Abstract
The success of gene therapy hinges on achievement of adequate transgene expression. To ensure high transgene expression, many gene-therapy vectors include highly active virus-derived transcriptional elements. Other vectors include tissue-specific eukaryotic transcriptional elements, intended to limit transgene expression to specific cell types, avoid toxicity, and prevent immune responses. Unfortunately, tissue specificity is often accompanied by lower transgene expression. Here we use eukaryotic (murine) transcriptional elements and a virus-derived posttranscriptional element to build cassettes designed to express a potentially therapeutic gene (interleukin-10) in large vessel endothelial cells (EC) at levels as high as obtained with the CMV immediate-early promoter, while retaining EC-specificity. The cassettes were tested by incorporation into helper-dependent adenoviral vectors, and transduction into bovine aortic EC in vitro and rabbit carotid EC in vivo. The murine endothelin-1 promoter showed EC-specificity, but expressed only 3% as much IL-10 mRNA as CMV. Inclusion of precisely 4 copies of an EC-specific enhancer and a posttranscriptional regulatory element increased IL-10 expression to a level at or above the CMV promoter in vivo, while retaining—and possibly enhancing—EC specificity, as measured in vitro. The cassette reported here will likely be useful for maximizing transgene expression in large vessel EC, while minimizing systemic effects.
Keywords: adenovirus, endothelium, carotid artery, interleukin-10, gene expression regulation
Introduction
Endothelial cells (EC) of large arteries play major roles in several important physiologic and pathologic processes including regulation of hemostasis and thrombosis, control of leukocyte trafficking, regulation of vascular tone, and modulation of lipoprotein entry to the vascular wall.1 Durable, high-level expression of recombinant genes in large-vessel EC, using in vivo gene transfer, is potentially a powerful tool for understanding these biological processes and for preventing and treating human diseases such as atherosclerosis and thrombosis.2-5 However, achievement of durable, high-level transgene expression in EC in vivo has been challenging.6-9
Several groups have focused on the problem of increasing transgene expression levels in EC while limiting expression in other cell types, in which transgene expression could be deleterious.10-20 Expression cassettes constructed by these groups contain EC-specific eukaryotic promoter and enhancer elements, expected to be active in EC and less active in other cell types. These cassettes express transgenes at high levels in EC in tumor vessels and in small vessels of ischemic muscle, estrogen-stimulated uterine tissue, liver, and lung; often with evidence of relative EC-specific expression.11,13-15,17-20 However, far less attention has been devoted to maximizing transgene expression in EC of large blood vessels, in which transcriptional regulation is likely different than in smaller vessels.21 Many investigators use the cytomegalovirus (CMV) immediate early promoter to drive transgene expression in large vessel EC despite its lack of tissue-specificity and its susceptibility to attenuation and shutdown.16,22 The need for an EC-specific expression cassette than outperforms CMV in large vessel EC is well enunciated; however, attempts to construct such a cassette have not been successful.16
Several years ago, we reported that helper-dependent adenovirus (HDAd) can express a transgene in EC of large arteries for at least 2 months—far longer than first or second-generation Ad vectors—and with negligible local inflammation.23 These results suggest that HDAd will be extremely useful for expressing transgenes in EC of large vessels and that HDAd is therefore an attractive platform for developing and testing highly active, EC-specific expression cassettes. Here we report use of a 1.36 kb enhancer-promoter fragment from the murine endothelin-1 (mET-1) gene,24 a truncated version of the ET-1 enhancer-promoter that contains 3 additional copies of a 45 bp EC-specific ET-1 enhancer element (“ETE”), a genomic (intron-containing) DNA sequence, and the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE)25 to attempt to build a cassette that expresses at least as well as CMV in large-vessel EC in vivo while retaining significant EC-specificity.
Results
Use of the mET-1 enhancer-promoter and a genomic IL-10 clone as a strategy to obtain high-level, EC-specific transgene expression
We used the potentially therapeutic26 rabbit interleukin-10 (IL-10) cDNA and gene as reporters in experiments aimed at developing improved expression cassettes. Our initial hypotheses were that the native mET-1 enhancer-promoter would express at a lower level than the CMV promoter (as is typically the case for cell-type-specific promoters),12,16 and that the native mET-1 enhancer-promoter would show relative EC-specificity compared to the 750 bp CMV promoter. Because inclusion of introns can boost transgene expression,16,27 we also hypothesized that vectors containing the complete IL-10 exon-intron sequence would express at higher levels than vectors containing the IL-10 cDNA. We began to test these hypotheses by transducing bovine aortic EC (BAEC) with HDAd containing four different IL-10 constructs (CMV-cIL-10, CMV-gIL-10, mET1-cIL-10 and mET1-gIL-10) as well as HDAdNull, as a negative control (Figure 1). We measured IL-10 mRNA in transduced cells and normalized expression both to GAPDH mRNA and to vector DNA measured in cells from the same well. Expression from CMV-cIL-10 was 30-fold higher than mET1-cIL-10 and was approximately 3-fold higher than CMV-gIL-10 (Figure 2a). Expression from the two mET-1 vectors (mET1-cIL-10 and mET1-gIL-10) was not significantly different. Two-way ANOVA confirmed overall effects of CMV versus mET-1 promoters (CMV > mET-1; P < 0.001); this effect was present for both cDNA and gDNA. There was also an overall effect of cDNA versus gDNA (cDNA > gDNA; P < 0.001); however, this was driven by the CMV results and was not evident at the low levels of expression obtained with the mET1 promoter.
Figure 1.
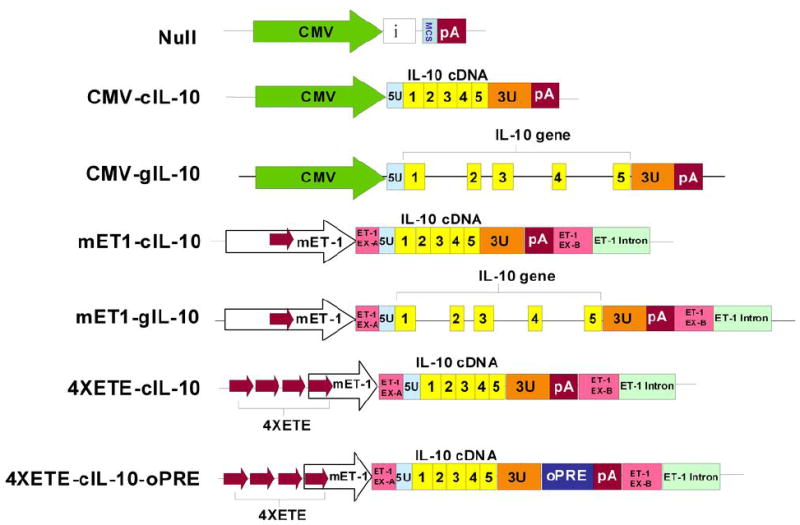
Expression cassettes. Cassettes were tested both in plasmid vectors in vitro and in HDAd in vitro and in vivo. Symbols: CMV = CMV promoter from pCI plasmid; i = β-globin/IgG chimeric intron from pCI; MCS = multiple cloning site; pA=SV40 poly A signal; 5U = 5′-untranslated region of rabbit IL-10 gene; 1, 2, 3, 4, 5 (yellow blocks) = rabbit IL-10 exons; 3U = 3′-untranslated region of rabbit IL-10 gene; mET-1 = native murine endothelin-1 enhancer-promoter; red arrows = ET-1 enhancer (ETE) in native position and with 3 additional ETE added as direct repeats (4XETE); ET-1 EX-A and ET-1 EX-B = 5′ and 3′ segments of mouse ET-1 exon 1 (untranslated); ET-1 intron = 5′ segment of mouse ET-1 intron 1; oPRE = optimized woodchuck hepatitis virus post-transcriptional regulatory element. Drawing is not to scale.
Figure 2.
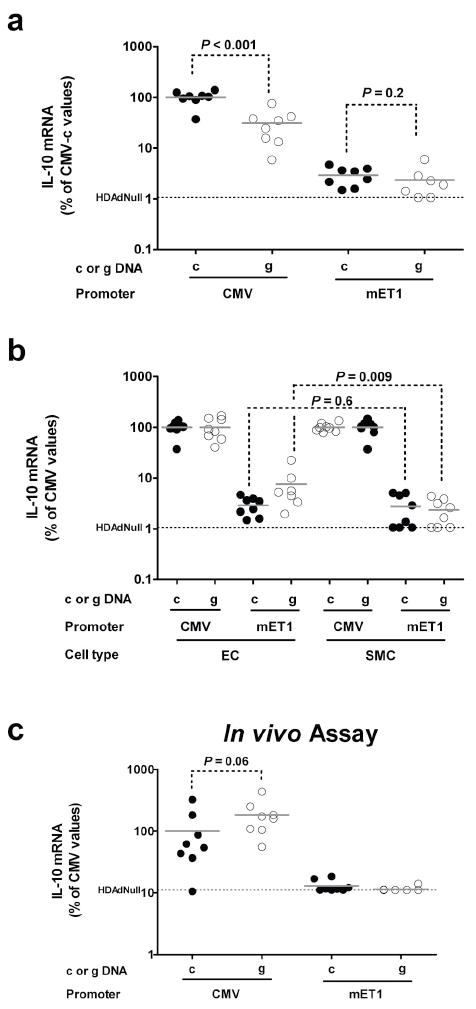
Expression of IL-10 cDNA (“c”) or genomic DNA (“g”) from the CMV promoter or the mET-1 enhancer-promoter in vitro and in vivo. (a) Bovine aortic endothelial cells (EC) were transduced with each of the 4 vectors, mRNA harvested after 24 hr, and IL-10 mRNA measured by qRT-PCR with mean expression from CMV-cIL-10 assigned a value of 100%. (b) EC or bovine aortic smooth muscle cells (SMC) were transduced, mRNA harvested after 24 hr, and IL-10 mRNA measured by qRT-PCR. For each cell type, mean IL-10 expression in each of the 2 CMV-containing-vector-transduced groups was assigned a value of 100%. Expression in individual wells transduced with this CMV-containing vector or with the analogous mET-1-containing vector is calculated as a percentage of this value. The EC data in (b) are the same data as in (a), replotted after making these calculations. Data points in (a) and (b) are individual wells. (c) In vivo IL-10 expression. RNA was harvested from carotid arteries 3 d after transduction and IL-10 mRNA was measured by qRT-PCR. Mean IL-10 expression in the CMV-cIL-10 group was assigned a value of 100%. Expression in each artery is plotted as a percentage of this value. The background IL-10 qRT-PCR signal [measured in cells (a-b) or in arteries (c) transduced with HDAdNull] is indicated. Bars in all panels are group means.
We next looked for evidence of EC specificity of the mET-1 enhancer-promoter. The same vectors were used to transduce bovine aortic smooth muscle cells (BASMC), and IL-10 expression was again measured. For both BAEC and BASMC, IL-10 expression from the CMV promoter-containing vectors (both cDNA and genomic) was assigned a value of 100%. Expression from each of the mET-1-containing vectors was then calculated as a fraction of the expression of the analogous CMV-containing vector in the same cell type. Using this calculation, EC specificity would manifest as a higher percentage of CMV-mediated expression in EC than in SMC. The mET1-gIL-10 cassette expressed IL-10 mRNA at a higher relative level in BAEC than BASMC (4-fold higher; P = 0.009) whereas the mET1-cIL-10 cassette expressed at a similar level relative to CMV in both cell types (Figure 2b; P = 0.6). Two-way ANOVA confirmed an overall effect of cell type (EC > SMC) on level of transgene expression from the mET-1 promoter (P = 0.03).
We next performed this same 4-vector comparison in our in vivo EC gene transfer model.9,28 The four IL-10-expressing vectors and the control vector HDAdNull were infused into surgically isolated rabbit carotid arteries and IL-10 mRNA was measured in arterial extracts 3 days later. In contrast to the in vitro results, expression from HDAd-CMV-gIL-10 was highest in vivo (2-fold above HDAd-CMV-cIL-10; P = 0.06; Figure 2c). Similar to the in vitro results, IL-10 mRNA levels in arteries infused with either of the mET-1 vectors were far below IL-10 mRNA levels in arteries infused with the CMV vectors. The background signal in this experiment was the q-RTPCR signal from arteries transduced with HDAdNull; the signal from arteries transduced with both of the mET-1 vectors was at this level (Figure 2c). Therefore, if either of the mET-1 vectors expressed IL-10 mRNA in vivo, the level of mRNA was too low for us to detect.
Increased IL-10 expression and preserved EC-specificity in vitro after addition of EC-specific enhancer elements
To increase expression from the mET-1 promoter, we constructed plasmids in which the ET-1 promoter sequence 5′ of the native 45 bp EC-specific ET-1 enhancer (the ETE; located at −364 to −319 in the mouse ET-1 gene; Figure 1)24 was replaced by 2, 3, or 4 additional copies of the ETE. In these plasmids the ETE were located upstream of a minimal (318 bp) mET-1 promoter and the rabbit IL-10 cDNA (Supplementary Figure 1). These plasmids were transfected into BAEC and expression of IL-10 mRNA quantified. The plasmid containing 3 copies of the ETE (the native ETE with 2 additional copies: “3XETE”) expressed 4–5-fold more IL-10 mRNA than the plasmid containing 1 ETE copy. The plasmid with 4 copies of the ETE (“4XETE”) expressed twice as much IL-10 mRNA as plasmids containing 3 or 5 copies (P < 0.001 for all comparisons; Figure 3a).
Figure 3.
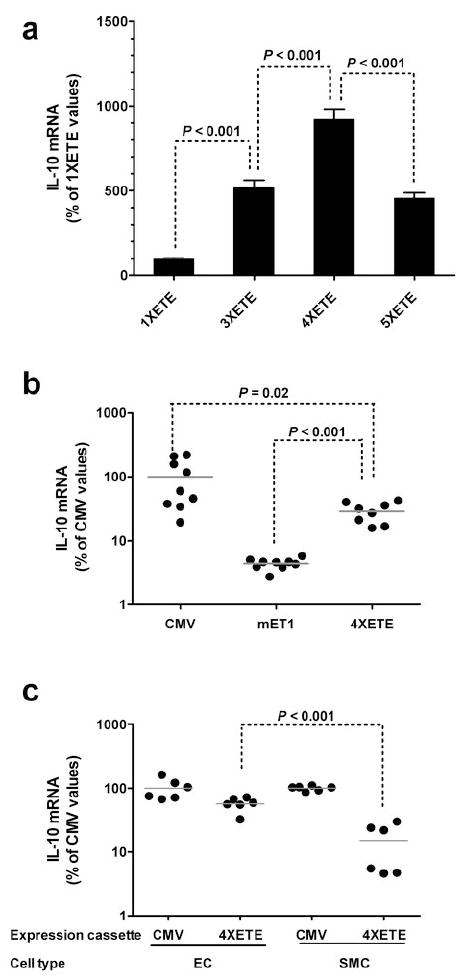
Enhancement of IL-10 expression and preservation of EC-specificity after addition of ET-1 enhancer (ETE) sequences to the mET-1 promoter. (a) EC were transfected with plasmids containing the 318 bp mET-1 promoter, the IL-10 cDNA, and 1, 3, 4, or 5 copies of the ETE (see Supplementary Figure 1). IL-10 mRNA was measured by qRT-PCR. Mean IL-10 expression for the 1XETE construct was assigned a value of 100%. Expression measured in individual wells was calculated as a percentage of this value and represented here as mean ± SD. (b) EC were transduced with HDAd containing the indicated expression cassettes (see Figure 1) and IL-10 mRNA measured by qRT-PCR. Mean IL-10 expression for the CMV-cIL-10 group was assigned a value of 100%. Values from individual wells are expressed as a percentage of this value. Bars are group means. (c) EC or SMC were transduced with HDAd containing the CMV-cIL-10 or the 4XETE-cIL-10 cassettes (Figure 1) and IL-10 mRNA measured by qRT-PCR. For each cell type, mean IL-10 expression for the CMV-cIL-10 group was assigned a value of 100%. IL-10 expression from individual wells is expressed as a percentage of this value. Bars are group means; SD are shown in (a).
Based on this result we constructed a new HDAd in which 3 copies of the ETE were added upstream of the endogenous ETE and minimal mET-1 promoter (4XETE-cIL-10; Figure 1). EC were transduced with one of 3 HDAd: 4XETE-cIL-10, CMV-cIL-10, or mET1-cIL-10 and IL-10 mRNA measured. In these experiments, in relation to CMV-cIL-10 expression set at 100%, inclusion of 4 ETE copies increased IL-10 expression by about 7-fold compared to HDAd-mET1-cIL-10 (Figure 3b; P < 0.001). However, this increased level of expression was still only about 30% of levels obtai?ned with HDAd-CMV-cIL-10 (P = 0.02).
To determine whether addition of 3 copies of the ETE and removal of the ET-1 promoter sequences 5′ of the native ETE (in HDAd4XETE-cIL-10) affected EC-specificity, we compared IL-10 expression from HDAd4XETE-cIL-10 in BAEC and BASMC. When normalized to CMV-directed expression in the same cell type, HDAd4XETE-cIL-10 expressed approximately 4-fold more IL-10 mRNA in BAEC than in BASMC (P < 0.001; Figure 3c). By comparing these data to data generated with the mET1-driven vectors (Figure 2b) we noted that addition of the 4X-ETE increased IL-10 expression not only in EC, but also in SMC. Therefore, the 4X-ETE is not completely EC-specific. To assess this observation more quantitatively, we repeated the measurements of IL-10 expression using a randomly selected subset of samples from the in vitro experiments with the mET1-cIL10 and 4XETE-cIL-10 vectors (samples from the experiments shown in Figure 2b and 3c). We measured IL-10 expression simultaneously in all samples, without normalization to CMV expression in the same cell type (Figure 4). These measurements revealed—as in Figure 2b—that expression from mET1 was low in both EC and SMC. However, here we detected significantly higher expression from mET1 in EC versus SMC (2-fold; P < 0.001). Addition of the 4X-ETE increased IL-10 expression in both cell types but far more in EC than SMC (280-fold versus 28-fold). Therefore, although the 4X-ETE is not completely EC-specific, it is far more active in EC than SMC and therefore contributes both higher expression and increased EC-specificity above that achieved with the mET1 promoter.
Figure 4.
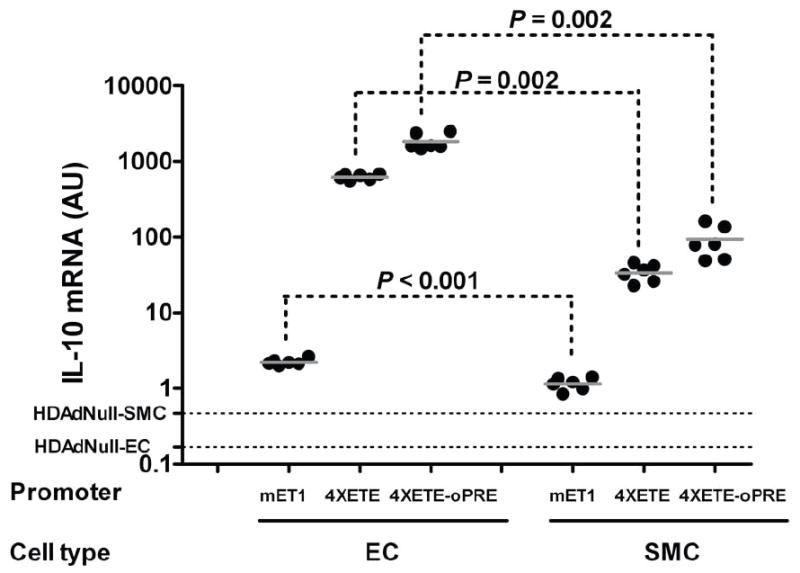
Relative activities of the 4XETE and oPRE in EC and SMC. Bovine EC or SMC were transduced with HDAd in which expression of the IL-10 cDNA is driven by the mET1 enhancer-promoter, the truncated mET1 enhancer-promoter with 3 additional copies of the ETE (4XETE), or the 4XETE enhancer-promoter with the oPRE added (see Figure 1). IL-10 mRNA was measured by qRT-PCR, normalized to GAPDH mRNA in the same extracts, and expressed as arbitrary units (AU). Background signal in this assay was determined by measurement of IL-10 mRNA in extracts of EC and SMC transduced with HDAdNull. Data points are from individual wells, bars are group means. The cell extracts assayed here are a subset of those represented in Figures 2b, 3c, and 5b. We repeated our measurements of IL-10 and GAPDH mRNA in these 3 sets of extracts (qRT-PCR was performed on all 3 sets simultaneously here) to generate data for this figure.
Increased IL-10 expression and preserved EC-specificity in vitro after addition of a posttranscriptional regulatory element
To further increase vector-mediated transgene expression while hopefully maintaining relative EC-specificity, we inserted both the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE)25 and an optimized version of this element (oPRE; in which the potentially oncogenic X protein sequences are deleted)29 into the IL-10 3′ UTR in plasmids containing the CMV-cIL-10 and 4XETE-cIL-10 expression cassettes. We tested both PRE in both orientations in plasmids containing both the CMV and 4XETE-mET-1 promoters. Results of these EC transfections were generally unimpressive, with increased IL-10 mRNA (< 2-fold) only from the WPRE in the forward orientation, and only with the CMV promoter (Supplementary Figure 2a,b).
Because our in vitro plasmid transfection data did not always predict relative expression levels when the same cassettes were transduced by HDAd in vivo (compare CMVcIL-10 and CMVgIL-10 in Figure 2a versus 2c), before abandoning the PREs we tested one of them in an HDAd. We selected the oPRE in the forward orientation, and used it to construct HDAd4XETE-cIL-10-oPRE (Figure 1). In this experiment, in relation to CMV-cIL-10 expression set at 100%, BAEC transduced with HDAd4XETE-cIL-10-oPRE expressed about 50% more IL-10 mRNA than BAEC transduced with HDAd4XETE-cIL-10 (Figure 5a; P = 0.01). This level of expression was about 60% of that obtained in parallel transductions with HDAd-CMV-cIL-10. At this stage of our experiments, we developed a western blot method for detection of rabbit IL-10 protein. Western analysis of media conditioned by BAEC transduced with HDAd4XETE-cIL-10-oPRE and HDAd-CMV-cIL-10 revealed that HDAd4XETE-cIL-10-oPRE produced at least as much IL-10 protein as HDAd-CMV-cIL-10 (Supplementary Figure 3), possibly reflecting a post-transcriptional effect of the oPRE.
Figure 5.
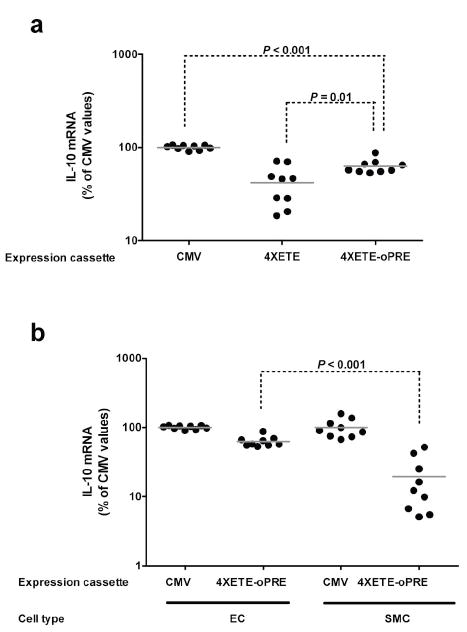
Enhancement of IL-10 expression and preservation of EC-specificity after addition of the optimized woodchuck hepatitis virus posttranscriptional regulatory element (oPRE). (a) EC were transduced with HDAd containing the CMV-c-IL-10 expression cassette or the 4XETE-cIL-10 cassette both without and with the oPRE. IL-10 mRNA was measured by qRT-PCR. Mean IL-10 expression for the CMV-cIL-10 cassette was assigned a value of 100%; IL-10 expression in individual wells was calculated as a percentage of this value. (b) EC or SMC were transduced with HDAd containing the CMV-cIL-10 or the 4XETE-cIL-10-oPRE cassettes (Figure 1) and IL-10 mRNA measured by qRT-PCR. For each cell type, mean IL-10 expression for the CMV-c-IL-10 group was assigned a value of 100%; IL-10 expression in individual wells was calculated as a percentage of this value. Bars in all panels are group means.
To determine whether addition of the oPRE affected EC-specificity, we compared IL-10 expression from HDAd4XETE-cIL-10-oPRE in BAEC and BASMC. When normalized to CMV-directed expression in the same cell type, HDAd4XETE-cIL-10-oPRE expressed approximately 3-fold more IL-10 mRNA in BAEC than in BASMC (P < 0.001; Figure 5b). Therefore, significant EC-specificity was retained after addition of the oPRE. To further examine the potency and specificity of the oPRE, we measured IL-10 mRNA in a randomly selected subset of samples from those portrayed in Figure 5b, and compared IL-10 mRNA in these samples with IL-10 mRNA in samples from EC and SMC transduced with the mET1-cIL-10 and 4XETE-cIL-10 vectors (Figure 4). This analysis revealed that addition of the oPRE increased IL-10 expression equally (3-fold) in both cell types. Therefore, unlike the 4XETE, the oPRE does not act preferentially in EC.
Increased in vivo IL-10 expression from vectors containing 4XETE and the oPRE
We next tested whether addition of the 4XETE and oPRE to the mET-1 promoter also increased transgene expression in rabbit EC in vivo. IL-10 mRNA was measured in rabbit carotids infused with one of 4 HDAd: CMV-cIL-10, mET1-cIL-10, 4XETE-cIL-10 and 4XETE-cIL-10-oPRE (Figure 6). As before, expression from mET1-cIL-10 was far below expression from CMV-cIL-10. Addition of the 3 ETE copies to the native mET-1 enhancer-promoter greatly increased IL-10 expression (9-fold; P < 0.001), yielding expression that was not significantly different from that obtained with the CMV promoter-containing vector (P = 1). Addition of the oPRE further increased IL-10 expression by 2-fold (P = 0.01 versus 4XETE-cIL-10) to a level 35% higher than CMV-cIL-10-transduced arteries, although this difference was of only borderline statistical significance (P = 0.1).
Figure 6.
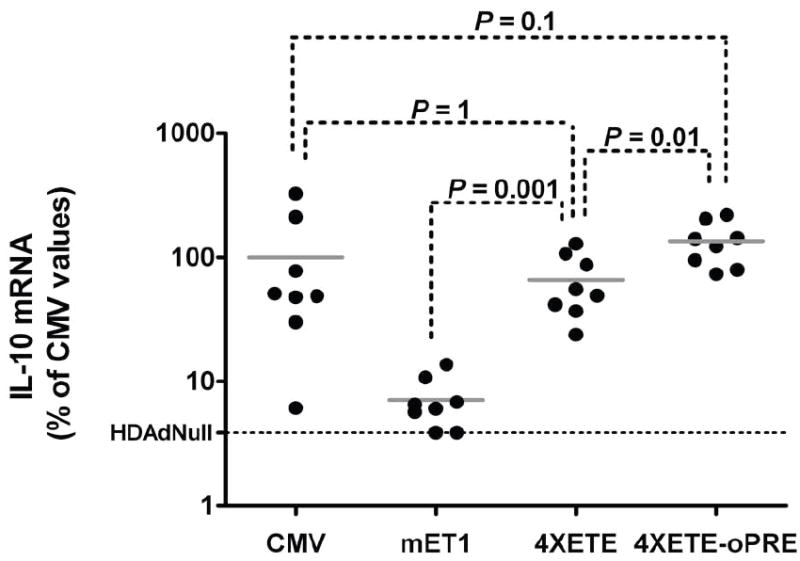
Enhancement of IL-10 expression in vivo after addition of enhancers and the oPRE. Rabbit EC were transduced in vivo by infusion of HDAd containing one of the four indicated expression cassettes, each containing the IL-10 cDNA (Figure 1). Three days later, transduced arteries were removed and IL-10 mRNA measured by qRT-PCR. Mean IL-10 expression for the CMV-cIL-10 group was assigned a value of 100%; IL-10 expression from individual arteries was calculated as a percentage of this value. The background IL-10 qRT-PCR signal (measured in arteries transduced with HDAdNull) is indicated. Data points are individual arteries; bars are group means.
Lack of systemic vector dissemination and transgene expression
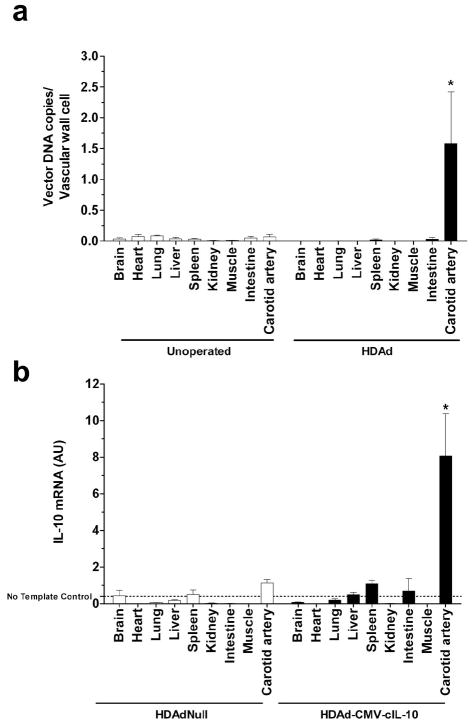
If vectors are released from the site of infusion, disseminate throughout the animal, and transduce cells in distant tissues, and if the transduced cells in these distant tissues are not EC, then transgene expression in distant tissues could provide an in vivo read-out of promoter specificity. We therefore determined whether vector DNA was present in distant tissues after carotid gene transfer, and if so whether the IL-10 transgene was expressed.
We measured vector DNA and IL-10 expression in tissues from rabbits after carotid artery infusion of either HDAd-CMV-cIL-10 or HDAd-Null. Vector DNA was below background levels (as determined by measurement of DNA in tissues of unoperated animals) in all tissues other than the carotids (Figure 7a). Because vector-directed RNA (present as numerous copies per cell) might be more easily detected than vector DNA (fewer copies per cell), we also measured IL-10 mRNA in these organs. IL-10 mRNA was not significantly above background levels (as determined by measurement of IL-10 mRNA in tissues from HDAdNull-infused rabbits) at any site other than the common carotid artery (Figure 7b).
Figure 7.
Lack of detectable systemic vector distribution and transgene expression in tissues distant from site of gene transfer. Carotid arteries were infused locally with an HDAd (either HDAdNull or HDAd-CMV-cIL-10). Three days later, DNA and RNA were isolated from carotid arteries and various distant tissues. Unoperated arteries were also included as controls. (a) Vector DNA (n = 16 per group) was measured by qPCR with probe/primers specific for the CMV promoter. * P < 0.01 compared to unoperated carotid artery. (b) IL-10 mRNA was measured by qRT-PCR of RNA from carotid arteries (n = 5 per group) infused with either HDAdNull or HDAd-CMV-cIL-10, and from other tissues (n = 4 – 5 per group) harvested from the same rabbits. The background q-RTPCR signal in this assay is that obtained with the “no template control” sample. Values near the no-template control signal are likely noise rather than signal. Among the HDAd-CMV-cIL-10 samples, only the signal from carotid artery was significantly different from the analogous HDAdNull sample (* P < 0.03). Values were normalized to GAPDH expression, expressed as arbitrary units (AU), and are shown as mean ± SD.
Discussion
The objective of this study was to construct an expression cassette that would drive high-level transgene expression in large vessel EC transduced by an HDAd vector and also provide significant cell-type specificity. The need for an EC-specific cassette that achieves expression levels in large vessel EC at least as high as those obtained with the CMV promoter is well stated in a recent article by White et al.16 We pursued this goal using rabbit IL-10 cDNA and genomic clones, murine ET-1 promoter and enhancer sequences, and a post-transcriptional regulatory element (PRE). Our major findings were: 1) The native murine 1.36 kb ET-1 enhancer-promoter expresses at a far lower level than the CMV promoter in EC both in vitro and in vivo; 2) inclusion of rabbit IL-10 introns in the expression cassette leads to a modest increase in IL-10 expression in rabbit EC in vivo, but lowers expression in BAEC in vitro; 3) The 1.36 kb native murine ET-1 enhancer-promoter provides modest EC-specificity of transgene expression; 4) Addition of ET-1 enhancer elements substantially increases transgene expression in EC both in vitro and in vivo and increases EC-specificity; 5) Addition of a PRE further increases transgene expression in EC; 6) Transgene expression from a cassette including a 364 bp ET-1 enhancer-promoter, 3 additional ET-1 enhancers, and a PRE is at least as high as is obtained with the CMV promoter in vivo, and—based on parallel in vitro studies—is more EC-specific.
The CMV promoter is often incorporated into gene-transfer vectors because of its high activity, small size, and ability to express transgenes in a wide range of cell types. CMV is especially useful for proof-of-concept studies that require only a burst of transgene expression, especially those carried out only in vitro. However, the utility of CMV in vivo is limited by its lack of tissue specificity, which allows transgene expression in nontargeted cell types including antigen-presenting cells.22,30 The CMV promoter is also susceptible to promoter attenuation and shutdown, far more so than eukaryotic promoters.22,31 Other disadvantages of CMV for cardiovascular applications include downregulation by β-blockers32 (drugs that are taken by many patients with cardiovascular diseases) and upregulation by inflammation,23 which many cardiovascular therapies aim to reduce.33
Because of the limitations of CMV and other viral promoters for EC-targeted gene transfer, several groups have constructed and tested vectors with putative EC-specific promoters including Flt-1, ICAM-1, ICAM-2, von Willebrand factor, Tie-1, Tie-2, flk-1, vascular endothelial cadherin (VE-cadherin), lysyl oxidase-1 (LOX-1), and endoglin.11-16 In several cases relative EC-specificity was achieved compared to viral or broadly active eukaryotic promoters such as phosphoglycerate kinase. However, EC-specificity was acquired at the cost of reliably lower transgene expression.12,14,16
In work published largely since we began this project, the ET-1 promoter and enhancer were used to target EC—with apparent success—by at least two groups. One group used the ET-1 promoter and a multimerized 3X ETE to construct Ad for use in EC-targeted gene therapy aimed at blocking tumor angiogenesis or promoting ischemic angiogenesis.17,18,20,34 The vectors appeared relatively EC-specific and dramatically outperformed the CMV promoter both in vitro (in BAEC and HUVEC) and in vivo. However, the exact sequences of these ET-1-based promoter/enhancer constructs are not published,17,18,20,34 and the in vivo expression data are largely limited to small vessels, with no quantitation of absolute expression levels achieved in large vessel endothelium.17,18,34 For these reasons it is difficult to compare our results with these precedents. A second group fused multimers of the ETE (4X and 7X) to the human Cdc-6 and cyclin A promoters, to generate expression cassettes specific for proliferating EC.19,35 The ETE multimers added EC specificity to these proliferation-sensitive promoters and conferred upon the Cdc-6 promoter an ability to express a reporter gene in tumors and estrogen-stimulated uterus at a level similar to that obtained with the CMV promoter. However, these expression levels appeared low; only about 1% of CMV expression in normal lung. Our study is the first to introduce a eukaryotic expression cassette in vivo in large vessel EC and unequivocally achieve transgene expression at least as high as obtained from the CMV promoter.
Several groups have shown that inclusion of introns in plasmid and viral vectors increase transgene expression.16,27,30,36,37 We were therefore surprised that the IL-10 cDNA vector expressed significantly more IL-10 mRNA in vitro than the genomic IL-10 vector (Figure 2a). In contrast, the genomic vector expressed more IL-10 mRNA than the cDNA vector in vivo (Figure 2c). The reason for this discrepancy is unclear, but could include use of EC from different species (bovine in vitro versus rabbit in vivo) or alteration in EC transcriptional regulation after removal from the vessel wall.21 It is possible that bovine—but not rabbit—EC express a transcriptional regulator that suppresses expression by binding to the genomic rabbit IL-10 sequences. We could not test the role of species specificity because rabbit endothelial cells are no longer available to us. Another example of in vitro/in vivo discrepancy is evident in Figures 5a and 6, in which the 4XETE-oPRE vector compares far more favorably to CMV in vivo than in vitro. This might be due to presence of ETE-binding transcription activators in rabbit EC in vivo versus their absence in cultured BAEC. Because we constructed the final 2 vectors (4XETE-cIL-10 and 4XETE-cIL-10-oPRE; Figure 1) based on in vitro results showing superiority of the IL-10 cDNA (Figure 2a) we may have missed an opportunity to construct vectors that—because they contained the genomic IL-10 sequence—expressed higher than both 4XETE-cIL-10-oPRE and CMV-cIL-10 in vivo (Figure 6). However, the increased expression level provided by the genomic DNA versus cDNA was relatively small (2-fold; Fig. 2c), of only borderline significance (P = 0.06) and was not found in transduced arteries of fat-fed rabbits (data not shown). Based on the comparatively dramatic effect of adding 3 copies of the ETE (> 200-fold increase; Figure 4) introduction of additional 5′ regulatory elements to the expression cassette is likely to be a more fruitful approach for increasing transgene expression.
Some of the features of the rabbit carotid model used here are worthy of comment. First, compared to the rat carotid gene transfer model with which we investigated transcriptional regulation in EC,38 gene transfer in the rabbit model is more reproducible and transgene expression levels are farther above background. Second, there is no evidence in the rabbit model of vector dissemination beyond the site of gene transfer (Figure 7). Although it is likely that a small amount of vector is released systemically and is present in distant tissues below the limit of assay detection, our data nevertheless suggest that surgically isolated blood vessels can be efficiently transduced in situ by Ad without causing a potentially toxic systemic exposure to adenovirus.39 Confinement of transgene expression to the site of gene transfer is particularly important when expressing immunosuppressive genes such as IL-10 which—if released systemically—could cause immunosuppression and susceptibility to opportunistic infection. The absence of detectable vector DNA and RNA at distant sites also suggests that local vascular IL-10 gene therapy can be accomplished without systemic side effects.
In summary, we report an animal model and a general approach (incorporation of EC-specific transcriptional elements and other cis-acting sequences) that dramatically increases HD-Ad-driven transgene expression in EC (Figure 6). In vivo expression levels with the final construct are at least as high as those obtained with CMV. To our knowledge expression from a eukaryotic-based promoter at a level equivalent to CMV has never been unequivocally demonstrated in EC of a large vessel. Moreover, in vitro studies (Figures 4 and 5b) provide strong support for preservation of substantial EC-specificity, even at the highest expression levels. This model and approach will be useful for testing additional vector modifications aimed at further increasing transgene expression,30,40 adding physiologic regulation,41 and enhancing duration of expression. Improved vectors, designed and tested with this approach, will increase the likelihood that vascular gene therapy will be clinically useful.
Materials and methods
Construction of IL-10 expression cassettes and HDAd vectors
A plasmid containing a rabbit IL-10 cDNA was a kind gift from Dr. Harvey Perkins (Australian National University, Canberra, AU).42 We cloned the rabbit IL-10 gene by PCR amplification of genomic DNA extracted from the skin of a New Zealand White rabbit. Plasmid pqET-1, containing the murine endothelin-1 (mET-1) promoter, was obtained from Dr. T. Quertermous (Stanford University, Palo Alto, CA). To increase expression from cassettes containing the mET-1 promoter, we inserted additional copies of a 45-bp endothelium-specific enhancer element (“ETE”)24 present in the mET-1 promoter upstream of the native ETE in the mET-1 promoter. Multimers of the ETE were constructed using standard recombinant DNA methods. The WPRE was obtained from Dr. David Russell (University of Washington, Seattle).43 We also used a truncated version of the WPRE, termed “oPRE” or “optimal Post-transcriptional Regulatory Element”, obtained from Dr. Axel Schambach (Hannover Medical School, Germany).29 The oPRE lacks the X Protein coding sequence and promoter, which are associated with oncogenic activity.44 To enable efficient transfer of the IL-10 expression cassettes into the HDAd backbone plasmid pC4HSU (Microbix Biosystems, Toronto, ON, Canada) we constructed a shuttle plasmid that we used to insert cloned sequences into pC4HSU by homologous recombination (Supplementary Figure 4). A detailed description of cloning of the rabbit IL-10 gene, construction of all of the expression cassettes (Figure 1), as well as generation and production of the HDAd vectors is provided in the Supplementary Information.
Characterization of HDAd vectors
Viral particle (part) concentration was measured by spectrophotometer and was typically 1 – 3 × 1012 part/ml.45 E1A and helper virus (H14) contamination were measured with a quantitative (q) PCR-based approach in which viral genomes were measured by qPCR for SV40 polyA sequences and both E1A-containing and H14 sequences were measured by qPCR for sequences present only in E1A or H14. Absolute quantification of copies was achieved by reference to standard curves constructed by performing qPCR on known amounts of plasmid DNA containing the target sequences. Probes for qPCR were from Applied Biosystems (Foster City, CA) or IDT (Coralville, IA), primers were from Invitrogen (Carlsbad, CA) or Bioneer (Alameda, CA), and qPCR reagents were from Abgene/Thermo Fisher (Waltham, MA). E1A contamination in the viral preparations was below 1 in 106 HDAd genomes, and helper virus contamination was less than 1% of HDAd genomes.
Quantification of IL-10 expression in vitro
Bovine aortic endothelial cells (BAEC) and bovine aortic smooth muscle cells (BASMC) were purchased (Cell Applications Inc, San Diego, CA) and expanded in culture. Cells were transduced at passages 6 – 8 by addition of HDAd at 1 × 1010 part/ml (MOI approximately 2 × 104) at t = 0. Six hrs later, the vector-containing medium was aspirated, and the cells washed and incubated with fresh medium. The cells were harvested at t = 24 hr. RNA was extracted and purified using Trizol (Invitrogen) or the RNeasy mini kit (Qiagen, Valencia, CA); DNA was extracted and purified with the DNeasy kit (Qiagen). RNA was first digested with DNase I to remove contaminating genomic DNA, then 100 ng of RNA was reverse-transcribed and amplified with a one-step qRT-PCR protocol and reagents from Abgene/Thermo Fisher. qRT-PCR probes were from Applied Biosystems or IDT and the primers from Bioneer. The IL-10 specific sequences are: forward primer, 5′-AGA ACA GCT GCA TTC ACT TTC CA-3′; reverse primer, 5′-CCT TCG ATT GAA AGA AAG TCT TCA C-3′; probe, 5′-6-FAM-CTC CGC GAG CTC CGT GCT GC-TAMRA-3′. Cycle threshold (Ct) values for IL-10 were normalized for the amount of input RNA using Ct values for GAPDH obtained with the same RNA sample. IL-10/GAPDH values were then normalized for transfection/transduction efficiency by dividing by the number of vector copies in the transfected/transduced cells, obtained by qPCR for the SV40 polyA sequence. In each set of experiments, IL-10 expression from a CMV promoter-containing vector (containing the IL-10 cDNA for all experiments except for the cell-type-specificity experiments, in which both the cDNA and genomic DNA-containing vectors were used) was assigned a value of 100%. Expression from mET-1 promoter-containing vectors was calculated as percentage of the expression of the analogous CMV promoter-containing vector, measured in the same cell type. All in vitro experiments were performed three times with an n = 3 per treatment each time. All data points (n = 9) were pooled for final analyses.
In vivo gene transfer
Specific pathogen-free male New Zealand White rabbits (3 – 4 kg, Western Oregon Rabbit Co.) were fed a normal chow diet. After at least 1 wk of adaption to new housing, rabbits underwent bilateral gene transfer to their common carotid arteries. This procedure, carried out under general anesthesia, includes surgical isolation and vector infusion into the lumens of isolated carotid segments for 20 min, and achieves gene transfer almost exclusively to luminal endothelial cells.9,28 All animal protocols were approved by the Office of Animal Welfare of the University of Washington.
Quantification of vector copies and IL-10 expression in vivo
Carotid arteries were harvested 3 d after gene transfer and cut into 4 equal segments designated A, B, C, and D from the proximal to distal end. To measure HDAd vector copy number, DNA was isolated from both segment B and segment D, using the DNeasy Blood and Tissue kit (Qiagen). Segments A and C were snap-frozen in liquid nitrogen for later extraction of RNA with the RNeasy mini kit (Qiagen).
To evaluate whether this carotid gene transfer protocol resulted in vector dissemination and ectopic expression, in some rabbits we harvested RNA and DNA from several organs (brain, heart, lung, liver, spleen, kidney, muscle, and intestine) at the same time the carotids were harvested. RNA and DNA from these tissues were extracted as described above.
Western analysis of IL-10
BAEC were transduced with 1 ×1010 part/ml of HDAdNull, HDAd-CMV-cIL-10, or HDAd-4XETE-cIL-10-oPRE for 6 hr. Medium was changed to DMEM and collected after 24 hr. Vector DNA isolated from the cells and quantified by qPCR revealed equivalent transduction in all 3 groups (data not shown). Equal volumes of conditioned medium were resolved under reducing conditions by electrophoresis on 0.1% SDS 15% polyacrylamide gels. Proteins were transferred electrophoretically to an Immobilon-P membrane (Millipore, Billerica, MA). After blocking in 10 mM Tris-HCl buffer, pH 8.0, containing 150 mM sodium chloride, 0.1% Tween 20, and 5% (w/v) nonfat dry milk, IL-10 protein was detected with goat anti-human IL-10 primary antibodies (R&D Systems, Minneapolis, MN) followed by incubation with HRP-conjugated Donkey anti-goat secondary antibodies (Rockland, Gilbertsville, PA). Bound antibody was detected with a chemiluminescence reagent kit (Immun-Star WesternC kit; Bio-Rad Laboratories, Hercules, CA).
Statistical methods
Data are expressed as Mean ± SD. Group means were compared by t-test. Multiple groups were compared by one or two-way ANOVA. Sigma plot 11 software (Systat Software Inc., San Jose, CA) was used for statistical analysis.
Supplementary Material
Acknowledgments
We thank AdVec, Inc for permission to use the HDAd reagents, David Russell for providing the WPRE, Axel Schambach for the oPRE sequence, Harvey Perkins for the rabbit IL-10 cDNA, and Thomas Quertermous for the murine ET-1 sequences. Sofia Penev provided valuable technical help, and we are grateful to Margo Weiss for administrative assistance. This work was supported by National Institutes of Health grant HL076226 and the John L. Locke, Jr. Charitable Trust. Jordan Kho was supported in part by a grant to the University of Washington from the Howard Hughes Medical Institute through the Undergraduate Science Education Program.
Footnotes
Conflict of interest Dr. Dichek’s work has been funded by the NIH.
Supplementary Information accompanies the paper on the Gene Therapy website (http://www.nature.com/gt)
References
- 1.De Caterina R, Libby P, editors. Endothelial Dysfunctions and Vascular Disease. Blackwell; Malden, MA: 2007. p. 416. [Google Scholar]
- 2.Yla-Herttuala S, Martin JF. Cardiovascular gene therapy. Lancet. 2000;355:213–222. doi: 10.1016/S0140-6736(99)04180-X. [DOI] [PubMed] [Google Scholar]
- 3.Baker AH. Development and use of gene transfer for treatment of cardiovascular disease. J Card Surg. 2002;17:543–548. doi: 10.1046/j.1540-8191.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 4.Gaffney MM, Hynes SO, Barry F, O’Brien T. Cardiovascular gene therapy: current status and therapeutic potential. Br J Pharmacol. 2007;152:175–188. doi: 10.1038/sj.bjp.0707315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 6.Wen S, Schneider DB, Driscoll RM, Vassalli G, Sassani AB, Dichek DA. Second-generation adenoviral vectors do not prevent rapid loss of transgene expression and vector DNA from the arterial wall. Arterioscler Thromb Vasc Biol. 2000;20:1452–1458. doi: 10.1161/01.atv.20.6.1452. [DOI] [PubMed] [Google Scholar]
- 7.Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I, et al. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 8.Channon KM, Qian H, Youngblood SA, Olmez E, Shetty GA, Neplioueva V, et al. Acute host-mediated endothelial injury after adenoviral gene transfer in normal rabbit arteries: impact on transgene expression and endothelial function. Circ Res. 1998;82:1253–1262. doi: 10.1161/01.res.82.12.1253. [DOI] [PubMed] [Google Scholar]
- 9.Newman KD, Dunn PF, Owens JW, Schulick AH, Virmani R, Sukhova G, et al. Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia. J Clin Invest. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modlich U, Pugh CW, Bicknell R. Increasing endothelial cell specific expression by the use of heterologous hypoxic and cytokine-inducible enhancers. Gene Ther. 2000;7:896–902. doi: 10.1038/sj.gt.3301177. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds PN, Nicklin SA, Kaliberova L, Boatman BG, Grizzle WE, Balyasnikova IV, et al. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat Biotechnol. 2001;19:838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 12.Nicklin SA, Reynolds PN, Brosnan MJ, White SJ, Curiel DT, Dominiczak AF, et al. Analysis of cell-specific promoters for viral gene therapy targeted at the vascular endothelium. Hypertension. 2001;38:65–70. doi: 10.1161/01.hyp.38.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Velasco B, Ramirez JR, Relloso M, Li C, Kumar S, Lopez-Bote JP, et al. Vascular gene transfer driven by endoglin and ICAM-2 endothelial-specific promoters. Gene Ther. 2001;8:897–904. doi: 10.1038/sj.gt.3301468. [DOI] [PubMed] [Google Scholar]
- 14.De Palma M, Venneri MA, Naldini L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum Gene Ther. 2003;14:1193–1206. doi: 10.1089/104303403322168028. [DOI] [PubMed] [Google Scholar]
- 15.Richardson TB, Kaspers J, Porter CD. Retroviral hybrid LTR vector strategy: functional analysis of LTR elements and generation of endothelial cell specificity. Gene Ther. 2004;11:775–783. doi: 10.1038/sj.gt.3302220. [DOI] [PubMed] [Google Scholar]
- 16.White SJ, Papadakis ED, Rogers CA, Johnson JL, Biessen EA, Newby AC. In vitro and in vivo analysis of expression cassettes designed for vascular gene transfer. Gene Ther. 2008:340–346. doi: 10.1038/sj.gt.3303058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varda-Bloom N, Shaish A, Gonen A, Levanon K, Greenbereger S, Ferber S, et al. Tissue-specific gene therapy directed to tumor angiogenesis. Gene Ther. 2001;8:819–827. doi: 10.1038/sj.gt.3301472. [DOI] [PubMed] [Google Scholar]
- 18.Greenberger S, Shaish A, Varda-Bloom N, Levanon K, Breitbart E, Goldberg I, et al. Transcription-controlled gene therapy against tumor angiogenesis. J Clin Invest. 2004;113:1017–1024. doi: 10.1172/JCI20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymanski P, Anwer K, Sullivan SM. Development and characterization of a synthetic promoter for selective expression in proliferating endothelial cells. J Gene Med. 2006;8:514–523. doi: 10.1002/jgm.875. [DOI] [PubMed] [Google Scholar]
- 20.Levanon K, Varda-Bloom N, Greenberger S, Barshack I, Goldberg I, Orenstein A, et al. Vascular wall maturation and prolonged angiogenic effect by endothelial-specific platelet-derived growth factor expression. Pathobiology. 2006;73:149–158. doi: 10.1159/000095561. [DOI] [PubMed] [Google Scholar]
- 21.Minami T, Aird WC. Endothelial cell gene regulation. Trends Cardiovasc Med. 2005;15:174–184. doi: 10.1016/j.tcm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 23.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- 24.Bu X, Quertermous T. Identification of an endothelial cell-specific regulatory region in the murine endothelin-1 gene. J Biol Chem. 1997;272:32613–32622. doi: 10.1074/jbc.272.51.32613. [DOI] [PubMed] [Google Scholar]
- 25.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terkeltaub RA. IL-10: An “immunologic scalpel” for atherosclerosis? Arterioscler Thromb Vasc Biol. 1999;19:2823–2825. doi: 10.1161/01.atv.19.12.2823. [DOI] [PubMed] [Google Scholar]
- 27.De Geest B, Van Linthout S, Lox M, Collen D, Holvoet P. Sustained expression of human apolipoprotein A-I after adenoviral gene transfer in C57BL/6 mice: role of apolipoprotein A-I promoter, apolipoprotein A-I introns, and human apolipoprotein E enhancer. Hum Gene Ther. 2000;11:101–112. doi: 10.1089/10430340050016193. [DOI] [PubMed] [Google Scholar]
- 28.Schneider DB, Vassalli G, Wen S, Driscoll RM, Sassani AB, DeYoung MB, et al. Expression of Fas ligand in arteries of hypercholesterolemic rabbits accelerates atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 2000;20:298–308. doi: 10.1161/01.atv.20.2.298. [DOI] [PubMed] [Google Scholar]
- 29.Schambach A, Bohne J, Baum C, Hermann FG, Egerer L, von Laer D, et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 2006;13:641–645. doi: 10.1038/sj.gt.3302698. [DOI] [PubMed] [Google Scholar]
- 30.Schiedner G, Hertel S, Johnston M, Biermann V, Dries V, Kochanek S. Variables affecting in vivo performance of high-capacity adenovirus vectors. J Virol. 2002;76:1600–1609. doi: 10.1128/JVI.76.4.1600-1609.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8:2019–2029. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 32.Salem HK, Ranjzad P, Driessen A, Appleby CE, Heagerty AM, Kingston PA. Beta-adrenoceptor blockade markedly attenuates transgene expression from cytomegalovirus promoters within the cardiovascular system. Arterioscler Thromb Vasc Biol. 2006;26:2267–2274. doi: 10.1161/01.ATV.0000239445.67579.19. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 34.Tal R, Shaish A, Rofe K, Feige E, Varda-Bloom N, Afek A, et al. Endothelial-targeted gene transfer of hypoxia-inducible factor-1alpha augments ischemic neovascularization following systemic administration. Mol Ther. 2008;16:1927–1936. doi: 10.1038/mt.2008.191. [DOI] [PubMed] [Google Scholar]
- 35.Shaw LC, Pan H, Afzal A, Calzi SL, Spoerri PE, Sullivan SM, et al. Proliferating endothelial cell-specific expression of IGF-I receptor ribozyme inhibits retinal neovascularization. Gene Ther. 2006;13:752–760. doi: 10.1038/sj.gt.3302718. [DOI] [PubMed] [Google Scholar]
- 36.Miao CH, Ohashi K, Patijn GA, Meuse L, Ye X, Thompson AR, et al. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- 37.Xu ZL, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T, Hayakawa T. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene. 2001;272:149–156. doi: 10.1016/s0378-1119(01)00550-9. [DOI] [PubMed] [Google Scholar]
- 38.Dong G, Schulick AH, DeYoung MB, Dichek DA. Identification of a cis-acting sequence in the human plasminogen activator inhibitor type-1 gene that mediates transforming growth factor-b1 responsiveness in endothelium in vivo. J Biol Chem. 1996;271:29969–29977. doi: 10.1074/jbc.271.47.29969. [DOI] [PubMed] [Google Scholar]
- 39.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Parks RJ, Bramson JL, Wan Y, Addison CL, Graham FL. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J Virol. 1999;73:8027–8034. doi: 10.1128/jvi.73.10.8027-8034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houston P, White BP, Campbell CJ, Braddock M. Delivery and expression of fluid shear stress-inducible promoters to the vessel wall: applications for cardiovascular gene therapy. Hum Gene Ther. 1999;10:3031–3044. doi: 10.1089/10430349950016429. [DOI] [PubMed] [Google Scholar]
- 42.Perkins HD, van Leeuwen BH, Hardy CM, Kerr PJ. The complete cDNA sequences of IL-2, IL-4, IL-6 AND IL-10 from the European rabbit (Oryctolagus cuniculus) Cytokine. 2000;12:555–565. doi: 10.1006/cyto.1999.0658. [DOI] [PubMed] [Google Scholar]
- 43.Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingsman SM, Mitrophanous K, Olsen JC. Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) Gene Ther. 2005;12:3–4. doi: 10.1038/sj.gt.3302417. [DOI] [PubMed] [Google Scholar]
- 45.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.