Abstract
How the directionality of vesicle traffic is achieved remains an important unanswered question in cell biology. The Sec23p/Sec24p coat complex sorts the fusion machinery (SNAREs) into vesicles as they bud from the endoplasmic reticulum. Vesicle tethering to the Golgi begins when the tethering factor TRAPPI binds to Sec23p. Where the coat is released and how this event relates to membrane fusion is unknown. Here we use a yeast transport assay to demonstrate that an ER-derived vesicle retains its coat until it reaches the Golgi. A Golgi-associated kinase, Hrr25p (CK1δ ortholog), then phosphorylates the Sec23p/Sec24p complex. Coat phosphorylation and dephosphorylation are needed for vesicle fusion and budding, respectively. Additionally, we show that Sec23p interacts in a sequential manner with different binding partners, including TRAPPI and Hrr25p, to ensure the directionality of ER-Golgi traffic and prevent the back-fusion of a COPII vesicle with the ER. These events are conserved in mammalian cells.
Membrane fusion is mediated by a highly conserved family of membrane proteins called SNAREs. The pairing of a SNARE on the vesicle (v-SNARE) with its cognate SNARE on the target membrane (t-SNARE) is required for fusion1, however, the same v-SNARE (Sec22p) can act in both anterograde endoplasmic reticulum (ER) to Golgi and retrograde Golgi-ER traffic2. This observation implies that factors other than the SNAREs define the direction of membrane flow.
Motifs in the ER-Golgi SNAREs, needed for fusion, are masked by Sec24p (subunit of the COPII coat) as these fusogens are sorted into ER-derived vesicles3. The COPII coat is assembled on the ER when the activated form of the GTPase Sar1p (Sar1p-GTP) recruits the Sec23p/Sec24p complex by binding to Sec23p, the GTPase-activating protein (GAP) for Sar1p4. Polymerization of the coat requires the recruitment of the Sec13p/Sec31p complex (coat outer shell) by the Sec23p/Sec24p complex, which leads to the hydrolysis of GTP on Sar1p and vesicle fission4.
The initial interaction of a vesicle with its target membrane is mediated by a class of proteins called tethers that work in conjunction with GTPases of the Rab family5. The tethering factor TRAPPI is a multimeric guanine nucleotide exchange factor (GEF) that recruits and activates the Rab GTPase Ypt1p6. Previous findings showed that the interaction of TRAPPI with the coat adaptor protein Sec23p is required for vesicle tethering6. These studies, however, did not address if the coat is shed before or after vesicles bind to the Golgi. Here we show that a Golgi-localized kinase, Hrr25p, displaces purified TRAPPI that is pre-bound to Sec23p and phosphorylates the Sec23p/Sec24p complex. Our findings show that the COPII coat subunit Sec23p interacts in a hierarchical manner with Sar1p, TRAPPI and Hrr25p to ensure the directionality of anterograde membrane flow.
The Golgi inhibits TRAPPI vesicle binding
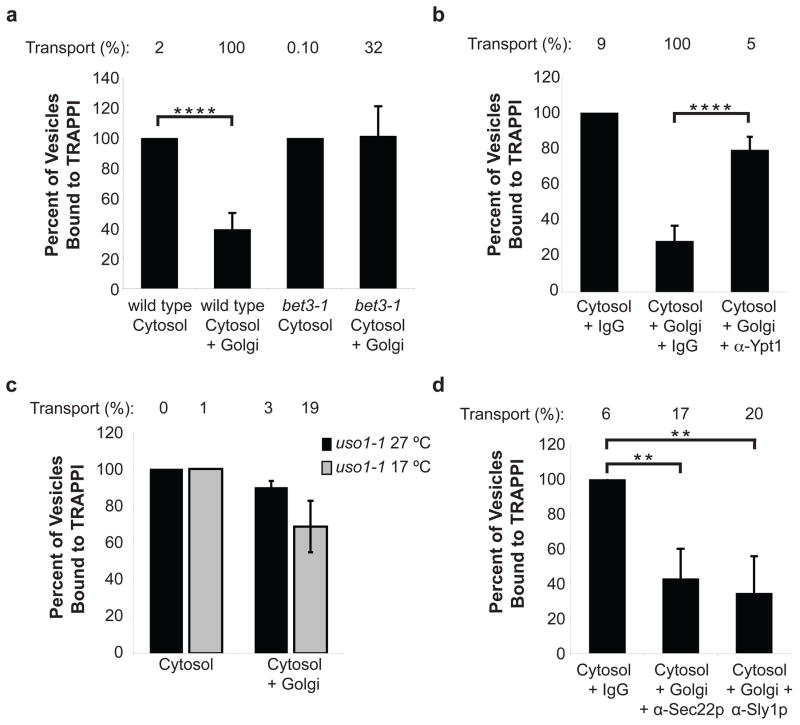
After COPII vesicles bud from the ER, Sar1p is released from vesicles when GTP is hydrolyzed, but the inner and outer shells of the coat are largely retained (Fig S1)7. To define the events that occur after TRAPPI binds to Sec23p, we immobilized TRAPPI on beads and asked when COPII vesicles lose their ability to bind. For these studies, the binding of pro-α-factor-containing vesicles formed with cytosol, was considered to be 100% (Fig. 1a, bar 1). We observed that vesicles formed in the presence of Golgi lost their ability to bind TRAPPI (Fig. 1a, bar 2). Since the binding of vesicles to TRAPPI is mediated by the COPII coat, this experiment suggests that COPII vesicles lose their ability to bind TRAPPI because the Golgi contains a factor that either releases or modifies the coat. To determine if vesicles must tether to the Golgi to lose their ability to bind to TRAPPI, we formed vesicles with bet3-1 mutant fractions. The bet3-1 mutant, which harbors a mutation in the Bet3p subunit of TRAPPI, is defective in vesicle tethering6. The defect in this mutant is partially complemented in vitro by the addition of purified recombinant TRAPPI (Fig. S2a). Vesicles formed from bet3-1 donor cells and cytosol, with or without Golgi, bound equally well to the TRAPPI-containing beads (Fig. 1a, last two bars). These findings suggest that the vesicles must tether to the Golgi to lose their ability to bind to TRAPPI.
Figure 1. COPII vesicles lose their ability to bind TRAPPI after Uso1p acts.
a, Vesicles formed in vitro with cytosol +/− Golgi were incubated with TRAPPI-containing beads and the precipitated radiolabeled cargo was counted. Cytosol and Golgi were isolated from either wild type or the bet3-1 mutant. Error bars represent S.D., N=6. In a–d, the percent transport observed for the total reaction is reported above the bar graphs. For b, and d, cytosol and Golgi fractions, derived from wild type, were formed with: anti-Ypt1p (12 μg), anti-Sec22p (12 μg), anti-Sly1p (20 μg) antibodies, or IgG (12 μg or 20 μg, respectively). Error bars represent S.D., N=4. c, Vesicles were formed with uso1-1 fractions at 27°C or 17°C and incubated with TRAPPI-containing beads. N=2, bars show the range. (**P<0.01, ****P<0.0001 Student’s t test).
COPII vesicle tethering requires TRAPPI, Ypt1p and Uso1p5, 6. To determine when COPII vesicles lose their ability to bind to TRAPPI, we blocked vesicle tethering and fusion at several different steps in vitro in the presence of Golgi membranes. The pro-α-factor-containing membranes, formed during these blocks, were then tested for their ability to bind to TRAPPI. The transport incompetent vesicles that formed, when Ypt1p function was blocked with anti-Ypt1p antibody, bound efficiently to TRAPPI (Fig. 1b). Disrupting Ypt1p function should also block the recruitment to vesicles of the long coiled-coil tether Uso1p (yeast ortholog of p115), a Ypt1p effector that links donor and acceptor membranes to each other in vitro5. When we formed vesicles with fractions from the uso1-1 mutant, transport incompetent vesicles retained their ability to bind TRAPPI at 27°C and 17°C (Fig. 1c).
We also blocked fusion in vitro with antibody directed against Sec22p (SNARE) or Sly1p, a Sec1-like protein that binds to SNAREs8. In vitro, neutralizing antibody to Sly1p blocks trans-SNARE complex formation8. Binding to TRAPPI decreased when vesicles were formed in the presence of anti-Sec22p or anti-Sly1p antibodies (Fig. 1d). These findings suggest that COPII vesicles lose their ability to bind TRAPPI after Uso1p functions, but before trans-SNARE complex formation. Uso1p does not appear to play a role in vesicle uncoating, as the membrane and soluble pools of Sec23p were unaltered in the uso1-1 mutant in vivo (Fig S3a) and Uso1p/p115 did not release Sec23 from membranes in vitro (see Figs. S3b, S3c). Together, these findings indicate that COPII vesicles retain their coat until they reach the Golgi.
Hrr25p phosphoregulates Sec23p/Sec24p
Despite the fact that only 36 +/− 0.65% of wild type vesicles uncoat in vitro in the presence of Golgi membranes (Fig. S3d), 61 +/−4% of the vesicles lose their ability to bind to TRAPPI (Fig. 1a). This observation suggests that the inability of COPII vesicles to bind TRAPPI is not just a consequence of vesicle uncoating. Because the COPII coat inner shell is known to be phosphorylated in mammalian cells9, we considered the possibility that phosphorylation of Sec23p may block the ability of COPII vesicles to bind TRAPPI.
To identify a kinase that could phosphorylate Sec23p, we searched the yeast database for an essential (Fig. S3e) Golgi localized kinase. Only one kinase, Hrr25p, was found to have an ortholog (CKIδ, human ortholog of casein kinase Iδ) that localizes at the Golgi and ER-Golgi interface in mammalian interphase cells10. Inhibiting CKIδ function was reported to block ER-Golgi traffic11, and a mutation that reduced the kinase activity of Hrr25p was shown to suppress the temperature-sensitive COPII vesicle budding defect in the sec12-4 mutant12. While these results implicated Hrr25p/CKIδ in ER-Golgi traffic, its role in membrane traffic is not well defined.
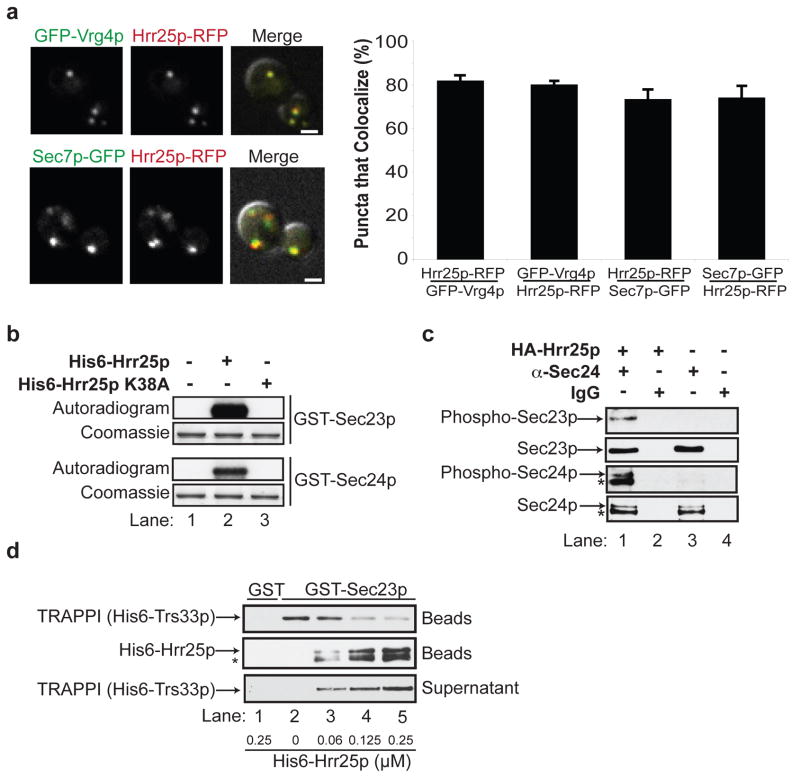
Hrr25p is known to reside in the nucleus in G1 arrested cells and, like CKIδ, it is found at the spindle pole body (SPB) in nocodazole treated (M-phase) cells10, 13. When we visualized Hrr25p-RFP (tagged genomic copy) in asynchronously grown cells, however, the majority (> 95%) was found on punctate structures that largely colocalize with early (Vrg4p) and late Golgi (Sec7p) markers (Figs. 2a). Occasionally, we observed Hrr25p-RFP in the nucleus, at puncta along the nuclear envelope (presumably the SPB), and at the bud neck and cortex, as previously reported13, 14. Consistent with its Golgi localization, all of the HA-Hrr25p co-fractionated with membranes in differential fractionation experiments (Fig. 5a, bottom).
Figure 2. Hrr25p resides on the Golgi and phosphorylates Sec23p/Sec24p.
a, Hrr25p-RFP colocalizes with GFP-Vrg4p (top) and Sec7p-GFP (bottom). The green and red channels are merged with the DIC image (right panel). The puncta that colocalize (S.D., N=3) are shown on the right. The scale bar is 2 microns. b, GST-Sec23p and GST-Sec24p were incubated without (lane 1), or with His6-Hrr25p (lane 2) or His6-Hrr25p-K38A (lane 3) and γP32-ATP. The autoradiogram and coomassie stained gel are shown. c, Lysates expressing (lanes 1 and 2) or not expressing HA-Hrr25p (lanes 3 and 4) were immunoprecipitated with anti-Sec24 antibody (lanes 1 and 3) or IgG (lanes 2 and 4) and immunoblotted with anti-phosphoSer/Thr, anti-Sec23p and anti-Sec24p antibodies. d, TRAPPI, pre-bound to GST-Sec23p-containing beads (top panel), was incubated with increasing concentrations of His6-Hrr25p. The beads were pelleted and the amount of TRAPPI in the supernatant (bottom panel) and pellet (top panel) was assessed. The Hrr25p that bound to the beads (middle panel) was also measured. TRAPPI, or Hrr25p, did not bind to GST (lane 1). The starred bands in c and d are degradation products of Sec24p and His6-Hrr25p.
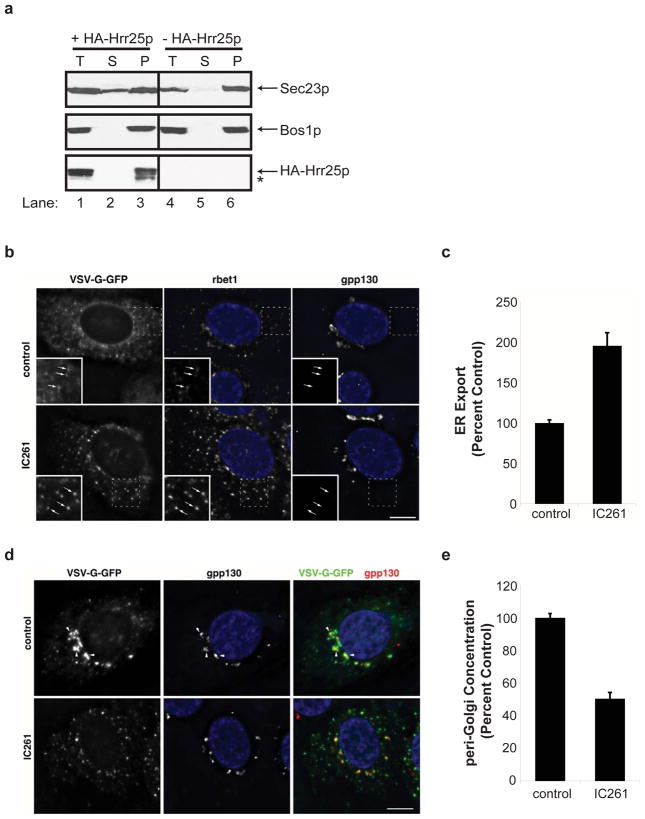
Figure 5. In the presence of IC261, cargo is exported more rapidly but remains at peripheral pre-Golgi sites.
a, The hrr25 mutant was grown in galactose (lanes 1–3), or glucose (lanes 4–6) supplemented media for 10 h. Total (T) lysates were centrifuged at 150,000 g and the supernatant (S) and pellet (P) fractions were analyzed. The starred band is a degradation product of HA-Hrr25p. b, NRK cells that accumulated VSV-G-GFP in the ER at 40°C were shifted to 15°C for 30 min in the presence of DMSO (top) or 100 μM IC261 (bottom) to allow cargo transit from the ER to the ER/Golgi interface. Left, VSV-G-GFP fluorescence; middle, rbet1; right, gpp130. Insert in left corner is an expansion of the dotted box. Arrows, peripheral cargo-containing punctate structures that colocalize with rbet1 but not gpp130. c, Quantitation, from Fig. 5b, of VSV-G-GFP fluorescence in pre-Golgi structures divided by VSV-G-GFP remaining in the ER (see METHODS for calculation). S.E.M., N=20 cells/bar, P<0.0001 Student’s t test. d, Same as b only cells were incubated at 15°C for 60 min. Left, VSV-G-GFP fluorescence; middle, gpp130; right, merge of VSV-G-GFP (green) and gpp130 (red). Arrowheads, cargo that concentrated in the Golgi area. e, Quantitation, from Fig. 5d, of VSV-G-GFP fluorescence in punctate structures overlapping the Golgi divided by total fluorescence in punctate structures (see METHODS for calculation). S.E.M., N=20 cells/bar, P<0.0001 Student’s t test. The nuclei are stained with DAPI and the scale bars are 10 microns.
Both GST-Sec23p and GST-Sec24p were phosphorylated by His6-Hrr25p (Figs. 2b, S3f, compare lanes 1 and 2), but not catalytically inactive His6-Hrr25p-K38A in vitro (Figs. 2b, S3f, lane 3). Phosphorylation in vivo was examined with a conditional allele of hrr2514. In this mutant, HA-tagged HRR25degron was placed behind the inducible GAL promoter as the sole copy of the gene. When this strain is grown in galactose, HA-Hrr25pdegron is expressed. In the presence of glucose, however, the expression of HA-Hrr25pdegron ceases and the protein is rapidly degraded. After 10 hr in glucose, when a delay in trafficking of carboxypeptidase Y between the ER-Golgi was observed, most of the Hrr25pdegron was degraded (not shown). Lysates prepared from the hrr25 mutant grown with galactose (+Hrr25p) or glucose (−Hrr25p) (Fig. S3g) were precipitated with anti-Sec24p (Fig. 2c, lanes 1 and 3), or IgG (lanes 2 and 4). Western blot analysis of the immunoprecipitates with anti-phospho Ser/Thr antibody revealed that phospho-Sec23p and phospho-Sec24p could only be detected in vivo when Hrr25p was expressed (Fig 2c, lane 1).
Since Sec23p binds to both TRAPPI and Hrr25p, we determined if TRAPPI and Hrr25p compete for binding to Sec23p. To do this, the 6 subunits of the TRAPPI complex were co-expressed in bacteria and purified from bacterial lysates as described before6. When similar amounts of purified His6-Hrr25p and TRAPPI were incubated together, Hrr25p effectively competed with TRAPPI for binding to purified GST-Sec23p (Fig. S4a, compare lanes 3–5 to GST controls in lanes 1 and 2). Binding was not dependent on kinase activity, as His6-Hrr25p-K38A bound as efficiently as His6-Hrr25p (not shown). Since His6-Hrr25p binds with higher affinity to GST-Sec23p (Kd= 0.043 +/−0.009 μM, Fig. S4b) than TRAPPI (Kd=0.63 +/− 0.15 μM, Fig. S4c), we determined if it displaces TRAPPI that is pre-bound to GST-Sec23p. Increasing amounts of His6-Hrr25p were mixed with GST-Sec23p beads pre-incubated with saturating amounts of TRAPPI (Fig. 2d). When the concentration of Hrr25p was increased (lanes 2–5), TRAPPI was released from the beads (top panel, lanes 2–5) into the supernatant (bottom panel, lanes 2–5) as His6-Hrr25p bound to GST-Sec23p (middle panel). These findings show that Hrr25p and TRAPPI compete for binding to Sec23p, and suggest that Hrr25p could displace TRAPPI from Sec23p when COPII vesicles tether to the Golgi. Consistent with the possibility that phosphorylation of the coat blocks the binding of TRAPPI to Sec23p, we observed a decrease in the binding of TRAPPI to GST-Sec23p that contained phosphomimetic mutations at two phospho sites (see below and Fig. S5b).
Conservation of phosphosites in Sec23p
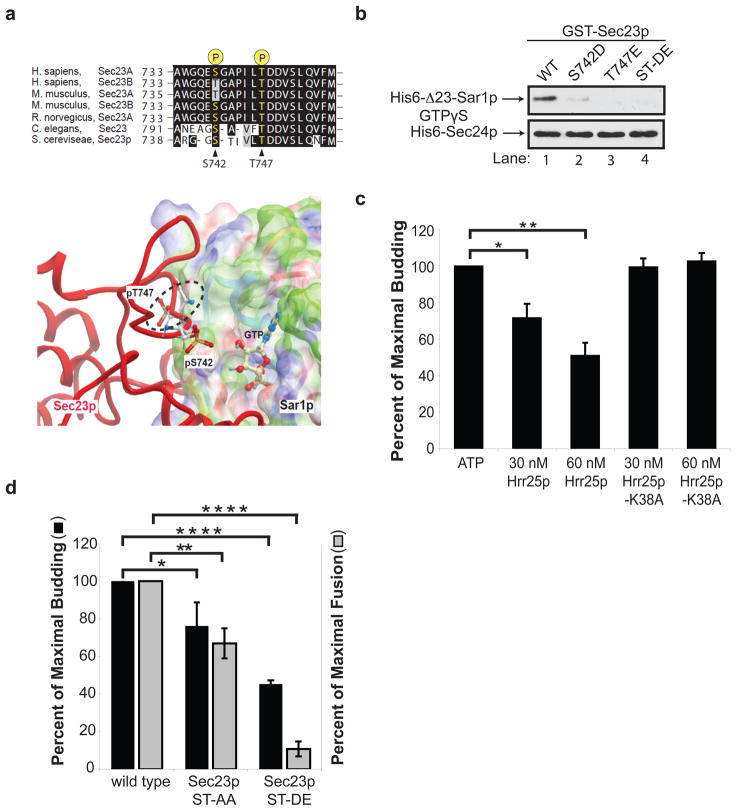
Because Hrr25p phosphorylates Sec23p more efficiently than Sec24p, we focused on Sec23p for subsequent studies. Three Hrr25p phosphorylation sites were identified in Sec23p by mass spectrometry: T555, S742 and T747. Two of these phospho residues, S742 and T747, are conserved from yeast to man and were analyzed further (Fig. 3a, top). The T747 residue is a known Sar1p contact site, while S742 is within a disordered loop in the established structure of Sec23p complexed with Δ23-Sar1p-GTP (PDB: 2QTV)15. Initially, we used computational modeling to predict the consequences of phosphorylation at these sites. We added phosphates on S742 and T747 of Sec23p using Molsoft-ICM software and modified their orientations by several rounds of Monte Carlo optimization. Phosphorylation at T747 presented significant steric clashes with the surface of Sar1p in all possible orientations and was deemed incompatible with Sec23p binding to Sar1p-GTP (Fig. 3a bottom, see dotted ellipsoid). Phosphorylated S742 is also located at the Sec23p-Sar1p interface, but its location within a flexible loop limited our ability to predict consequences (Fig 3a, bottom)15. We found that His6-Δ23-Sar1p-GTPγS bound preferentially to GST-Sec23p (Fig. S5a), and failed to bind GST-Sec23p harboring the phosphomimetic S742D, T747E or S742D/T747E (ST-DE) mutations (Fig. 3b top, lanes 1–4). Binding of His6-Sec24p to GST-Sec23p was unaffected by the phosphomimetic mutations (Fig. 3b, bottom).
Figure 3. Phosphorylation of S742 and T747 blocks ER-Golgi traffic in vitro.
a, Top, The sequence flanking S742 and T747 in Sec23p was aligned with Sec23 orthologues. Bottom, Phosphorylated S742 and T747 in Sec23p (red) are located at its interface with Sar1p. The electrostatic potential of the Sec23p-binding surface of Sar1p is colored according to solvation properties of the residues (white, hydrophobic; green, polar; blue, basic; red, acidic). The dotted ellipsoid marks the steric clash between Sec23p and Sar1p-GTP. b, Top, wild type (WT) and mutant GST-Sec23p fusion proteins were incubated with 10 nM of His6-Δ23-Sar1p-GTPγS or His6-Sec24p. A truncated form of Sar1p was used for these studies because the full length protein aggregates15. c, Increasing amounts of purified His6-Hrr25p or His6-Hrr25p-K38A were added in vitro at the beginning of a complete transport reaction and vesicle budding was measured. Error bars represent S.D., N=4. d, Fractions prepared from the indicated strains were assayed for vesicle budding and fusion as before6. Error bars represent S.E.M., N=4 (*P<0.05, **P<0.01,, ****P<0.0001 Student’s t test).
If Hrr25p phosphorylates Sec23p at Sar1p contact sites, the addition of His6-Hrr25p to the transport assay should disrupt the binding of Sec23p to Sar1p-GTP and inhibit vesicle budding in vitro. When His6-Hrr25p was added to the assay at the beginning of the reaction, budding was inhibited as the concentration of His6-Hrr25p was increased (Fig. 3c). Inhibition was dependent on kinase activity, as no effect was seen with catalytically inactive His6-Hrr25p-K38A (Fig 3c). Consistent with this observation, a yeast strain harboring the S742D/T747E mutations disrupted vesicle budding and fusion in vitro (Fig. 3d) and displayed a severe growth defect at 33°C (Fig. S5c). The defect in fusion may be the consequence of blocking the cycling of Sec23p on and off membranes (see Fig. 5a).
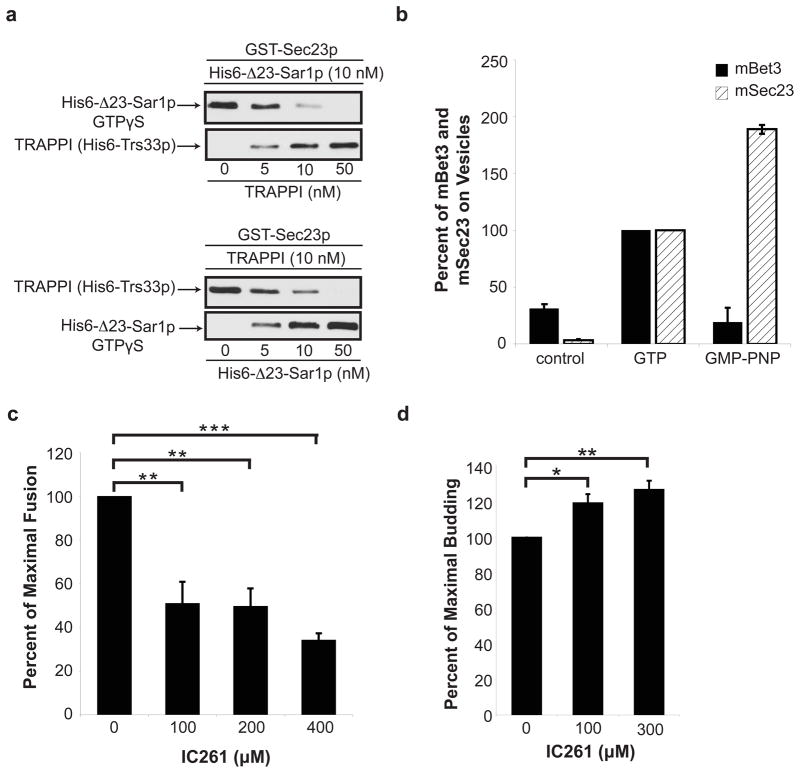
Since Hrr25p phosporylates Sec23p at Sar1p contact sites, and Hrr25p competes with TRAPPI for binding to Sec23p, we wanted to address if TRAPPI also competes with His6-Δ23-Sar1p-GTPγS for binding to Sec23p. When we incubated a constant amount of His6-Δ23-Sar1p-GTPγS with increasing amounts of TRAPPI, TRAPPI effectively competed with Δ23-Sar1p-GTPγS for binding to Sec23p (Fig. 4a, top). Similar results were obtained when increasing amounts of His6-Δ23-Sar1p-GTPγS were incubated with a constant amount of TRAPPI (Fig. 4a, bottom). These results imply that TRAPPI and Sar1p-GTP bind to the same or overlapping site(s) on Sec23p. Consistent with this hypothesis, we found a decrease in the binding of TRAPPI to GST-Sec23p harboring the S742D/T747E mutations (Fig. S5b). Together, these findings imply that TRAPPI binds to Sec23p after Sar1p-GTP is released from membranes. This event appears to be conserved in higher eukaryotes (Fig. 4b) since we could only detect Bet3 on immuno-isolated tagged mammalian COPII vesicles (VSV-G-myc) formed in vitro with GTP (−Sar1), but not GMP-PNP (+Sar1) or control vesicles that lacked VSV-G-myc.
Figure 4. TRAPPI and Sar1p-GTP cannot bind to Sec23p simultaneously.
a, GST-Sec23p beads were incubated with 10 nM of His6-Δ23Sar1p-GTPγS and increasing concentrations of TRAPPI (top), or 10 nM TRAPPI and increasing concentrations of His6-Δ23Sar1p-GTPγS (bottom). b, mBet3 is on COPII vesicles formed with GTP (−Sar1), but not GMP-PNP (+Sar1). Vesicles formed in vitro with GTP or GMP-PNP were immunoisolated with an antibody against the cargo VSV-G-myc and blotted for mBet3 and mSec23. The data was normalized to cargo yield (see METHODS). N=2, bars show the range. c, In vitro transport was performed with increasing concentrations of IC261 and fusion was measured. Error bars represent S.D., N=4. d, The in vitro transport assay was performed as in Fig. 4c with IC261 and budding was measured. Error bars represent S.D., N=3 (*P<0.05, **P<0.01,***P<0.001 Student’s t test)
Loss of Hrr25p activity inhibits fusion
To address the role of Hrr25p phosphorylation in ER-Golgi traffic in vitro, we used the ATP competitive inhibitor IC261, which selectively inhibits the highly conserved kinase domain of CK1δ11, 16. As seen in Fig. 4c, increasing concentrations of IC261 inhibited fusion. To address if IC261 inhibits membrane fusion or vesicle tethering, we formed vesicles in the presence of Golgi with or without inhibitor, and then fractionated the reaction product on a sucrose velocity gradient that separates free vesicles (Fig. S2b, top) from vesicles that are bound to the Golgi (Fig. S2b, bottom). Subsequently, each fraction was treated with ConA Sepharose to precipitate radiolabeled pro-α-factor. Most of the vesicles bound to the Golgi in the presence of IC261, indicating that the inhibitor largely blocks fusion and not tethering (Fig. S2b bottom, compare dotted and solid lines).
Interestingly, when the transport assay was performed with concentrations of IC261 that inhibited fusion, a stimulation in vesicle budding was observed (Fig. 4d). This finding suggests that dephosphorylation is needed for budding and is consistent with an earlier report showing that phosphorylated mammalian Sec24 cannot form a pre-budding complex9. A prediction of this result is that loss of kinase activity should stimulate cargo release in vivo (see below) and could explain why a kinase loss of function mutation was identified as a suppressor of the sec12-4 mutant12. We were unable to address the role of CK1δ in vitro because the mammalian COPII vesicle tethering assay did not work robustly at the lower ATP concentrations needed to test the inhibitor.
To address if phosphorylation and dephosphorylation alter the distribution of Sec23p on membranes in vivo, a differential fractionation experiment was performed with the conditional hrr25 mutant after growth in galactose or glucose containing medium. The SNARE Bos1p served as a membrane marker for these studies. This analysis revealed the presence of a soluble pool of Sec23p when HA-Hrr25p was expressed (Fig 5a, lanes 1–3). In the absence of HA-Hrr25p, however, all of the Sec23p was membrane-bound (Fig. 5a, lanes 4–6). Although Hrr25p activity appears to play a role in releasing Sec23p from membranes in vivo, we found it was not sufficient to uncoat the vesicles in the absence of Golgi membranes in vitro (data not shown).
In mammalian cells, COPII vesicles fuse to each other or with COPI (Golgi) vesiclesto form a pre-Golgi compartment that matures into a Golgi17. To address the role of CKIδ in vivo, we accumulated ts O45 VSV-G-GFP in the ER of NRK cells at 40°C and then shifted the cells in the presence and absence of IC261 to 15°C, a temperature that selectively slows traffic at the pre-Golgi compartment step18. In the presence of inhibitor, the pre-Golgi (marked by rbet1) but not early Golgi (marked by gpp130)was dramatically dispersed (Fig. 5b). Consistent with the observation that inhibiting CK1δ function stimulates COPII vesicle budding and blocks fusion, VSV-G-GFP was more rapidly depleted from the ER and concentrated at peripheral sites of the pre-Golgi compartment (Figs. 5b, 5c), the site where COPII vesicles fuse. The VSV-G-GFP remained at the peripheral sites in the IC261-treated cells and failed to concentrate in the peri-Golgi region (Figs. 5d, 5e). Together, these findings imply that the events we describe here are conserved in higher cells.
Discussion
The CKI family of kinases represents a unique group of highly conserved serine/threonine kinases that regulate a variety of cellular processes, including membrane traffic11,12. Here we report that Sec23p, a component of the inner shell of the COPII coat, sequentially interacts with three different binding partners, Sar1p, TRAPPI and Hrr25p, to control the direction of ER-Golgi traffic. These interactions define three different stages in vesicle traffic: budding, tethering and a pre-fusion step.
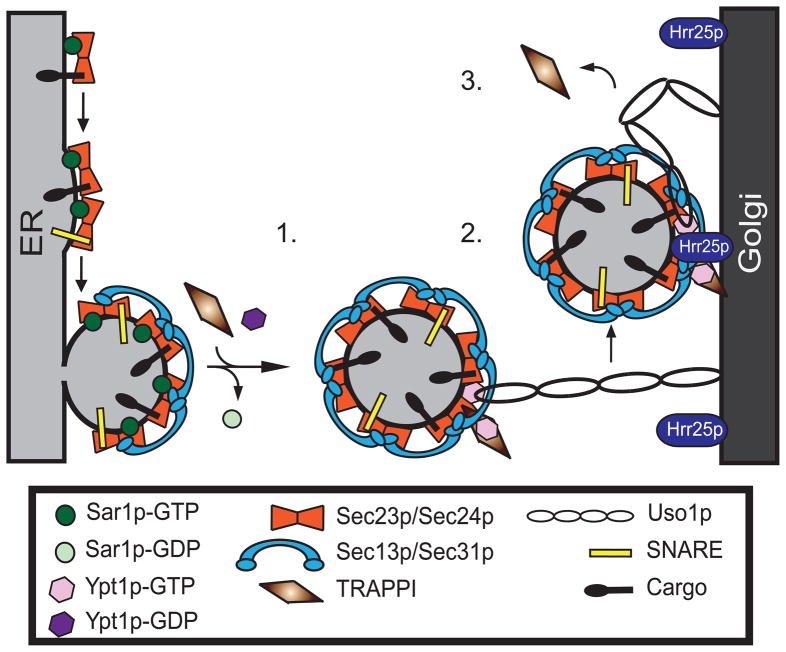
Since Sar1p is required for fission19,20, our findings imply that TRAPPI can only bind to Sec23p after vesicle fission and the release of Sar1p from membranes (Fig. 6, 1.). This ensures that COPII vesicle tethering is only initiated after a vesicle buds from the ER. Subsequently, TRAPPI activates Ypt1p on the vesicle (Fig. 6, 2.). Genetic studies and a kinetic analysis of GEF activity have revealed that TRAPPI is more than a GEF21. GEFs typically release Rabs soon after they activate them. TRAPPI, however, forms a relatively stable ternary complex (TRAPPI-Ypt1p-nucleotide) with the Rab21, implying that it is also a Ypt1p effector. In parallel, the pool of Ypt1p-GTP that is released from TRAPPI can then recruit the long coiled-coil tether Uso1p (Fig. 6, 2.). When Uso1p bends, the vesicle comes into proximity with the Golgi, triggering the release of TRAPPI from the vesicle (Fig 6, 3.). Hrr25p, which concentrates on the Golgi in yeast, could facilitate this release. Phosphorylation of the Sec23p/Sec24p complex by Hrr25p may be required, but is not sufficient for COPII vesicle uncoating. Another kinase could also be involved in this event as Sec31p, a known phosphoprotein22, appears to be phosphorylated by a different kinase12 and Hrr25p cannot uncoat COPII vesicles in vitro.
Figure 6. Sec23p ensures the direction of ER-Golgi traffic.
1. TRAPPI binds to Sec23p after GTP is hydrolyzed on Sar1p. 2. Ypt1p is activated by TRAPPI and Uso1p is recruited to the vesicle. Subsequently, Uso1p binds to the Golgi. 3. When Uso1p bends, it brings the vesicle to the Golgi. TRAPPI is then released from the vesicle and Hrr25p phosphorylates the Sec23p/Sec24p complex.
Fusogenic SNARE motifs that bind to the coat must be unmasked before trans-SNARE complex formation can proceed at the target membrane3. Phosphorylation of Sec24p by Hrr25p/CKIδ could play a role in disengaging the SNAREs from the coat at the Golgi. As the COPII coat only acts in anterograde traffic4, the compartmentalization of a kinase that regulates membrane fusion at the Golgi, ensures an ER-Golgi v-SNARE will only pair with its cognate t-SNARE. The directionality imposed by this cycle also prevents the back-fusion of a COPII vesicle with the ER. The findings we report here describe a new role for Hrr25p/CKIδ that may extend to other CKI family members and coats.
METHODS SUMMARY
Yeast COPII vesicles were formed in vitro with donor cells, cytosol, +/− Golgi for 90 min at 20°C or 27°C or 120 min at 17°C as described before6. The vesicle binding assays were performed as described before42. Additional information is provided in the METHODS
Protein and antibody purifications were performed as before6. Mass spec analysis, in vitro bindings assays, kinase and immunoprecipitation assays, microscopy, the construction of phosphomimetic mutations and all studies with mammalian cells are described in the METHODS.
Supplementary Material
Acknowledgments
We thank Randy Schekman, Martha Cyert, Liz Miller, Ben Glick, Martin Lowe and Emi Mizuno-Yamasaki for strains, constructs, purified protein and antibody; Karin Reinisch for stimulating discussions; Shuliang Chen, Huaqing Cai and Mikel Garcia-Marcos for advice; Wenyun Zhou and Andrea Lougheed for technical assistance. This work was supported by the Howard Hughes Medical Institute. Salary support for S. F-N., D.B. and S.M. was provided by the Howard Hughes Medical Institute, P.G. was funded by the Burroughs Wellcome Fund and M.G. by the SRP Super Fund Research Program. Work at the University of Montana was supported by National Institutes of Health grant GM-059378 (to J.C.H.) and the National Institutes of Health COBRE Center grant RR-015583.
Footnotes
Author Contributions C.L., D.B., S.M., D.N. and J.H. performed experiments and analyzed data. M.G. did the mass analysis and P.G. did the computational modeling and analyzed data. S.F.N. directed the project, analyzed data and wrote the paper. C.L., D.B., J.H. and P.G. co-wrote the paper.
References
- 1.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee MCS, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 5.Whyte JRC, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 7.Barlowe C, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan JJ, Barlowe C. Cysteine-disulfide cross-linking to monitor SNARE complex assembly during endoplasmic reticulum-Golgi transport. J Biol Chem. 2006;281:2281–2288. doi: 10.1074/jbc.M511695200. [DOI] [PubMed] [Google Scholar]
- 9.Dudognon P, Maeder-Garavaglia C, Carpentier J, Paccaud J. Regulation of a COPII component by cytosolic O-glycosylation during mitosis. FEBS Lett. 2004;561:44–50. doi: 10.1016/S0014-5793(04)00109-7. [DOI] [PubMed] [Google Scholar]
- 10.Milne DM, Looby P, Meek DW. Catalytic activity of protein kinase CK1 delta (casein kinase 1delta) is essential for its normal subcellular localization. Exp Cell Res. 2001;263:43–54. doi: 10.1006/excr.2000.5100. [DOI] [PubMed] [Google Scholar]
- 11.Yu S, Roth MG. Casein kinase I regulates membrane binding by ARF GAP1. Mol Biol Cell. 2002;13:2559–2570. doi: 10.1091/mbc.E02-04-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami A, Kimura K, Nakano A. The Inactive Form of a Yeast Casein Kinase I Suppresses the Secretory Defect of the sec12 Mutant. J Biol Chem. 1999;274:3804–3810. doi: 10.1074/jbc.274.6.3804. [DOI] [PubMed] [Google Scholar]
- 13.Lusk CP, et al. Nup53p is a target of two mitotic kinases, Cdk1p and Hrr25p. Traffic. 2007;8:647–660. doi: 10.1111/j.1600-0854.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 14.Kafadar KA, Zhu H, Snyder M, Cyert MS. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes &Development. 2003;17:2698–2708. doi: 10.1101/gad.1140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 16.Mashhoon N, et al. Crystal Structure of a Conformation-selective Casein Kinase-1 Inhibitor. J Biol Chem. 2000;275:20052–20060. doi: 10.1074/jbc.M001713200. [DOI] [PubMed] [Google Scholar]
- 17.Xu D, Hay JC. Reconstitution of COPII vesicle fusion to generate a pre-Golgi intermediate compartment. J Cell Biol. 2004;167:997–1003. doi: 10.1083/jcb.200408135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweizer A, et al. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- 19.Bielli A, et al. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MCS, et al. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Chin HF, et al. Kinetic analysis of the guanine nucleotide exchange activity of TRAPP, a multimeric Ypt1p exchange factor. J Mol Biol. 2009;389:275–288. doi: 10.1016/j.jmb.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salama NR, Chuang JS, Schekman RW. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.