Abstract
Summary
It is unclear whether optimal levels of 25-hydroxyvitamin D (25(OH)D) in whites are the same as in minorities. In adult participants of NHANES, the relationships between 25(OH)D, bone mineral density (BMD), and parathyroid hormone (PTH) differed in blacks as compared to whites and Mexican-Americans, suggesting that optimal 25(OH)D levels for bone and mineral metabolism may differ by race.
Introduction
Blacks and Hispanics have lower 25-hydroxyvitamin D concentrations than whites. However, it is unclear whether 25(OH)D levels considered “optimal” for bone and mineral metabolism in whites are the same as those in minority populations.
Methods
We examined the relationships between 25(OH)D and parathyroid hormone in 8,415 adult participants (25% black and 24% Mexican-American) in the National Health and Nutrition Examination Surveys 2003–2004 and 2005–2006; and between 25(OH)D and bone mineral density in 4,206 adult participants (24% black and 24% Mexican-American) in the 2003–2004 sample.
Results
Blacks and Mexican-Americans had significantly lower 25(OH)D and higher PTH concentrations than whites (P<0.01 for both). BMD significantly decreased (P<0.01) as serum 25(OH)D and calcium intake declined among whites and Mexican-Americans, but not among blacks (P=0.2). The impact of vitamin D deficiency (25 (OH)D≤20 ng/ml) on PTH levels was modified by race/ethnicity (P for interaction, 0.001). Whereas inverse relationships between 25(OH)D and PTH were observed above and below a 25(OH)D level of 20 ng/ml in whites and Mexican-Americans, an inverse association between 25(OH)D and PTH was only observed below this threshold in blacks, with the slope of the relationship being essentially flat (P=0.7) above this cut-point, suggesting that PTH may be maximally suppressed at lower 25(OH)D levels in blacks than in whites or Mexican-Americans.
Conclusions
The relationships between 25(OH)D, BMD, and PTH may differ by race among US adults. Whether race-specific ranges of optimal vitamin D are needed to appropriately evaluate the adequacy of vitamin D stores in minorities requires further study.
Keywords: Bone mineral density, Ethnic differences, Parathyroid hormone, Vitamin D
Introduction
The central role of vitamin D in the maintenance of calcium homeostasis and bone health as well as recent data linking lower serum 25-hydroxyvitamin D (25(OH) D) concentrations to a variety of chronic illnesses, has fueled burgeoning interest in the importance of maintaining adequate vitamin D stores in health and disease [1]. Although there remains no consensus as to the best method for determining optimal vitamin D status for bone health, many investigators define the threshold for vitamin D sufficiency as the lowest serum concentration of 25(OH) D that maximally suppresses parathyroid hormone (PTH) secretion and/or optimizes bone mineral density (BMD) [2–7]. Based upon these criteria, most experts suggest that 25(OH)D levels of 21 to 30 ng/ml are indicative of relative vitamin D insufficiency, while levels ≤20 ng/ml constitute vitamin D deficiency [1].
Blacks and Hispanics consistently manifest lower serum concentrations of 25(OH)D than non-Hispanic whites [8–12], primarily because increased skin pigmentation inhibits cutaneous synthesis of cholecalciferol, the metabolic precursor to 25(OH)D [13]. This has led many investigators to conclude that blacks and Hispanics are at higher risk of vitamin D deficiency than whites [8–12], and as a result, may also have increased risk of developing associated chronic disease conditions such as hypertension, diabetes, and cancer [14, 15]. These conclusions have largely been predicated upon comparisons of serum 25(OH)D concentrations in blacks and Hispanics against “optimal” ranges established in predominantly white, elderly, female populations. However, it is unclear whether extrapolating 25(OH)D thresholds derived in white populations to racial and ethnic minorities is appropriate. For example, blacks maintain higher BMD and lower skeletal fracture risk than whites despite lower 25(OH)D concentrations and higher PTH concentrations [16–18], and Mexican-Americans manifest similar though less pronounced differences compared to non-Hispanic whites [19–21]. These observations suggest that vitamin D ranges considered optimal for bone and mineral metabolism among whites may not be the same as those among blacks and Hispanics [22].
Previous studies examining the associations between vitamin D, BMD, and PTH by race and ethnicity either limited the analyses to single-gender or elderly populations, or inconsistently accounted for critical factors that might impact these relationships, such as dietary calcium intake [7, 9, 19]. Accordingly, we studied cross-sectional associations between 25(OH)D and PTH among adult participants in the 2003–2004 and 2005–2006 National Health and Nutrition Examination Surveys (NHANES), and between 25(OH)D and BMD in the 2003–2004 sample.
Methods
Study population
We studied participants in NHANES from 2003–2004 and 2005–2006. The design and operation of NHANES has been described previously [23]. Briefly, NHANES provides nationally representative cross-sectional data on the health status of the civilian, non-institutionalized US population. After selection in a complex survey design, eligible participants were interviewed and examined, and participants 12 years of age or older had blood collected for measurement of a biochemistry profile including serum PTH, 25(OH)D, calcium, phosphate, albumin, and creatinine. A dietary interview collected data for estimating the daily intake of nutrients on the basis of foods and beverages consumed during the 24-h period preceding the interview (midnight to midnight). Participants were also queried about the use of supplemental calcium and vitamin D. Participants self-classified their race–ethnicity as non-Hispanic white, non-Hispanic black, Hispanic, and Other Race, including multi-racial. Hispanic ethnicity was further sub-classified into Mexican-American or Other Hispanic. Protocols to recruit and study participants of NHANES were reviewed and approved by the National Center for Health Statistics Institutional Review Board.
Measurement of laboratory parameters
Serum PTH concentrations were measured with the Elecsys 1010 analyzer (Roche, Basel, Switzerland) using the Origen electrochemiluminescent process. Three levels of controls were used to assess the quality of each serum PTH run, and coefficients of variation were <10% for each run [23]. After rapid extraction of 25(OH)D and other hydroxylated metabolites with acetonitrile, serum concentrations of 25(OH)D were measured using an equilibrium radioimmunoassay procedure (DiaSorin, Still-water, MN). Two levels of controls were used to test 25 (OH)D concentrations during the study periods examined [23]. Assay coefficients of variation for these quality control pools were 7–13% at the lower end of 25(OH)D values (9–25 ng/ml) and 8–11% at the higher end of 25 (OH)D values (32–45 ng/ml). Serum calcium, albumin, phosphate, and creatinine were measured using a Beckman Synchron LX20 chemistry analyzer (Beckman Coulter, Fullerton, CA, USA). Estimated glomerular filtration rate (eGFR) was calculated according to the simplified Modification of Diet in Renal Disease Study (MDRD) prediction equation [24]. We corrected serum creatinine in the NHANES 2005–2006 sample to align values with the creatinine assay used in the development of the MDRD equation [25] using the following formula: corrected serum creatinine=−0.016+(0.978×measured serum creatinine). Serum creatinine in the NHANES 2003–2004 sample did not require correction.
Measurement of bone mineral density
BMD was measured using whole body dual-energy X-ray absorptiometry (DXA). We used whole body BMD for these analyses in order to examine a single composite measure of the skeleton, and because a previous analysis in NHANES showed that racial differences in BMD were qualitatively the same whether analyzing whole body BMD or specific subregions such as pelvis or lumbar spine [26]. The DXA examination protocol is detailed extensively in the January 2004 Body Composition Procedures Manual at http://www.cdc.gov/nchs/nhanes.htm. Briefly, whole body DXA scans were acquired using a Hologic QDR 4500A fan-beam densitometer (Hologic, Bedford, MA, USA) and the scan for each participant was reviewed and analyzed by the University of California, San Francisco Department of Radiology. Because of non-random patterns to missing DXA data, NHANES imputed missing and invalid BMD data using multiple-imputation methodology. Accordingly, all analyses of DXA data were performed according to the processes detailed in the technical documents at http://www.cdc.gov/nchs/nhanes.htm. BMD data for the purposes of this study were only available for the NHANES 2003–2004 cycle.
Statistical analyses
Because older individuals, Hispanics, and blacks were intentionally over-represented, NHANES 2003–2004 and 2005–2006 were not simple random samples of the US population. Therefore, appropriate sample weights were used to obtain weighted regression estimates, allowing the results of these analyses to be generalizable to the US population.
For all analyses, we excluded participants less than 18 years old or with missing data on key study variables of interest. In addition, in order to focus the analysis on differences in whites, blacks, and Mexican-Americans, we excluded participants self-identified as Other Race or as Hispanic ethnicity other than Mexican-American. Furthermore, we excluded participants with chronic kidney disease (eGFR <60 mL/min/1.73 m2), which is strongly linked with abnormalities in PTH and vitamin D metabolism [1], and participants with PTH concentrations in the highest 1% of values in the study sample (PTH >134 pg/ml) in order to minimize the influence of outliers potentially linked with primary parathyroid disorders known to impair PTH regulation. Within each category of race–ethnicity, we calculated the percentage of each categorical demographic characteristic and the mean (±standard error) of age, body mass index (BMI), dietary calcium and phosphorus intake, BMD, and each laboratory measurement. In addition, we used linear regression to compare mean values of dietary calcium intake, serum PTH, and BMD across quantiles of 25(OH)D according to race and ethnicity. Since age may modify the relationship between 25(OH)D and BMD [27], we tested for interaction between age and 25(OH)D in these models. When interaction was detected (P<0.05), we analyzed stratified models.
Locally weighted estimated scatterplot smoothing (LOWESS) plots were used to examine the relationship between serum PTH and 25(OH)D concentrations by race and ethnicity. LOWESS is a non-parametric technique for describing the relationship between two continuous variables, and so does not require a priori assumptions about the functional form of the relationship [28]. For these plots, we utilized an automated algorithm to choose the most appropriate smoothing parameter according to objective criteria [29]. Of note, we excluded a single black participant with an unusually high 25(OH)D value (64 ng/ml) from the LOWESS analyses since this outlier resulted in an implausible extrapolation of the smoothed curve relating 25(OH)D and PTH among blacks. We also examined racial and ethnic differences in the relationship between 25(OH)D and PTH in linear regression models adjusted for age, sex, BMI, creatinine, calcium intake, and season of blood draw (coded as a binary variable depending on whether the blood draw occurred between November 1 to April 30 or May 1 to October 31), all of which may influence PTH, 25(OH)D, or both. In addition, we examined whether the impact of vitamin D deficiency (as defined by 25(OH)D ≤20 ng/ml [1]) on PTH levels was modified by race/ethnicity, BMI, and age by testing the significance of interaction terms. When interaction was detected (P<0.05), we analyzed stratified models.
Because 25(OH)D results from 2000–2006 NHANES may have been affected by drifts in assay performance (as detailed at http://www.cdc.gov/nchs/data/nhanes/nhanes3/VitaminD_analyticnote.pdf), we performed several sensitivity analyses. First, we examined LOWESS plots of the relationship between 25(OH)D and PTH in the 2003–2004 dataset as compared to the 2005–2006 dataset, and found that there were minimal differences in the smoothed curves describing these relationships. Second, we examined the multivariable-adjusted relationships between 25(OH)D and PTH in the 2003–2004 sample as compared to the 2005–2006 sample, and found that the relationships were qualitatively the same between the 2-year datasets. Therefore, we only present the results of the combined 2003–2004 and 2005–2006 datasets. All analyses were performed using SUDAAN version 9.0 (Research Triangle Park, NC, USA) and SAS version 9.1 statistical software (SAS Institute, Cary, NC, USA). Two-tailed P values <0.05 were considered statistically significant for all analyses.
Results
Population characteristics
A total of 19,593 participants in NHANES 2003–2004 and 2005–2006 were available for analysis. Of these participants, 11,178 (57%) met one or more exclusion criteria, leaving 8,415 participants in the final study sample, 25% of whom were black and 24% of whom were Mexican-American. There were minimal differences in the characteristics of participants in the final study sample as compared to participants who were excluded from the analysis (data not shown).
Table 1 depicts the demographic, laboratory, and dietary intake characteristics of the study sample according to race and ethnicity. Both blacks and Mexican-Americans had higher serum PTH concentrations, lower 25(OH)D concentrations, and lower dietary calcium intake as compared to whites (P<0.05 for all comparisons). When the relationships between PTH and race/ethnicity were adjusted for age, gender, BMI, dietary calcium intake, and serum concentrations of calcium, albumin, and 25(OH)D, serum PTH concentrations remained 3.3 pg/ml higher in blacks (95% CI 1.7 to 4.9 pg/ml) and 3.7 pg/ml higher in Mexican-Americans (95% CI 2.3 to 5.0 pg/ml) compared to whites. Similarly, 25(OH)D concentrations remained significantly lower in blacks and Mexican-Americans as compared to whites after adjustment for age, gender, and BMI. Further adjusting for supplemental calcium and vitamin D intake did not materially change the results. Although blacks and Mexican-Americans both had lower 25(OH)D, higher PTH, and lower dietary calcium intake than whites, blacks had significantly higher mean BMD as compared to whites (P<0.01), whereas there were no significant differences in BMD between Mexican-Americans and whites (P=0.6).
Table 1. Participant characteristics by race and ethnicity.
| White | Mexican-American | Black | |
|---|---|---|---|
| N | 4,309 | 2,025 | 2,081 |
| Age, years | 45.3±0.5 | 37.0±0.7 | 41.3±0.6 |
| BMI, kg/m2 | 28.0±0.2 | 28.5±0.2 | 30.2±0.2 |
| Female, % | 50 | 48 | 54 |
| Dietary calcium intake, mg/d | 977±14 | 923±23 | 748±16 |
| Dietary phosphorus intake, mg/d | 1,414±13 | 1,448±20 | 1,187±18 |
| Serum calcium, mg/dl | 9.54±0.01 | 9.46±0.01 | 9.54±0.01 |
| Serum albumin, g/dl | 4.3±0.01 | 4.3±0.02 | 4.1±0.01 |
| Serum phosphate, mg/dl | 3.8±0.01 | 3.8±0.01 | 3.8±0.01 |
| Serum 25-hydroxyvitamin D, ng/ml | 25.6±0.4 | 19.5±0.5 | 14.8±0.4 |
| Serum parathyroid hormone, pg/ml | 39.9±0.4 | 44.4±0.5 | 46.5±0.7 |
| Whole body bone mineral density (g/cm2)a | 1.02±0.01 | 1.02±0.01 | 1.09±0.01 |
Values are means±standard errors
Data for bone mineral density from 2003–2004 sample only
Distribution of 25(OH)D, PTH, and BMD by race/ethnicity
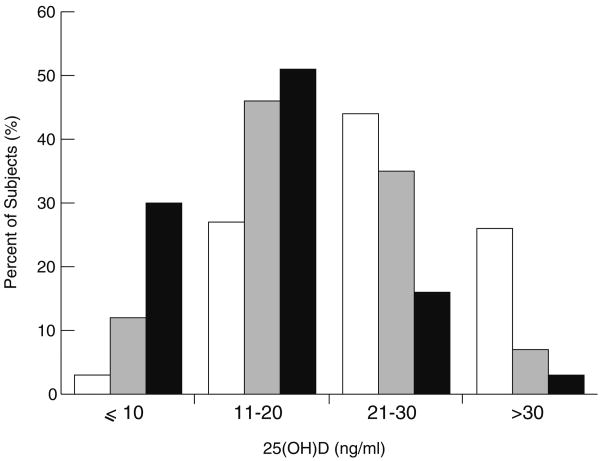
Figure 1 depicts the distribution of 25(OH)D concentrations in the study sample by race and ethnicity. Using one common definition of vitamin D deficiency (25(OH)D ≤20 ng/ml) [1], 50% of overall study participants would be classified as vitamin D deficient. However, when restricted to whites, only 28% of participants would be classified as vitamin D deficient, whereas the analogous percentages among Mexican-Americans and blacks would be 58% and 81%, respectively.
Fig. 1.
Distribution of 25(OH)D concentrations in the study sample by race and ethnicity. White bars indicate white participants (N= 4,309), gray bars indicate Mexican-American participants (N=2,025), and black bars indicate black participants (N=2,081)
Table 2 depicts the overall and group-specific mean calcium intake, PTH concentrations, and BMD across quantiles of 25(OH)D in 4,206 participants from the 2003–2004 cycle (BMD data were not available for the 2005–2006 cycle). Among all groups, lower dietary calcium intake and higher mean PTH concentrations were significantly associated with lower concentrations of 25 (OH)D. In addition, BMD significantly decreased (P<0.01) as 25(OH)D concentrations and dietary calcium intake declined in whites and Mexican-Americans. In contrast, there was no significant decrease in BMD (P=0.2) as 25 (OH)D concentrations and dietary calcium intake declined among black participants. These relationships did not materially change in analyses adjusted for gender, BMI, and creatinine. Furthermore, age did not modify the relationships between 25(OH)D and BMD among blacks or Mexican-Americans. However, significant interaction between age and 25(OH)D was detected among whites (P for interaction=0.02). When stratified by age ≥ vs. <50 years, 25(OH)D remained linearly associated with BMD among whites ≥50 years old (P<0.01), but not among whites <50 years of age (P=0.13).
Table 2. Mean PTH concentrations, dietary calcium intake, and whole body BMD according to quantiles of 25(OH)D in NHANES 2003–2004, stratified by race and ethnicitya.
| Categories of 25-hydroxyvitamin D (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|
| >31 | 30−26 | 25−21 | 20−16 | 15−11 | ≤10 | P valuesb | |
| All race/ethnicity, N (total=4,206) | 882 | 701 | 844 | 780 | 618 | 381 | <0.01 |
| Dietary calcium intake, mg/d | 1,001±29 | 927±28 | 954±27 | 831±23 | 784±25 | 666±28 | <0.01 |
| Serum parathyroid hormone, pg/ml | 35±0.9 | 41±0.7 | 42±1 | 44±0.9 | 49±1 | 55±2 | <0.01 |
| BMD g/cm2 | 1.03±0.01 | 1.03±0.01 | 1.03±0.01 | 1.03±0.01 | 1.03±0.01 | 1.03±0.01 | 0.79 |
| White, N (total=2,239) | 736 | 477 | 480 | 337 | 152 | 57 | |
| Dietary calcium intake, mg/d | 1,007±29 | 924±29 | 967±31 | 853±33 | 830±47 | 645±59 | <0.01 |
| Serum parathyroid hormone, pg/ml | 35±0.9 | 40±0.7 | 41±1 | 44±1 | 49±2 | 58±4 | <0.01 |
| BMD, g/cm2 | 1.03±0.01 | 1.03±0.01 | 1.03±0.01 | 1.02±0.01 | 0.99±0.01 | 0.96±0.02 | <0.01 |
| Mexican-American, N (total=989) | 106 | 162 | 236 | 223 | 183 | 79 | |
| Dietary calcium intake, mg/d | 979±69 | 996±41 | 985±72 | 889±68 | 816±28 | 819±126 | <0.01 |
| Serum parathyroid hormone, pg/ml | 37±1 | 45±2 | 45±1 | 49±2 | 50±2 | 50±1 | <0.01 |
| BMD, g/cm2 | 1.03±0.01 | 1.03±0.01 | 1.03±0.01 | 1.01±0.01 | 1.00±0.01 | 0.99±0.01 | <0.01 |
| Black, N (total=978) | 40 | 62 | 128 | 220 | 283 | 245 | |
| Dietary calcium intake, mg/d | 715±54 | 856±90 | 783±52 | 698±40 | 700±45 | 645±48 | 0.03 |
| Serum parathyroid hormone, pg/ml | 41±4 | 41±2 | 42±2 | 44±1 | 47±2 | 54±2 | <0.01 |
| BMD, g/cm2 | 1.08±0.02 | 1.15±0.03 | 1.09±0.01 | 1.09±0.01 | 1.10±0.01 | 1.08±0.01 | 0.21 |
Whole body BMD values from 2005–2006 were unavailable
P value from univariate linear regression analyses with 25(OH)D as the dependent variable and either serum PTH, dietary calcium intake, or BMD as the independent variable
Relationships between PTH and 25(OH)D
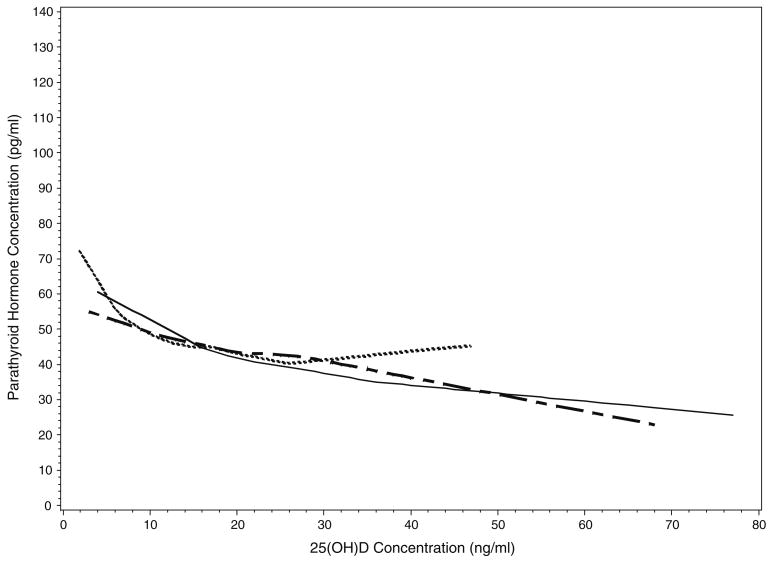
Figure 2 depicts the LOWESS plots of serum PTH as a function of serum 25(OH)D stratified by race and ethnicity. PTH was inversely related to 25(OH)D throughout the range of 25(OH)D values in whites and Mexican-Americans. In contrast, an inverse relationship between PTH and 25(OH)D was only apparent when 25(OH)D values fell below 26 ng/ml among blacks.
Fig. 2.
LOWESS regression plots showing serum PTH concentrations as a function of 25 (OH)D concentrations in whites (solid line), Mexican-Americans (checkered line), and blacks (dotted line)
The impact of vitamin D deficiency (25(OH)D ≤20 ng/ml) on PTH levels was modified by race/ethnicity (P for interaction, 0.001). Table 3 depicts the relationships between PTH and 25(OH)D in multivariable regression models stratified by a 25(OH)D level above or below 20 ng/ml. In the overall sample, PTH values were inversely associated with 25(OH)D values above and below this threshold. However, these associations differed when stratified by race. Whereas inverse associations between 25(OH)D and PTH values were observed above and below this cut-point among whites and Mexican-Americans, a significant inverse relationship between 25(OH)D and PTH values was only observed when 25(OH)D concentrations were below 20 ng/ml among blacks. Indeed, the slope of the relationship between 25(OH)D and PTH was essentially flat (P=0.71) above this threshold in black participants.
Table 3. Multivariable-adjusted change in PTH (95% CI) per 1 ng/ml change in 25(OH)D above and below 20 ng/ml in the population overall and stratified by race and ethnicity.
| Population | N | Δ PTH, pg/ml (95% CI)a | P value | N | Δ PTH, pg/ml (95% CI)a | P value |
|---|---|---|---|---|---|---|
| 25(OH)D ≤20 ng/ml | 25(OH)D >20 ng/ml | |||||
| All Participants | 3,903 | −0.80 (−1.00, −0.60) | <0.01 | 4,101 | −0.30 (−0.39, −0.22) | <0.01 |
| White | 1,203 | −0.85 (−1.15, −0.54) | <0.01 | 2,938 | −0.29 (−0.37, −0.21) | <0.01 |
| Mexican-American | 1,121 | −0.50 (−0.78, −0.22) | <0.01 | 802 | −0.52 (−0.71, −0.34) | <0.01 |
| Black | 1,579 | −0.90 (−1.28, −0.52) | <0.01 | 361 | 0.09 (−0.41, 0.59) | 0.71 |
Multivariable linear regression analyses, adjusted for age, gender, BMI, creatinine, calcium intake, and season of blood draw (either November 1 to April 30 or May 1 to October 31)
Discussion
In this cross-sectional analysis of relatively young adults in NHANES, BMD significantly decreased with declining 25 (OH)D concentrations in whites and Mexican-Americans, but not in blacks. In addition, there were significant racial differences in the relationship between 25(OH)D and PTH above and below a 25(OH)D threshold commonly used to define vitamin D deficiency (20 ng/ml). These results may have important implications for evaluating vitamin D adequacy in minority populations. Indeed, vitamin D stores in racial minorities are routinely assessed by comparing their serum 25(OH)D concentrations against “normal” ranges that have largely been derived from the mathematical modeling of PTH and/or BMD as a function of 25(OH)D in predominantly white populations. In contrast, the results of this study suggest that optimal ranges for vitamin D among whites may not be the same as those among blacks, at least with respect to optimizing bone and mineral metabolism.
Racial differences in the relationship between 25(OH)D and BMD have been reported by previous investigators. For example, Aloia et al. analyzed the change in BMD in 208 postmenopausal African-American women randomly assigned to receive either vitamin D3 supplementation or placebo for 3 years [30]. In contrast to analogous studies of white subjects [31, 32], these investigators found no significant differences in BMD loss between the intervention groups despite a significant increase in 25(OH)D concentrations in the active group as opposed to the control group [30]. Similarly, in a study examining the relationships between 25(OH)D and BMD in elderly white, black, and Hispanic men residing in Boston, serum 25(OH)D concentrations were independently correlated with BMD in the white participants, but not the black participants [19]. Our findings mirror these data in that we found that BMD decreased in parallel with declining serum 25(OH)D and dietary calcium intake among whites and Mexican-Americans, yet it remained remarkably preserved even within the lowest categories of calcium intake and 25(OH) D levels among blacks. These observations likely reflect known racial differences in calcium economy. Blacks are more efficient than whites in absorbing dietary sources of calcium, preserving calcium in the bones, and retaining calcium in the kidney, especially during adolescence [33–35], leading some to suggest that blacks may require less dietary calcium than whites to maintain bone health [36]. Accordingly, it is possible that blacks may also require a lower “set-point” for serum 25(OH)D to optimize calcium metabolism. Indeed, blacks maintain similar if not higher concentrations of 1,25-dihydroxyvitamin D than whites even at comparable concentrations of PTH [37–39], suggesting that blacks may be able to maintain adequate calcium homeostasis at lower circulating 25(OH)D levels than whites. In addition, it is possible that higher BMI among blacks may also contribute to racial differences in the relationships between 25(OH)D and BMD [40].
We observed highly significant inverse relationships between 25(OH)D and PTH above and below a 25(OH)D concentration of 20 ng/ml in whites and Mexican-Americans, but not among blacks. Indeed, while 25(OH)D and PTH were inversely related when 25(OH)D values were ≤20 ng/ml, the slope of this relationship was essentially flat above this threshold among blacks. These findings suggest that PTH concentrations may be maximally suppressed at a lower 25(OH)D concentration among blacks than among whites or Mexican-Americans. In support of this possibility is the study by Aloia et al. which found that PTH values appeared to plateau at a 25(OH)D concentration between ∼16 and 20 ng/ml in African-American women [41]. Moreover, women who began this study with 25(OH)D concentrations <17 ng/ml demonstrated significant reductions in PTH in response to vitamin D supplementation, whereas those who began the study >17 ng/ml did not, suggesting a threshold effect for PTH suppression at a 25(OH)D concentration of ∼17 ng/ml, in contrast to analogous studies in white populations [3, 4]. Collectively, these data suggest that 25(OH)D threshold values derived from the mathematical modeling of PTH as a function of 25(OH)D in white populations may not be appropriate for blacks, potentially leading to inaccurate estimations of the prevalence of vitamin D insufficiency and deficiency in minorities.
It is important to note that numerous investigators have cautioned against the use of “bone-” or “mineral-based” 25 (OH)D thresholds as the primary determinant of optimal vitamin D levels, particularly considering the potential non-mineral benefits of vitamin D on outcomes such as hypertension, diabetes, cancer, immune function, and death [7, 42, 43]. Although we agree with this concern, 25(OH)D thresholds derived from skeletal or mineral endpoints in white populations remain widely utilized for evaluating vitamin D stores in racial minorities, resulting in alarmingly high prevalence rates of vitamin D deficiency in minority populations [8–10]. Indeed, 81% of blacks would have been categorized as vitamin D deficient, as compared to only 28% of whites, if one commonly used cut-off for vitamin D deficiency were indiscriminately applied to participants in this study. Yet utilizing this “one-size-fits-all” approach to assessing vitamin D stores in minority populations overlooks critical racial differences in bone and mineral metabolism as detailed above. Thus, while we cannot advocate different threshold values for vitamin D sufficiency in blacks based upon the results of this study alone, it would be similarly inappropriate to assume that standardized thresholds for vitamin D sufficiency in whites can be directly extrapolated to minority populations. Further studies are needed to test whether uniform targets for 25(OH)D adequacy result in similar or discordant outcomes by race.
Our study has limitations. First, variability in the performance of 25(OH)D assays in NHANES 2000–2006 is a potential limitation of this study. While sensitivity analyses suggested that the results of this study remained qualitatively the same when comparing the 2003–2004 sample to the 2005–2006 sample, variability from assay drift could still have impacted these results. Future studies will need to confirm these relationships when appropriate corrections for 25(OH)D results across NHANES 2000–2006 cycles are established. Second, we did not have measurements of 1,25-dihydroxyvitamin D or fibroblast growth factor 23, both of which influence 25(OH)D, PTH and bone metabolism and have been shown to differ by race [37, 44]. In addition, we did not have information with respect to parathyroid gland morphology, and so we could not determine whether parathyroid gland hyperplasia among blacks may have partly accounted for our results [45]. Further studies will need to determine to what extent these factors may have impacted the findings of this study. Finally, we did not have prospective data concerning the associations between low 25(OH)D concentrations and the development of adverse health outcomes such as hypertension, diabetes, or cancer. Thus, we were unable to determine whether these relationships differ by race/ethnicity. For example, it is possible that blacks require higher 25(OH)D levels than whites for certain non-skeletal outcomes such as cardiovascular health, cancer prevention, or immune function. However, if so, this would only strengthen the case against assuming that vitamin D threshold values in whites can be extrapolated across racial and ethnic groups without first determining whether this currently widespread assumption is in fact appropriate.
In summary, we found that the relationships between 25(OH)D, BMD, and PTH varied by race among US adults. Further studies are needed to confirm these findings, and to determine whether race- and/or ethnic-specific ranges of optimal 25(OH)D are required to appropriately evaluate the adequacy of vitamin D stores in diverse populations.
Acknowledgments
The authors thank UCLA Statistical Consulting for their assistance in these analyses.
Sources of funding: Research support was obtained from grants K23DK081673 (to OMG) and K08DK073381 (to ENT) from the National Institutes of Health. In addition, this study was supported with resources and facilities at the Veterans Affairs Medical Center, Boston, MA.
Footnotes
Conflicts of interest: Dr. Gutiérrez reports accepting honoraria from Abbott Laboratories.
Contributor Information
O. M. Gutiérrez, Email: ogutierrez2@med.miami.edu, Division of Nephrology and Hypertension, Department of Medicine, University of Miami Miller School of Medicine, 1120 NW 14th street, CRB (C-221) Room 815, Miami, FL 33136, USA
W. R. Farwell, Department of Medicine, Veterans Affairs Medical Center, Boston, MA, USA; Division of Aging, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA
D. Kermah, Department of Medicine, Charles Drew University of Medicine and Science, Los Angeles, CA, USA
E. N. Taylor, Division of Nephrology and Transplantation, Maine Medical Center, Portland, ME, USA; Channing Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 4.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N Engl J Med. 1989;321:1777–1783. doi: 10.1056/NEJM198912283212602. [DOI] [PubMed] [Google Scholar]
- 5.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 6.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–2341. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67:1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 9.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85:4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 10.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 11.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(S5):97–101. [PubMed] [Google Scholar]
- 13.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 14.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Liu Y, Willett WC. Cancer incidence and mortality and vitamin D in black and white male health professionals. Cancer Epidemiol Biomark Prev. 2006;15:2467–2472. doi: 10.1158/1055-9965.EPI-06-0357. [DOI] [PubMed] [Google Scholar]
- 16.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 17.Nelson DA, Jacobsen G, Barondess DA, Parfitt AM. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 18.Aloia JF, Vaswani A, Ma R, Flaster E. Comparison of body composition in black and white premenopausal women. J Lab Clin Med. 1997;129:294–299. doi: 10.1016/s0022-2143(97)90177-3. [DOI] [PubMed] [Google Scholar]
- 19.Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93:40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leder BZ, Araujo AB, Travison TG, McKinlay JB. Racial and ethnic differences in bone turnover markers in men. J Clin Endocrinol Metab. 2007;92:3453–3457. doi: 10.1210/jc.2006-2695. [DOI] [PubMed] [Google Scholar]
- 21.Bauer RL, Diehl AK, Barton SA, Brender J, Deyo RA. Risk of postmenopausal hip fracture in Mexican American women. Am J Public Health. 1986;76:1020–1021. doi: 10.2105/ajph.76.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88:545S–550S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: (2003–2004) National Health and Nutrition Examination Survey Data. [Google Scholar]
- 24.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 25.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 26.Looker AC, Melton LJ, 3rd, Harris T, Borrud L, Shepherd J, McGowan J. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int. 2009;20:1141–1149. doi: 10.1007/s00198-008-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 28.Cleveland W, Devlin S. Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 29.Hurvich C, Simonoff J. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J Roy Stat Soc. 1998;60:271–293. [Google Scholar]
- 30.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–1623. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 32.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115:505–512. doi: 10.7326/0003-4819-115-7-505. [DOI] [PubMed] [Google Scholar]
- 33.Bell NH, Yergey AL, Vieira NE, Oexmann MJ, Shary JR. Demonstration of a difference in urinary calcium, not calcium absorption, in black and white adolescents. J Bone Miner Res. 1993;8:1111–1115. doi: 10.1002/jbmr.5650080912. [DOI] [PubMed] [Google Scholar]
- 34.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, Smith DL, Peacock M, Weaver CM. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88:1043–1047. doi: 10.1210/jc.2002-021367. [DOI] [PubMed] [Google Scholar]
- 35.Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, Lindsay R, Parisien M. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12:958–966. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 36.Heaney RP. The importance of calcium intake for lifelong skeletal health. Calcif Tissue Int. 2002;70:70–73. doi: 10.1007/s00223-001-0032-3. [DOI] [PubMed] [Google Scholar]
- 37.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76:470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson-Hughes B, Harris S, Kramich C, Dallal G, Rasmussen HM. Calcium retention and hormone levels in black and white women on high- and low-calcium diets. J Bone Miner Res. 1993;8:779–787. doi: 10.1002/jbmr.5650080702. [DOI] [PubMed] [Google Scholar]
- 40.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr. 2006;84:602–609. doi: 10.1093/ajcn/84.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghandur-Mnaymneh L, Cassady J, Hajianpour MA, Paz J, Reiss E. The parathyroid gland in health and disease. Am J Pathol. 1986;125:292–299. [PMC free article] [PubMed] [Google Scholar]