Abstract
Grb2 (growth-factor receptor-bound protein-2) is a signaling adaptor that interacts with numerous receptors and intracellular signaling molecules. However, its role in B-cell development and function remains unknown. Here we show that ablation of Grb2 in B cells results in enhanced B-cell receptor signaling; however, mutant B cells do not form germinal centers in the spleen after antigen stimulation. Furthermore, mutant mice exhibit defects in splenic architecture resembling that observed in B-cell–specific lymphotoxin-β–deficient mice, including disruption of marginal zone and follicular dendritic cell networks. We find that grb2−/− B cells are defective in lymphotoxin-β expression. Although lymphotoxin can be up-regulated by chemokine CXCL13 and CD40 ligand stimulation in wild-type B cells, elevation of lymphotoxin expression in grb2−/− B cells is only induced by anti-CD40 but not by CXCL13. Our results thus define Grb2 as a nonredundant regulator that controls lymphoid follicle organization and germinal center reaction. Loss of Grb2 has no effect on B-cell chemotaxis to CXCL13, indicating that Grb2 executes this function by connecting the CXCR5 signaling pathway to lymphotoxin expression but not to chemotaxis.
Keywords: B-cell signaling and activation, follicular dendritic cell development, germinal center development, regulation of lymphotoxin expression, chemokine signaling
Germinal center (GC) reaction is critical for effective humoral immune responses and for the generation of memory B lymphocytes. This process requires close interactions between antigen-specific B and T cells, and follicular dendritic cells (FDCs) (1–3). Multiple B-cell intrinsic signals are involved in orchestrating this process. The signal that initiates GC formation is delivered through the B-cell receptor (BCR), with a group of signaling proteins modulating the strength of BCR signaling and immunological synapse formation (4–6). These proteins include Lyn, SHP1, and DOCK8, as well as the coreceptor CD19, which collectively drive B-cell activation, proliferation, and differentiation to either memory B cells or antibody-producing plasma cells in GCs (7–9). In addition to BCR signaling, signals derived from the surrounding niche induce activated B cells to undertake the GC differentiation pathway and support them to complete the GC maturation program. B-cell–expressing molecules that are involved in this process include ICOSL, CD40, PD-1 ligands, and cytokine receptors (10–13). However, how these signaling pathways converge in B cells to elicit an optimal GC response is not known.
Only a small fraction of antigen-activated B cells will differentiate into GC cells. It is not clear what selects a B-cell to commit to the GC fate, although it is suggested that B cells compete for antigens, cognate T-cell help, and for other growth and survival signals from the specialized follicular microenvironment to establish GCs. Thus, the precise localization of B cells during immune responses is decisive for GC maturation. Chemokine receptors CXCR5 and CXCR4, lymphotoxin (LT), and TNF play a crucial role in organizing B-cell follicles and GCs (14). Of a particular interest, it has been shown that activation of CXCR5 induces naive B cells to produce LT that is required for establishing the FDC network and GC formation (15). Currently, how the CXCR5 signal is connected to LT expression still remains elusive.
The mammalian growth-factor receptor-bound protein-2 (Grb2) is a simple adaptor that consists of one central SH2 domain flanked by two SH3 domains (16). It is broadly expressed in many tissues and is essential for embryo development and multiple cellular functions (17–19). Grb2 has been identified as a major mediator in Ras-MAPK activation induced by numerous receptors because of its association with son of sevenless, a GDP-GTP exchange factor for Ras (18–20). Studies using T-cell–specific Grb2-deficient mice show that Grb2 plays a broad role in early T-cell development as both thymic-positive and -negative selection are impaired in the absence of Grb2. Grb2 is a positive regulator for TCR signaling, and it does not directly affect the activities of Ras and Erk1/2, but rather amplifies the activity of Lck, a member of the Src family of tyrosine kinases that functions at the top of the TCR signaling cascade (21). In chicken B cells, it has been reported that Grb2 regulates Ca2+ influx upon BCR activation (22). Mice deficient in the hematopoietic adaptor protein downstream of kinase-3 (Dok-3), an adaptor that recruits Grb2 to the membrane, exhibit enhanced BCR-induced Ca2+ mobilization (23, 24), suggesting that Grb2 may participate in BCR signaling also in mammalian B cells (for review, see refs. 18 and 19). However, the physiological role of Grb2 in B-cell development and function remains unclear. Here we show that Grb2 negatively regulates BCR signaling. grb2−/− B cells are hyperreactive; however, mutant splenic B cells did not form GCs. We find that Grb2 is necessary for CXCL13-induced LT expression in B cells. Our findings establish the CXCL13/CXCR5-Grb2-LT signaling axis in B cells as a nonredundant pathway that controls lymphoid follicle organization and GC reaction.
Results
Grb2BKO Mutation Impairs B-Cell Maturation and Enhances B-Cell Responses to Activating Stimuli.
To determine the function of Grb2 in B cells, we generated B-cell–specific grb2 knockout (Grb2BKO) mice by crossing grb2 floxed mice to CD19-Cre transgenic mice (Fig. S1) (21). Grb2 deficiency did not perturb B-cell development at an early stage (Table S1 and Fig. S2), but impaired further maturation of B cells after immature B cells egressed from the bone marrow (BM) into the spleen. The peripheral transitional T1 and T2 B cells in mutant mice were significantly reduced compared with that of WT mice (Fig. 1A and Table S1). As a result, follicular B cells declined to ∼70% of the number in WT mice (Fig. 1A and Table 1), and the subset of mature recirculating B cells (B220hiIgM+/−) in the mutant BM was only 40% of the WT counterpart (Table S1 and Fig. S2). We noted that the populations of marginal-zone B cells and B1 B cells were only mildly altered in the spleen of mutant mice. These results indicate that Grb2 exerts a differential regulatory role in B-cell lineage development, with follicular B cells being most profoundly affected by the Grb2BKO mutation.
Fig. 1.
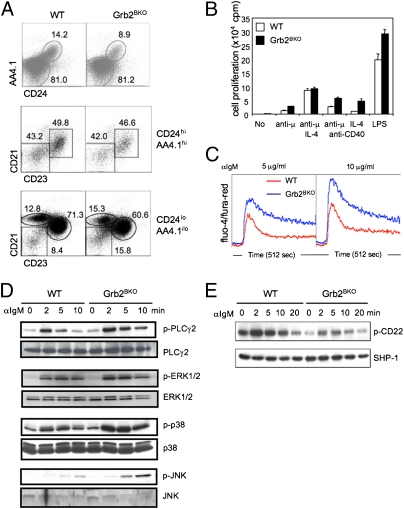
Altered development and antigen-receptor signaling of grb2−/− B cells. (A) Dot plots show B220+-gated splenic cells of WT and Grb2BKO mice. (Top) Mature (AA4.1loCD24lo) and immature (AA4.1hiCD24hi) B cells. (Middle) transitional T1 (CD21hiCD23hi) and T2 (CD21loCD23lo) B cells within the AA4.1hiCD24hi-gated immature B-cell population. (Bottom) marginal-zone B (CD21hiCD23lo), follicular B (CD21hiCD23hi), and B1 B (CD21loCD23lo) cells within the AA4.1loCD24lo-gated mature B cells. Percentages of each subset are indicated in the plots. (B) Purified B cells (B220+AA4.1loCD24loCD21hi CD23hi) were stimulated with anti-IgM F(ab)2, anti-CD40, IL-4, and LPS either alone or in combination as indicated. Cell proliferation was measured by 3H-thymidine incorporation; error bars with SD (n = 3). (C) Intracellular Ca2+ in B cells was measured by fluorescence intensity of Fluo-4 vs. Fura-red (n = 8). (D and E) Purified B cells from WT or Grb2BKO mice were stimulated with anti-IgM for various periods. Tyrosine phosphorylation of PLCγ2 and CD22 was determined by immunoprecipitation followed by Western blotting against phosphotyrosine (4G10). Active forms of Erk1/2, JNK, and p38 were directly determined using specific antibodies against individual phosphorylated kinases. The results represent more than three independent experiments.
To evaluate the role of Grb2 in B-cell activation, we compared the proliferative responses of grb2−/− and WT B cells to various activating stimuli. We found that mature B cells exhibited enhanced proliferation in response to anti-IgM or anti-IgM plus anti-CD40 (twofold), or IL-4 plus anti-CD40 (threefold) (Fig. 1B). When B cells were stimulated with LPS, proliferation responses of both mutant and WT B cells were drastically increased, and even then, mutant B cells showed stronger proliferation (Fig. 1B). These same stimuli also induced increased proliferation in grb2−/− immature B cells, even though responses of both WT and mutant immature B cells to these stimuli were overall much lower than that of mature B cells (Fig. S3).
Next, we sought to determine which BCR signaling event was interfered with by Grb2 deficiency. BCR stimulation activates the tyrosine kinase Lyn, which then phosphorylates immunoreceptor tyrosine-based activation motifs (ITAM) in Igα and Igβ. The phosphorylated ITAMs recruit and activate Syk kinase, leading to subsequent tyrosine phosphorylation of signaling effector molecules, including BLNK/p65 and PLCγ2 (25). We first examined whether Grb2 deficiency affected proximal BCR signaling. We found that while the kinetics and the level of tyrosine phosphorylation of total proteins in grb2−/− and WT B cells were comparable, the active form of Lyn phosphorylation in mutant B cells was slightly reduced than that of WT B cells after anti-IgM stimulation (Fig. S4). When distal signaling transduction molecules were examined, we found that phosphorylation of PLCγ2, activation of MAP kinases Erk1/2, p38, and JNK, and mobilization of Ca2+ were all significantly elevated in grb2−/− B cells (Fig. 1 C and D). These data indicate that Grb2 is a negative regulator and regulates BCR signaling at the top of the BCR signaling cascade.
How does Grb2 negatively regulate BCR signaling? It has been previously shown that Grb2 is associated with CD22, a membrane receptor that negatively regulates BCR signaling (26). Upon BCR stimulation, CD22 becomes tyrosine phosphorylated at its cytoplasmic tail by Lyn, which then recruits tyrosine phosphatase SHP-1 to the membrane to suppress BCR downstream signaling molecules, including BLNK and Igα/Igβ (27). Therefore, we examined this negative regulatory circuit of BCR signaling. We found that tyrosine phosphorylation of CD22 in the mutant B cells was reduced compared with that occurring in WT cells (Fig. 1E). These results thus indicate that Grb2 is involved in the regulation of CD22 tyrosine phosphorylation, and suggest that Grb2 may control BCR signaling by integrating the CD22-SHP-1 negative-feedback loop in B cells.
Grb2BKO Mice Have Disrupted Lymphoid Follicles and Cannot Form GCs in the Spleen.
To directly assess the impact of Grb2 deficiency on T-cell–dependent (TD) antibody responses, we immunized mice with a TD antigen NP-KLH (4-hydroxy-3-nitrophenylacetyl–keyhole limpet hemocyanin). At day 10 after immunization, WT B cells had undergone extensive clonal expansion and some of the antigen-activated B cells differentiated into GC cells (28). Grb2BKO mice generated an equivalent number of BrdU+ B cells in the spleen during 4-h BrdU pulse, even though they have ∼40% fewer mature B cells compared with controls. Thus, proliferating B cells were proportionally increased ∼twofold in Grb2BKO mice (Fig. 2A). Although mutant B cells responded strongly to antigen stimulation, these cells did not give rise to GC cells (Fig. 2B). We further analyzed the antigen-specific B-cell compartment for the generation of IgG1lowCD38low GC and the IgG1highCD38+ memory B cells (29, 30) and found that both subsets were reduced in the spleen of the mutant mice (Fig. 2C). As a consequence of the enhanced antigen-activated B-cell proliferation but an impaired GC reaction, Grb2BKO mice generated more NP-specific IgM-producing and fewer IgG-producing plasma cells in the spleen (Fig. 2D).
Fig. 2.
Impairment of GC reaction in Grb2BKO spleen. Data were obtained from WT and Grb2BKO mice (8- to 10-wk-old) at day 10 after NP-KLH immunization. (A) (Left) Contour plots show BrdU+IgD+ and BrdU+IgD− populations of the B220+-gated cells. (Right) Frequencies and absolute numbers of BrdU+ B cells in WT (white bars) and Grb2BKO (gray bars) mice are shown as mean with SD (n = 7). (B) Plots are B220+-gated splenocytes. GC B cells are shown as either CD95+PNA+ population in contour plots or CD95+GL7+ cells in dot plots. (C) Splenocytes were stained with lineage markers (CD4, CD8, Gr1, F4/80), anti-B220, NP, and anti-CD38. (Upper) B220 vs. NP staining of gated-lineage marker negative (Lin−) splenocytes. The percentages of Lin− NP-binding B220+ cells are indicated. The frequencies of IgG1+CD38+ memory (Mem) and IgG1+CD38lo GC subsets within the antigen specific (B220+NP+) B-cell compartment are shown in the dot plots (n = 7). (D) Numbers of NP-specific IgM and IgG secreting plasma cells in the spleen of WT (white bars) and Grb2BKO (gray bars) mice. Each symbol represents a mean value of triplicate samples of an individual mouse.
To determine whether the lack of GC responses impaired long-term humoral immunity, we tested primary and recall responses to TD antigens in Grb2BKO and WT mice. When the serum level of NP-specific antibodies was measured at different time points during the primary immune response, we found that the NP-specific IgM titer was slightly higher in the mutant mice compared with that of controls (Fig. S5A). To our surprise, anti-NP responses of other Ig isotypes including IgG1, IgG2b, IgG2c, and IgG3 were unaffected by the loss of Grb2 (Fig. S5A). Generation of high-affinity anti-NP antibody occurred normally in mutant mice (Fig. S5B), despite the apparent lack of splenic GCs. The mutant mice also elicited normal recall responses (Fig. S5C), suggesting that the development of memory B cells and long-lived plasma cells is intact in Grb2BKO mice.
Our serological findings that Ig class switch and antibody-affinity maturation occurred normally in Grb2BKO mice in the absence of splenic GCs suggested that these processes might take place in other tissues than in the spleen, or grb2−/− GC B cells could not express the characteristic cell-surface markers. We, therefore, examined the GC structure in both spleen and lymph nodes by immunohistology with anti-CD35 that highlights FDC clusters in the light zone of the GC. At day 10 after immunization with sheep red blood cells (SRBC), WT mice formed large GCs in both the spleen and lymph nodes (Fig. 3 A–D), visualized as PNA+ B-cells and CD35+ FDC clusters. In contrast, neither PNA+ B cells nor FDC networks were detected in the spleen of mutant mice (Fig. 3 A and B). Interestingly, the GC structure was intact in lymph nodes of Grb2BKO mice (Fig. 3 C and D). These data demonstrate that B-cell–specific deletion of grb2 only affects GC formation locally in the spleen. The observed Ig switch recombination and antibody-affinity maturation in mutant mice can be attributed to normal GC reaction in lymph nodes. In addition to the absence of FDC networks in the spleen, further inspection of the spleen follicles revealed that the marginal zone was also severely disrupted in Grb2BKO mice (Fig. 3E). This result thus indicates that Grb2 is responsible for general organization of the spleen follicles.
Fig. 3.
Defective spleen architecture and GC reaction in Grb2BKO mice. Ten days after SRBC immunization, sections of WT and Grb2BKO spleens and mesenteric lymph nodes were prepared for immunofluorescence histological analysis. (A and B) Spleen sections at 4× magnification. (C and D) Lymph-node sections at 10× magnification. (E) Marginal zones in the spleen of WT and Grb2BKO mice are highlighted by MOMA-1 staining (red). B-cell follicles (B220+) and T-cell (CD4+, CD8+) zones are shown in blue and green, respectively.
Naive grb2−/− B Cells Are Deficient in LTβ Expression.
The phenotypes of Grb2BKO mice, such as defective marginal zone, diminished FDC networks, and failure of GC formation in the spleen but unaffected lymph-node architecture, bear a striking resemblance to that of B-cell–specific LTβ-deficient mice (31–33). We hence decided to examine whether Grb2 was required for the expression of LTβ and other immediate members of the TNF family. Quantitative RT-PCR analysis shows that although grb2−/− and WT naive B cells (IgM+ IgD+) had comparable levels of LTα and TNF-α transcripts, LTβ expression was clearly defective in the mutant B cells compared with the WT counterparts (Fig. 4A). When cell-surface LT was examined by a soluble form of the LT receptor, LTβR-Ig, which binds to the membrane-anchored LTα1β2, we could not detect LT on the cell surface of grb2−/− B cells (Fig. 4B). These results demonstrate that Grb2-mediated signaling is critical for LT expression in naive B cells.
Fig. 4.
Regulation of CXCR5 signaling and LT expression by Grb2. (A) IgM+IgD+ naive splenic B cells were purified from WT and Grb2BKO mice by FACS. Transcripts of LTα, LTβ, and TNF-α were quantified by qRT-PCR and are shown as relative units after being normalized to the expression of β-actin in the corresponding samples; P < 0.005, n = 5. (B) Splenocytes were stained with anti-B220 and LTβR-Ig (n = 7). The percentages of cells within each quadrant are indicated in the plots. (C) Splenocytes were rested on ice for 8 h to disengage the chemokine signaling. Cells were then stimulated with either 3 μg/mL of CXCL13 or 10 μg/mL of anti-CD40 at 37 °C for 16 h and then subjected to LTβR-Ig and anti-B220 staining. Shown are percentages of LT-positive (B220+LTβR-Ig+) B cells in a B220+-gated population. n = 4.
Grb2 Connects CXCR5 Signaling to LT Expression.
Previous studies have shown that CXCR5 signaling is essential for the expression of LT on naive B cells to control the formation of lymphoid follicles (15), whereas CD40L and other stimulatory molecules further up-regulate LT expression on activated lymphocytes during immune responses (34, 35). To determine whether Grb2 connected signaling from CXCR5 or CD40 to LT up-regulation in B cells, we examined LT expression after stimulating splenic B cells with the CXCR5 ligand CXCL13 or antibody to CD40. We found that anti-CD40 treatment drastically up-regulated LT expression on both WT and grb2−/− B cells (Fig. 4C). In contrast, CXCL13 induced LT expression only in WT but not in grb2−/− B cells (Fig. 4C and Fig. S6), demonstrating that Grb2 is an essential adaptor downstream of the CXCR5 signal-transduction pathway leading to LT expression. It is important to note that chemotaxis of grb2−/− B cells to either CXCL13 or CXCL12 stimulation was normal (Fig. S7). These results indicate that Grb2 specifically controls a branch of CXCR5 signaling in B cells that induces LTβ transcription but not chemotaxis.
grb2−/− B Cells Are Not Intrinsically Deficient in Differentiation into GC Cells.
The above results let us posit that grb2−/− B cells may undergo GC differentiation if the positive-feedback loop of LT and chemokines were restored in the spleen of Grb2BKO mice by WT B cells. To test this hypothesis, we established BM chimeras by cotransplanting an equal number of WT (CD45.1+) and Grb2BKO (CD45.2+) BM cells into lethally irradiated B6.SJL mice (CD45.1+) and examined GC formation after immunization. In this experimental setting, B cells derived from WT BM stem cells should express LT and, therefore, were expected to restore the splenic FDC networks. We found that indeed, in chimeras that received both WT and Grb2BKO BM cells, splenic GC cells contained not only WT but also grb2−/− B cells (Fig. 5A). In contrast, no GC population could be found in the spleen of mice receiving only Grb2BKO BM cells (Fig. 5B). This finding thus indicates that grb2−/− B cells are not intrinsically defective in GC differentiation. The GC defect caused by the Grb2BKO mutation can be rectified by the presence of WT B cells that presumably provide the necessary LT signals for the genesis of FDC networks and pattern formation of lymphoid follicles.
Fig. 5.
Restoration of GC development of Grb2BKO B cells by WT B cells in BM chimeras. At day 10 after SRBC immunization, splenocytes from WT and Grb2BKO BM chimeras were examined for the generation of GC cells. Grb2BKO (CD45.2+) and WT (CD45.1+) B cells were gated, respectively, and displayed as contour plots for GC B cells (CD95+PNA+). (A) BM chimeras with mixed WT and Grb2BKO donor cells. (B) BM chimeras with only Grb2BKO donor cells. n = 4.
Enforced LTβ Expression Restores Splenic Defects in Grb2BKO Mice.
To further test whether enforced expression of LTβ in Grb2BKO mice is sufficient to restore the FDC networks and GC reaction, we reconstituted Grb2BKO BM cells with either murine stem cell virus (MSCV) or LTβ expressing MSCV retroviral vector and generated BM chimeras. At day 11 after immunization, we examined GC B cells by flow cytometry and spleen architecture by immunofluorescent staining. As shown in Fig. 6, both GCs and FDC networks were fully restored in the BM chimeras transduced with LTβ-MSCV but not with empty MSCV vector. This result clearly shows that impaired LTβ expression in Grb2BKO mice is responsible for the defects of splenic architecture and GC reaction caused by Grb2 deficiency.
Fig. 6.
Enforced LTβ expression restores GC reaction and FDC networks in the spleen of Grb2BKO mice. WT and Grb2BKO BM cells were transduced with MSCV empty (MSCV) or LTβ-expressing (MSCV-LTβ) vector and transplanted into irradiated RAG-1−/− recipients. Two months after transplantation, BM chimeras were immunized with SRBC. (A) (Upper) Enforced LT expression in WT and Grb2BKO splenocytes. (Lower) B220+-gated plots. The percentages of GC B cells are indicated (n = 4). (B) Spleen sections from WT and Grb2BKO BM chimeras were stained with anti-B220, -CD35 together with anti-CD4 and -CD8 antibodies.
Discussion
Grb2 has been asserted to be a key adaptor for multiple cellular functions by virtue of its physical association with a variety of receptors and their downstream signaling molecules. However, its function in the immune system has not been fully characterized. While Grb2 positively regulates TCR signaling, it acts as a negative regulator in B-cell activation. Although the detailed mechanism by which Grb2 negatively regulates BCR signaling is still unclear, our observation of a reduction of CD22 phosphorylation in grb2−/− B cells after BCR stimulation may provide an explanation. CD22 is an inhibitory receptor for BCR signaling. It has been shown that Lyn phosphorylates CD22 after BCR stimulation. Phosphorylated CD22 then recruits the tyrosine phosphatase SHP-1 to the CD22/BCR complex to exert suppressive effects on Ca2+ flux and MAPK activation. Thus, it is likely that Grb2 is required for strengthening the Lyn-CD22-SHP-1 negative-feedback loop. Our data showing that the active form of Lyn was slightly reduced in the mutant B cells is consistent with this notion.
It has been shown that a strong BCR signal in immature B cells may lead to apoptosis, a mechanism being used to eliminate autoreactive B cells. Notably, Grb2BKO mice show about 60% loss of immature B cells at T1 and T2 stages, despite normal B-cell genesis. Because Grb2 deficiency enhances BCR signaling without affecting BAFF dependent survival of B cells, it is possible that a strong BCR signal in grb2−/− immature B cells assumes negative selection and purge ∼60% of immature B cells in the periphery of Grb2BKO mice. Alternatively, it is also likely that the splenic microenvironment of Grb2BKO mice is less supportive for grb2−/− B cells. Consistent with this idea, we observed that grb2−/− B cells were preferentially increased compared with the WT counterpart in Grb2BKO and WT mixed BM chimeras (Fig. S8), suggesting that the presence of WT B cells may correct the defective spleen environment (such as the lack of FDCs and perhaps also other accessory cells). More experiments are needed to clarify this issue.
While progressing through the T2 stage, maturing B cells migrate to and reach the B-cell follicles. After encountering antigens and with T-cell help, mature follicular B cells initiate GC reaction and develop into either memory B cells or long-lived plasma cells. While much progress has been made in the past decades, the mechanism that governs B cells into these two developmental pathways has not been fully understood. It is surprising that Grb2 deficiency in B cells abrogates GC formation in the spleen, even though mutant B cells respond to antigens vigorously. Our further studies demonstrate that Grb2-mediated signaling is not involved in dictating the GC-cell fate of antigen-activated B cells; rather, it delivers signals from CXCR5 to induce expression of LT, a well-known lymphokine that is essential for the development of proper lymphoid follicle structures, including the marginal zone, FDC networks, and GCs. Indeed, grb2−/− B cells are able to form GCs when coexisting with WT B cells, suggesting that the CXCR5-Grb2-LT signaling axis in WT naive B cells is required for laying out functional spleen architecture that accommodates mutant B cells to undergo the GC maturation program. Because grb2−/− B cells can form GCs, our results thus indicate that reverse signaling from the membrane-anchored LT, if any, is not required for the GC reaction.
Why is the defective development of FDC and GC confined to the spleen but not to lymph nodes of Grb2BKO mice? One explanation for such a phenotype is that deletion of Grb2 in B cells impairs LT expression only in B cells but not in other lineages of cells. It is known that different secondary lymphoid organs require different LT- and LTβR-expressing hematopoietic and stromal cells for proper organogenesis. For example, the architecture of the spleen but not of lymph nodes relies on B-cell–derived LT (31–33). On the other hand, LT produced by lymphoid tissue-inducer cells during days 11 to 16 gestation is pivotal for lymph node development (36, 37); however, lack of lymphoid tissue-inducer cells did not affect the formation of splenic B-cell follicles (38). We will be interested to determine whether Grb2 also functions in other cell lineages to control LT expression for the formation of lymphoid follicles and GCs in lymph nodes. It should also be noted that although our results establish Grb2 as an essential integrator of CXCR5 signaling pathways, there are phenotypic differences between Grb2BKO mice and CXCL13- or CXCR5-mutant mice, as the latter show more distorted follicular organization and a diminishment—but not absence—of GC reaction in the spleen (15, 39, 40). In vitro data have shown that chemokines other than CXCL13 can also up-regulate LT expression in naive B cells (15). It is therefore likely that ablation of Grb2 in B cells disconnects signaling from all chemokine receptors to LT expression (41), hence completely eliminating splenic FDC networks and GCs.
Grb2BKO mutation selectively affects humoral immunity in the spleen but not lymph nodes. It remains unclear whether such a differential regulation has any biological impact on immunity against pathogens or autoantigens. It is generally believed that lymph nodes normally mediate immunity against pathogens from their draining peripheral tissues. In contrast, the spleen encounters mostly blood-borne antigens. Interestingly, B-cell–specific LTβ-deficient mice, which have normal lymph nodes but a similar defect in the splenic architecture as Grb2BKO mice, fail to develop effective humoral responses against a low dose of vesicular stomatitis virus (33). In this regard, splenic GC reaction may play a more profound role in immune responses against those blood-borne pathogens. In addition to immunity against pathogens, we have noticed that Grb2BKO mice produce high titers of anti-dsDNA IgM and have a high incidence of antibody deposits in kidneys, but do not develop lupus-like nor other autoimmune diseases. It is therefore worthwhile to examine whether lack of splenic GC function in these mice may attenuate autoimmune responses.
In summary, our data demonstrate that Grb2 plays an important role in B cells via integrating various signaling transduction pathways of the BCR and CXCR5. Our Grb2BKO mice thus provide a useful model to further dissect the signalosomes downstream of these receptors. Importantly, we additionally reveal a Grb2-containing CXCR5 signalosome, distinct from those required for chemotaxis, which regulates LT expression. In this regard, targeting Grb2 may differentially modify chemokine receptor signaling and immune responses to achieve more selective therapeutic goals.
Materials and Methods
Mice, Adoptive Transfer, and Immunization.
grb2-floxed (grb2fl) mice, generated in our laboratory, have been back crossed to C57BL/6 for more than 12 generations, and further crossed to CD19-Cre transgenic mice to generate B-cell–specific grb2 knockout (grb2fl/fl CD19-Cre heterozygous) mice, termed here as Grb2BKO mice. Grb2BKO mice were healthy with no obvious abnormality. To generate BM chimeras, 106 BM cells from WT B6.SJL and Grb2BKO mice were injected intravenously, either alone or at 1:1 ratio, into 750 Rad-irradiated B6.SJL recipients (The Jackson Laboratory). To immunize mice with TD antigens, 50 μg of NP30-KLH (Biosearch Technologies) precipitated in Imject Alum (Pierce) were injected into mice intraperitoneally. To immunize mice with SRBC, 200 μL of PBS-washed 10% SRBC were injected into each mouse intraperitoneally. All animals were housed in the specific pathogen-free barrier facility at Columbia University in accordance with institution approved protocols.
Biochemistry and Antibodies.
For Western blot analysis, 50 to 100 μg of total proteins were loaded and size-fractionated on a 12% SDS-PAGE gel by electrophoresis and transferred to a PVDF membrane. For immunoprecipitation, 1 to 2 μg of antibody and 50 μL of protein G agarose beads was added to total cell lysate from 2 to 5 × 106 cells and rotated at 4 °C overnight. After washing, proteins were denatured by boiling and fractionized on 12% SDS-PAGE gel. Immunobloting was performed according to our previous protocol (42). The following antibodies were used for biochemical study: anti-IgM F(ab)2 (Biosource); anti-phsphotyrosine (4G10) (Upstate Biotech), anti–p-Erk, anti-Erk1/Erk2, anti-pJNK, anti-JNK, anti-p38, anti-Grb2, and anti-PLCγ2 (Santa Cruz); and anti–p-p38 (Biosource).
In Vitro 3H-Thymidine Incorporation Assay.
Mature (B220+AA4.1loCD24lo) splenic B cells were purified by FACS sorting, and seeded in triplicates into a 96-well plate at 5 × 104 cells per well, in the presence of goat anti-IgM F(ab)2 (Organon Teknica Corp.; 10 μg/mL), anti-IgM F(ab)2 plus anti-CD40 (BD Pharmingen; 10 μg/mL), or IL-4 (Biosources; 20 U/mL), anti-CD40 plus IL-4, or LPS (Sigma-Aldrich; 30 μg/mL). Forty-eight hours later, 3H-thymidine (1 μCi) was added to each well and incubated for an additional 6 h. Cells were harvested on a cell harvester and 3H-thymidine incorporation was measured on a β-counter.
Flow Cytometry and BrdU Labeling.
Single-cell suspensions were prepared from total BM, spleen, or mesenteric lymph nodes. After staining, cells were analyzed on a BD LSR II. The following antibodies are used for the staining: anti-B220, anti-CD21, anti-CD23, anti-CD24, anti-CD38, anti-GL7, anti-AA4.1, anti-IgG1 (BD Pharmingen); anti-IgM, anti-IgD (Southern Biotech); PNA (Vector); NP-PE (gift from M. Shlomchik, Yale University, New Haven, CT). For cell-surface LT staining, cells were incubated for 1 h on ice with staining solution containing 10% goat and rat serum to block Fc receptors. After washing, cells were incubated with 50 μL of LTβR-Ig (10 μg/mL) on ice for 45 min. Stained cells were further revealed by anti-human IgG (eBioscience). For BrdU-labeling, mice were injected with BrdU (1 mg per mouse) intraperitoneally. Four hours later, mice were killed for analysis. BrdU staining was performed using a kit (BD Pharmingen) according to the manufacturer's instructions.
Ca2+ Mobilization.
Splenic B cells were labeled with Fluo-4 and Fura-red (Molecular Probes) according to the manufacturer's instructions. Cells were then stimulated with anti-IgM F(ab)2 at 37 °C and subjected to FACS analysis. Intracellular Ca2+ concentration was presented as the ratio of fluorescence intensities of Fluo-4 vs. Fura-red.
Immunofluorescence Histology.
Cryosections of spleen and mesenteric lymph nodes were fixed in 4% paraformaldehyde at room temperature for 10 min, blocked with 10% goat serum in PBS containing 0.2% Triton X-100 for 30 min at room temperature, and then stained with corresponding antibodies in PBS containing 0.1% Tween-20 in a humidity chamber at RT for 1 h. Antibodies used are: anti-CD4, anti-CD8, anti-CD35, and B220 (BD Pharmingen); PNA-biotin (Vector); anti–Moma-1 (Serotec Inc.), anti-IgD (clone 1.3–5; gift from R. Pelanda, University of Colorado, Denver, CO), streptavidin-Alexa 568 or streptavidin-Cy5 (Molecular Probes). Data were obtained with a Nikon TE-2000E microscope using NIS-Elements 2.3 software.
Transwell Assay.
Total splenic cells were suspended in DMEM containing 10% FCS (107 cells/mL). Cells (100 μL) were loaded into the upper chamber of a 3-μM pore-size transwell (Corning Costar). The lower chamber was filled with 600 μL of medium or medium containing either CXCL13 (1 μg/mL) or CXCL12 (500 ng/mL) (PeproTech). After incubation at 37 °C for 3 h, cells in the lower chamber were counted and examined by flow cytometry.
ELISA and ELISPOT.
For ELISA, serum was collected from mice at different time points after immunization. Samples were diluted serially and added to 96-well plates (50 μL per well) precoated with NP30-BSA (Biosearch Technologies; 10 μg/mL). After incubation at 37 °C for 1 h, bound antibodies were revealed by AP-conjugated anti-mouse Ig of different isotypes (Southern Biotech). Results were presented as relative units taking the average of day-7 immunized-WT samples as a control. For an affinity maturation assay, the plates were coated with either NP30-BSA or NP2–3-BSA (Biosearch Technologies; 10 μg/mL). The serum titers of IgG1 against each conjugates were determined as above. Results were presented as ratios of IgG1 titers against NP2–3-BSA vs. NP30-BSA. For ELISPOT, 96-well MultiScreen filtration plates (Millipore) were coated with NP30-BSA. Total splenic cells were seeded into the plate (104 cells per well) and cultured overnight at 37 °C. After washing, AP-conjugated anti-mouse IgM or IgG (Southern Biotech) was added to the plates and incubated at 37 °C for 1 h. Antibody bound plaques were revealed using a BCIP/NBT kit (Vector) and quantified under a microscope.
Supplementary Material
Acknowledgments
We thank M. Shlomchik for kindly providing the NP-PE, C. Swanson and R. Pelanda for anti-mouse IgD, M. McHeyzer-Williams for discussion, and B. Diamond and A. Davidson for reading and comments on the manuscript. This work was supported by an Irene Diamond Fund (to H.G.) and by an Institution Fund (to Y.Z.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016451108/-/DCSupplemental.
References
- 1.Kosco-Vilbois MH, Bonnefoy JY, Chvatchko Y. The physiology of murine germinal center reactions. Immunol Rev. 1997;156:127–136. doi: 10.1111/j.1600-065x.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 2.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 3.Tarlinton D. Germinal centers: Form and function. Curr Opin Immunol. 1998;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- 4.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser AE, Shlomchik MJ, Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nat Rev Immunol. 2007;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- 6.MacLennan IC, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 7.Carter RH, Myers R. Germinal center structure and function: Lessons from CD19. Semin Immunol. 2008;20:43–48. doi: 10.1016/j.smim.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato J, et al. Affinity maturation in Lyn kinase-deficient mice with defective germinal center formation. J Immunol. 1998;160:4788–4795. [PubMed] [Google Scholar]
- 9.Randall KL, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Good-Jacobson KL, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAdam AJ, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 12.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 13.Wong SC, Oh E, Ng CH, Lam KP. Impaired germinal center formation and recall T-cell-dependent immune responses in mice lacking the costimulatory ligand B7-H2. Blood. 2003;102:1381–1388. doi: 10.1182/blood-2002-08-2416. [DOI] [PubMed] [Google Scholar]
- 14.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: Phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 16.Sadowski I, Stone JC, Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986;6:4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 18.Jang IK, Zhang J, Gu H. Grb2, a simple adapter with complex roles in lymphocyte development, function, and signaling. Immunol Rev. 2009;232:150–159. doi: 10.1111/j.1600-065X.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 19.Neumann K, Oellerich T, Urlaub H, Wienands J. The B-lymphoid Grb2 interaction code. Immunol Rev. 2009;232:135–149. doi: 10.1111/j.1600-065X.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- 20.Engels N, et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol. 2009;10:1018–1025. doi: 10.1038/ni.1764. [DOI] [PubMed] [Google Scholar]
- 21.Jang IK, et al. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc Natl Acad Sci USA. 2010;107:10620–10625. doi: 10.1073/pnas.0905039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stork B, et al. Grb2 and the non-T cell activation linker NTAL constitute a Ca(2+)-regulating signal circuit in B lymphocytes. Immunity. 2004;21:681–691. doi: 10.1016/j.immuni.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Cong F, Yuan B, Goff SP. Characterization of a novel member of the DOK family that binds and modulates Abl signaling. Mol Cell Biol. 1999;19:8314–8325. doi: 10.1128/mcb.19.12.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurosaki T. Functional dissection of BCR signaling pathways. Curr Opin Immunol. 2000;12:276–281. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 26.Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J Biol Chem. 2001;276:44315–44322. doi: 10.1074/jbc.M105446200. [DOI] [PubMed] [Google Scholar]
- 27.Tsubata T. Co-receptors on B lymphocytes. Curr Opin Immunol. 1999;11:249–255. doi: 10.1016/s0952-7915(99)80041-7. [DOI] [PubMed] [Google Scholar]
- 28.Camacho SA, Kosco-Vilbois MH, Berek C. The dynamic structure of the germinal center. Immunol Today. 1998;19:511–514. doi: 10.1016/s0167-5699(98)01327-9. [DOI] [PubMed] [Google Scholar]
- 29.Ridderstad A, Tarlinton DM. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J Immunol. 1998;160:4688–4695. [PubMed] [Google Scholar]
- 30.Toyama H, et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17:329–339. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 31.Endres R, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–168. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumanov A, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 33.Junt T, et al. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur J Immunol. 2006;36:2061–2075. doi: 10.1002/eji.200626255. [DOI] [PubMed] [Google Scholar]
- 34.Worm M, Geha RS. CD40 ligation induces lymphotoxin alpha gene expression in human B cells. Int Immunol. 1994;6:1883–1890. doi: 10.1093/intimm/6.12.1883. [DOI] [PubMed] [Google Scholar]
- 35.Boussiotis VA, Nadler LM, Strominger JL, Goldfeld AE. Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc Natl Acad Sci USA. 1994;91:7007–7011. doi: 10.1073/pnas.91.15.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 37.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 39.Voigt I, et al. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur J Immunol. 2000;30:560–567. doi: 10.1002/1521-4141(200002)30:2<560::AID-IMMU560>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 40.Förster R, et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 41.Ohl L, et al. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197:1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naramura M, et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.