byrnes C, Dieb R, Qaiser R, et al. Electroporative transfection with KGF-1 DNA improves wound healing in a diabetic mouse model. Gene Ther. 2004 Oct 7; doi: 10.1038/sj.gt.3302383. [DOI] [PubMed] [Google Scholar]
- 25.Medi BM, Hoselton S, Marepalli RB, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits. I: efficacy. Int J Pharm. 2005 Apr 27;294(1–2):53–63. doi: 10.1016/j.ijpharm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006 Feb;13(2):320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007 Feb 26;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandermeulen G, Staes E, Vanderhaeghen ML, Bureau MF, Scherman D, Preat V. Optimisation of intradermal DNA electrotransfer for immunisation. J Control Release. 2007 Dec 4;124(1–2):81–87. doi: 10.1016/j.jconrel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, Wu X, Song N, Cao Y, Liu W. Electroporation-mediated plasmid gene transfer in rat incisional wound. J Dermatol Sci. 2007 Aug;47(2):161–164. doi: 10.1016/j.jdermsci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008 Jan 17;26(3):440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, et al. Age-dependent impairment of HIF-1alpha expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008 Nov;217(2):319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andre F, Gehl J, Sersa G, Preat V, Hojman P, Eriksen J, et al. EFFICIENCY OF HIGH AND LOW VOLTAGE PULSE COMBINATIONS FOR GENE ELECTROTRANSFER IN MUSCLE, LIVER, TUMOR AND SKIN. Hum Gene Ther. 2008 Aug 21; doi: 10.1089/hum.2008.060. [DOI] [PubMed] [Google Scholar]
- 33.Draghia-Akli R, Khan AS, Brown PA, Pope MA, Wu L, Hirao L, et al. Parameters for DNA vaccination using adaptive constant-current electroporation in mouse and pig models. Vaccine. 2008 Sep 19;26(40):5230–5237. doi: 10.1016/j.vaccine.2008.03.071. [DOI] [PubMed] [Google Scholar]
- 34.Martinon F, Kaldma K, Sikut R, Culina S, Romain G, Tuomela M, et al. Persistent immune responses induced by a human immunodeficiency virus DNA vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum Gene Ther. 2009 Nov;20(11):1291–1307. doi: 10.1089/hum.2009.044. [DOI] [PubMed] [Google Scholar]
- 35.Vandermeulen G, Richiardi H, Escriou V, Ni J, Fournier P, Schirrmacher V, et al. Skin-specific promoters for genetic immunisation by DNA electroporation. Vaccine. 2009 Jul 9;27(32):4272–4277. doi: 10.1016/j.vaccine.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, et al. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4(9):e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos AK, Eriksson F, Walters DC, Pisa P, King AD. Optimization of skin electroporation in mice to increase tolerability of DNA vaccine delivery to patients. Mol Ther. 2009 Sep;17(9):1637–1642. doi: 10.1038/mt.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gothelf A, Hojman P, Gehl J. Therapeutic levels of erythropoietin (EPO) achieved after gene electrotransfer to skin in mice. Gene Ther. 2010 Apr 22; doi: 10.1038/gt.2010.46. [DOI] [PubMed] [Google Scholar]
- 39.Daugimont L, Baron N, Vandermeulen G, Pavselj N, Miklavcic D, Jullien MC, et al. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J Membr Biol. 2010 Jul;236(1):117–125. doi: 10.1007/s00232-010-9283-0. [DOI] [PubMed] [Google Scholar]
- 40.Hengge UR, Walker PS, Vogel JC. Expression of naked DNA in human, pig, and mouse skin. J Clin Invest. 1996 Jun 15;97(12):2911–2916. doi: 10.1172/JCI118750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heller L, Jaroszeski MJ, Coppola D, Pottinger C, Gilbert R, Heller R. Electrically mediated plasmid DNA delivery to hepatocellular carcinomas in vivo. Gene Therapy. 2000 May;7(10):826–829. doi: 10.1038/sj.gt.3301173. [DOI] [PubMed] [Google Scholar]
- 42.Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol. 1997 Oct;66(4):523–531. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]


