Abstract
In a previous study, we measured the redox potential of the primary electron acceptor pheophytin (Phe) a of photosystem (PS) II in the chlorophyll d–dominated cyanobacterium Acaryochloris marina and a chlorophyll a–containing cyanobacterium, Synechocystis. We obtained the midpoint redox potential (Em) values of −478 mV for A. marina and −536 mV for Synechocystis. In this study, we measured the redox potentials of the primary electron acceptor quinone molecule (QA), i.e., Em(QA/QA−), of PS II and the energy difference between [P680·Phe a−·QA] and [P680·Phe a·QA−], i.e., ΔGPhQ. The Em(QA/QA−) of A. marina was determined to be +64 mV without the Mn cluster and was estimated to be −66 to −86 mV with a Mn-depletion shift (130–150 mV), as observed with other organisms. The Em(Phe a/Phe a−) in Synechocystis was measured to be −525 mV with the Mn cluster, which is consistent with our previous report. The Mn-depleted downshift of the potential was measured to be approximately −77 mV in Synechocystis, and this value was applied to A. marina (−478 mV); the Em(Phe a/Phe a−) was estimated to be approximately −401 mV. These values gave rise to a ΔGPhQ of −325 mV for A. marina and −383 mV for Synechocystis. In the two cyanobacteria, the energetics in PS II were conserved, even though the potentials of QA− and Phe a− were relatively shifted depending on the special pair, indicating a common strategy for electron transfer in oxygenic photosynthetic organisms.
Keywords: photosynthesis, photochemical reaction
Chlorophylls (Chls) are key pigments for photosynthesis, and oxygenic photosynthetic organisms containing Chls are found all over the world—they sustain all terrestrial life through the primary production of organic molecules and the production of oxygen. Chl a and its derivatives, pheophytin (Phe) a and Chl a epimer (Chl a′), are used as the electron transfer components in photosystem (PS) II and PS I, respectively, except for in two taxonomic groups, marine cyanobacteria Prochlorococcus spp. containing divinyl-Chl a (1) and marine cyanobacteria Acaryochloris spp. containing Chl d (2). Consequently, experimental and theoretical analyses of the electron transfer system in oxygenic photosynthesis have been exclusively performed in Chl a–containing organisms, and the basic concept of the photosynthetic electron flow has been constructed on the basis of Chl a. However, our understanding of photosynthetic electron transfer is not yet complete because the reaction systems with divinyl-Chl a and Chl d have not been fully analyzed as yet.
Since the discovery of Chl d in Acaryochloris marina in 1996 (3, 4), questions have arisen on the whether the electron transfer system in this organism is significantly different from those with Chl a. Interestingly, the functional “special pair” are Chl d molecules in the reaction centers of PS I and II in A. marina (5, 6); however, in PS II, Phe a is the primary electron acceptor. Chl d gains light energy at longer wavelengths and with a lowered redox potential, by ~80 mV, compared with that of Chl a in Synechocystis sp. PCC 6803 (hereafter referred to as Synechocystis) and spinach (7–11). However, the overall conditions for the water-cleavage reaction are satisfied even with the difference in the redox potentials (12).
In PS I, the redox potential of P740 (corresponding to P700 for other oxygenic photosynthetic organisms) is the same as that of P700 (6, 13), suggesting that no specific mechanism exists to compensate for the lower energy from the longer wavelength absorption of Chl d. Thus, one can conclude that the redox potentials of the special pair in oxygenic photosynthetic organisms are determined by the oxidizing side of the PS and not by the reducing side. These considerations suggest that a determining factor for the potential of the special pair with a specific pigment(s) might be related to an oxidation reaction that includes ligands and/or hydrogen bonds from the amino acid(s) of proteins to the pigments.
Numerous values have been reported for the essential electron transfer components (primary, QA, and secondary, QB) of PS II on the quinone molecules with redox potentials values ranging from −340 to +120 mV (14). QA in PS II is particularly important and may even act in one-gated electron transfer. Because QA is a critical component in electron transfer in all oxygenic photosynthetic organisms, including A. marina and Synechocystis, its potential might be suitably adjusted to those of the special pair and Phe a− in each organism. Thus, we determined the redox potentials of QA/QA− and the energetics between [P680·Phe a−·QA] and [P680·Phe a·QA−] (i.e., ΔGPhQ) in these two cyanobacteria under several critical conditions with particular attention to the water-oxidation activity, Mn depletion and reconstitution with Mn and bicarbonate, and the effect of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). It was found that, by adjusting the potentials of Phe a− (12), the redox potentials of QA/QA− in A. marina were positively shifted by 59–66 mV, comparable to that of Synechocystis and in line with the energy gain by the special pair of Chl a (P680) and Chl d (P713).
Results
Midpoint Redox Potential (Em) of QA/QA− of A. marina and Synechocystis.
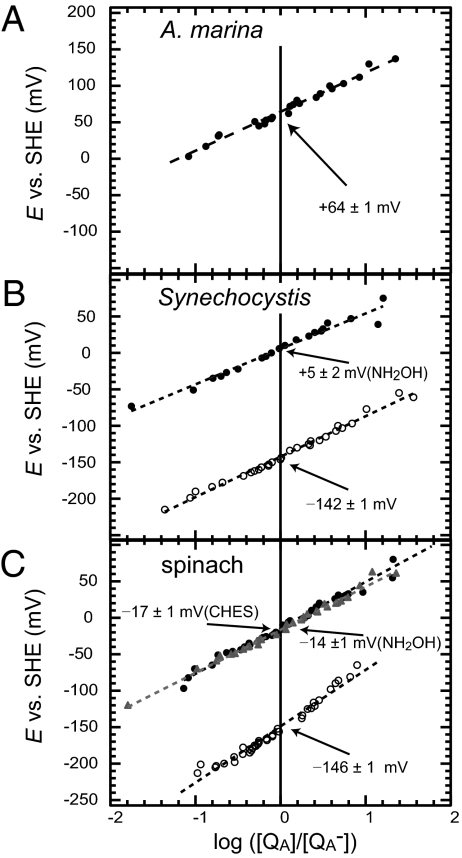
First, we estimated the Em value for the QA/QA− of PS II complexes in A. marina. These samples consisted of PsbA, PsbB, PsbD, PsbE, and small peptides PsbI and PsbF, but did not contain any extrinsic proteins (Fig. S1) or a Mn cluster. The amount of QA− under a certain potential condition was monitored by a PS II fluorescence intensity detected only with short excitation pulses (measuring light) of a double-modulation fluorometer. The intensity of the measuring flash was very low, ~0.01 μE m−2·s−1, and did not influence the QA− by a photochemical reaction. The fluorescence intensity changed as a function of the redox potential in the presence of sodium dithionite and potassium ferricyanide and gave a straight line as a function of log[(QA]/[QA−)] (Fig. 1). Based on the trace obtained, we estimated that the potential value of Em(QA/QA−) in A. marina is +64 mV (Fig. 1A and Table 1). The addition of DCMU (10−5 M) induced a potential shift to +89 mV (Fig. S2 and Table 1). The SD was estimated to be, at most, 1 mV in respective measurements.
Fig. 1.
Redox titration of QA/QA− in PS II complexes with and without the Mn cluster in A. marina (A), Synechocystis (B), and spinach (C) PS II particles. ○, PS II complex with the Mn cluster; ● and ▲, PS II complex without a Mn cluster. NH2OH and CHES indicate the hydroxyl amine treatment for the Mn depletion.
Table 1.
Redox potential of QA in various samples
| Samples and conditions | Em, mV |
| PS II complex from A. marina | |
| Control (without Mn clusters) | +64 ± 1 |
| Control (without Mn clusters) + DCMU | +89 ± 1 |
| Control (with Mn clusters) | −66 to approx. −86* |
| PS II core complex from Synechocystis | |
| Control (with Mn clusters) | −142 ± 1 |
| Control (with Mn clusters) + DCMU | −114 ± 1 |
| Mn depletion (with NH2OH) | +5 ± 2 |
| Mn depletion (with NH2OH) + DCMU | +36 ± 1 |
| Spinach PS II (BBY) particles | |
| Control (with Mn clusters) | −146 ± 1 |
| Control (with Mn clusters) + DCMU | −99 ± 1 |
| Mn depletion (with NH2OH) | −14 ± 1 |
| Mn depletion (with NH2OH) + DCMU | +24 ± 1 |
| Mn depletion (with CHES) | −17 ± 1 |
| Mn depletion (with CHES) + DCMU | +25 ± 1 |
| Restored after NH2OH treatment with Mn and HCO3− | −146 ± 1 |
*Represents a value estimated from the value for Synechocystis.
In contrast, the potential of QA/QA− in intact PS II core complexes isolated from Synechocystis was estimated to be −142 mV (Fig. 1B and Table 1). In this case, the complexes retained the Mn cluster and showed an oxygen-evolving activity [2,123 ± 67 μmol O2·(mg Chl)−1·hr−1] in their initial rates (Fig. S3). An effect of DCMU on the potentials was similarly observed in this sample, and the magnitude of increase was 28 mV, a value very similar to the case of A. marina (25 mV). When the Mn cluster was depleted by treatment with NH2OH, the Em(QA/QA−) was shifted significantly from −142 mV to +5 mV in the absence of DCMU (Fig. 1B and Table 1). The difference in magnitude was ~150 mV, indicating that depletion of the Mn cluster or partial destruction of the PS II structure severely affected the potential of QA. This finding is consistent with the result reported by Krieger and Rutherford (14, 15), even though the absolute values of the potentials are different [−80 mV in active PS II without DCMU by Krieger and Rutherford (15)]. A DCMU-induced up-shift of the potential of QA/QA− was also observed, from +5 mV to +36 mV, even after the Mn depletion (Table 1 and Fig. S2), but its magnitude was smaller than that observed in higher plants (15).
Changes in the Em(QA/QA−) of Spinach PS II Particles.
By redox titration measurements of QA/QA− couples from spinach that lacked QB molecules but retained oxygen-evolving activity, we found that they had a redox potential of −146 mV under the control condition (Fig. 1C and Table 1), which is consistent with Em(QA/QA−) values of −162 ± 3 mV from spinach PS II particles reported by Shibamoto et al. (16). However, it is substantially different from the value of −84 ± 16 mV, reported in 1995 by Krieger et al. (14). The discrepancy is not clear, but it might be because of the degree of intactness and differences in PS II particle isolation from spinach (SI Discussion).
The potential of QA/QA− was significantly affected by the depletion of Mn. When samples were treated with NH2OH (10 mM), the potential was up-shifted to −14 mV (Fig. 1C and Table 1). This value remained almost constant, even with the addition of CaCl2 and MnCl2 to a titration buffer in the dark or the light. Similarly there was an up-shift of potential to −17 mV with 2-(cyclohexylamino)ethanesulfonic acid (CHES), another Mn cluster–depleting agent (17, 18), and a result almost identical to the treatment with NH2OH (Fig. 1C and Table 1). The magnitude of the shift caused by the Mn depletion was ~130 mV, which is consistent with the value observed for the PS II complexes isolated from Synechocystis (147 mV; Fig. 1B) and the value in a previous report (14).
The DCMU effect was also similar for the above three samples. The magnitude of the up-shift was between 25 mV (A. marina; Fig. S3) and 47 mV (spinach, control; Fig. S2); a similar difference in the shift was also observed for the case of Mn depletion by NH2OH (ΔEm = 38 mV, spinach; Table 1) and CHES (ΔEm = 41 mV, spinach; Table 1 and Fig. S2). It is reasonable to infer that the addition of DCMU to the complexes induced a change that gave a similar shift in potential, irrespective of the samples. A larger ΔEm (~50 mV) in higher plants than in cyanobacteria is consistent with the report by Krieger and Rutherford (15).
Restoration of the Em(QA/QA−) Potential and Oxygen-Evolving Activity in Spinach PS II Particles.
It has been reported that oxygen-evolving activity can be restored in spinach PS II particles by the photo-induced reconstitution of Mn clusters in the Mn-depleted PS II particles in the presence of free Mn2+ and Ca2+ and that the addition of bicarbonate (1–5 mM) causes a considerable increase in both the rate and the yield of the photo-assembled oxygen-evolving complex (17, 19–21), even though bicarbonate was not found in the complex of recent crystallographic analysis. The stimulatory effect of bicarbonate in the photo-assembly of the oxygen-evolving complex is evidently based on the formation of easily oxidizable Mn–bicarbonate complexes (22), its participation in proton exchange within the oxygen-evolving complex (17, 21–23), and its suppression of photodamage to PS II (17, 23, 24). Here we observed a restoration of the Em(QA/QA−) and oxygen-evolving activity (Fig. S3). Under control conditions, the QA/QA− potential was −146 mV (Table 1), but it shifted to −17 mV after depletion of the Mn clusters by CHES. With the addition of MnCl2 (200 μM) and NaHCO3 (5 mM), as well as light (photo-activation), we observed a clear restoration to −146 mV (Table 1 and Fig. S2E) with the magnitude of the potential being fully recovered. These results clearly show that the restoration of the potential of Em(QA/QA−) is positively correlated with oxygen-evolving activity.
Restoration of the Phe a/Phe a− Potential in Synechocystis.
In a previous study (12), we examined the redox potentials of Phe a/Phe a− in the presence of a stabilizer (1 M betaine) in three preparations (Synechocystis, A. marina, and spinach): we found that the stabilizer induced a positive shift with a magnitude of, at most, −80 mV. These observations strongly indicate that the structure of the Mn cluster seriously affects the potentials of Phe a/Phe a−, even if the spatial loci are very far from the sites of the Mn cluster and Phe a. In the present study, we observed a reversible change in the redox potentials of QA/QA− caused by the presence of the Mn cluster in Synechocystis (Table 1). In terms of a long-range effect of the Mn cluster on the potentials of the components in the reducing side of PS II, these two effects are comparable. Therefore, we examined the potentials of Phe a/Phe a− after reconstitution of the Mn clusters.
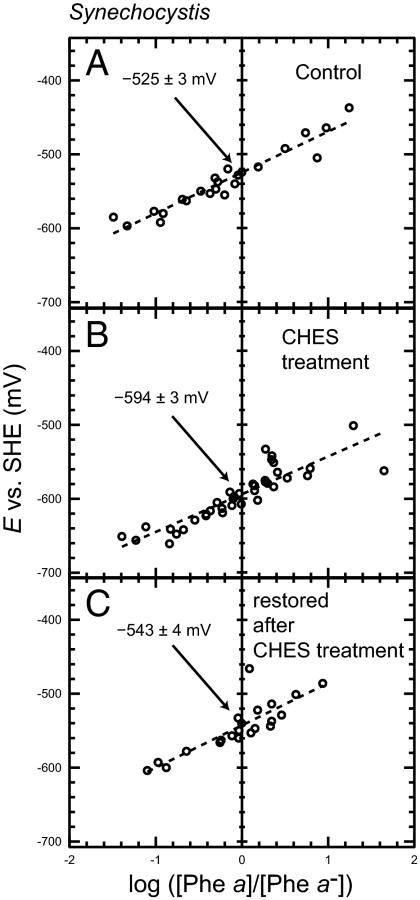
We used PS II complexes from Synechocystis, and the potentials were titrated. Under the control condition, the potential was estimated to be −525 mV (Table 2), and the SD was estimated to be, at most, 5 mV in the respective measurements. This potential value is consistent with our previous study, which found −536 mV (12), confirming a reproducibility of our measurements with different batches of samples. In this article, we describe the potential of Phe a/Phe a− to be −525 mV. For three kinds of treatment for the depletion of Mn clusters (i.e., CHES, NH2OH, and Tris), the magnitudes of down-shift of the potential of Phe a/Phe a− were −69, −84, and −55 mV, respectively (Table 2). Treatment with Tris resulted in irreversible damage to the samples, leading to the idea that reconstitution of the Mn cluster was not possible after Tris treatment, even though the magnitude of the potential shift was small. However, it is interesting to note that, after CHES and NH2OH treatment, the presence of MnCl2, NaHCO3, and light, the oxygen-evolving activity was recovered and, at the same time, the potential of Phe a/Phe a− was recovered to −543 and −557 mV, respectively (Fig. 2C, Fig. S3 B and C, and Table 2).
Table 2.
Redox potential of Phe a in the PS II core complex from Synechocystis sp. PCC 6803 and A. marina
| Samples and conditions | Em, mV |
| Synechocystis | |
| Control | −525 ± 3 |
| CHES treatment | −594 ± 3 |
| Restored after CHES treatment with Mn and HCO3− | −543 ± 4 |
| NH2OH treatment | −609 ± 2 |
| Restored after NH2OH treatment with Mn and HCO3− | −557 ± 3 |
| Tris treatment | −580 ± 5 |
| A. marina | |
| Control (without Mn clusters) | −478 ± 4 |
| Control (with Mn clusters) | −401 ± 5* |
The redox state of Phe a was estimated by the absorption change either at 683 nm (Synechocystis) or at 452 nm (A. marina) (Materials and Methods).
*Represents a value estimated from the value for Synechocystis.
Fig. 2.
Redox titration of Phe a/Phe a− in the PS II complexes in Synechocystis control (A), after CHES treatment (B), and restoration of the Mn cluster after CHES treatment (C).
Discussion
Evaluation of the Redox Potential in A. marina in the Presence of the Water-Oxidizing Complex.
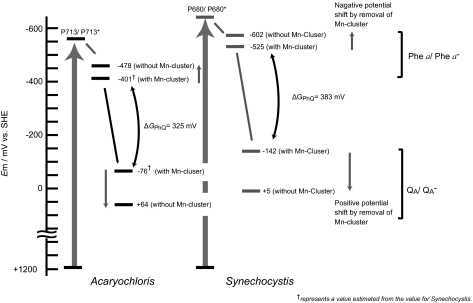
Here we showed that Em(QA/QA−) for PS II complexes from A. marina was approximately +64 mV (Figs. 1A and 3 and Table 1). These complexes did not retain a Mn cluster. Additionally, we showed that the Em(QA/QA−) values of the PS II samples from Synechocystis and spinach, −142 mV and −146 mV, respectively, were up-shifted by ~130–150 mV by Mn depletion (Fig. 1 B and C and Table 1), which is consistent with a recent result for Thermosynechococcus elongatus (25). Because the A. marina PS II sample did not retain a Mn cluster, it is reasonable to assume that the Em(QA/QA−) was down-shifted when the PS II complexes from A. marina with Mn were evaluated; an expected value would be approximately −76 mV when the observed difference in the Mn-depleted potentials (−130 mV for spinach and −150 mV Synechocystis) is applied (Table 1). If this down-shift were the case, the difference in the Em(QA/QA−) between Synechocystis and A. marina with Mn clusters would be approximately −66 mV (−142 mV vs. −76 mV); this value corresponds to the difference in energy gain by light absorption between the special pairs consisting of Chl a (1.82 V, P680) and Chl d (1.74 V, P713).
Fig. 3.
Overall energetics of PS II in the two cyanobacteria A. marina and Synechocystis.
This consideration may be extended to the potential of Phe a/Phe a−. As shown in the Synechocystis PS II complexes (Fig. 2), the Mn-depleted down-shift of the Em(Phe a/Phe a−) was approximately −77 mV (−525 mV vs. −602 mV, the latter being an average of −594 and −609 mV). In our previous study, the Em(Phe a/Phe a−) of A. marina was −478 mV (12). If this difference in the potential were applied to A. marina, an expected value would be −401 mV when the Mn cluster is present (Table 2). Compared with the observed Em(Phe a/Phe a−) of Synechocystis (−525 mV), the difference in the potential would be ~124 mV, which is slightly larger than the difference in the energy gain of Chl a and Chl d.
Overall Energetics of the Electron Transfer from Phe a− to QA−.
The energy differences between the electron transfer components are one of the key indices for the overall energetics of the photosynthetic electron transfer system. We surveyed the potentials of Em(QA/QA−) and Em(Phe a/Phe a−) of the Chl d–dominated cyanobacterium A. marina and obtained a value of −401 mV for Phe a and −76 mV for QA. The corresponding values in Synechocystis were −525 mV for Phe a and −142 mV for QA. The energy gap, ΔGPhQ, between [P680·Phe a−·QA] and [P680·Phe a·QA−] was calculated to be −325 and −383 mV for A. marina and Synechocystis, respectively (Fig. 3). These values are generally consistent with previous reports on the ΔGPhQ estimated by charge recombination: −310 mV for Synechocystis (26) and −340 mV for spinach (27). Because this energy gap is one of the essential factors that drive forward, as well as backward (charge recombination), electron transfers, the consistency of these values suggests that the overall energetics in the electron transfer pathway from the P* to QA− in A. marina are essentially identical to those in other Chl a–containing photosynthetic organisms. In oxygenic photosynthetic organisms, this ΔGPhQ corresponds to the reaction step. The consistency of the ΔGPhQ between A. marina and Synechocystis might be an important factor for their overall energetics in PS II. This conclusion may be confirmed with further studies on the electron transfer rate between Phe a and QA in related organisms.
The exact nature of primary electron donor in A. marina is still controversial. In our previous work, we assigned the Chl d dimer to the special pair based on results from absorption change, more sensitive/modern method FTIR spectroscopy, and redox potential of Phe a using highly purified samples (5, 12). In addition, some other studies using partially purified samples support our view (28, 29). However, for another study using partially purified samples, a different component (Chl a and Chl d heterodimer) was proposed for the special pair (30). Additionally, in an earlier study, Shevela et al. (31) proposed that the redox potentials of the water-oxidizing complex in its different Si states (donor side of PS II) are almost the same for A. marina cells and spinach thylakoids. However, experimental evidence for this interpretation was completely indirect. Moreover, it has been shown that the potential of P740 in A. marina is almost the same as that in Synechocystis (+435 mV) (6, 13). The cytochrome b6f complexes are situated between the PS II and PS I complexes, although we have no data on the potential of these complexes in A. marina. As the properties of the potentials of this complex are resolved, we will be able to clarify whether changes in the potentials of PS II in A. marina are limited to PS II, which will be closely related to the structural and functional sequence of the complexes in cyanobacteria.
Materials and Methods
Preparation of Complexes.
Core complexes from A. marina.
PS II core complexes from A. marina MBIC 11017 were isolated as described previously (5) but with slight modifications (12). The number of Chl d and Chl a per two Phe a molecules was estimated to be 29.6 and 1.9, respectively.
Core complexes from Synechocystis sp. PCC 6803.
PS II core complexes of Synechocystis sp. PCC 6803 were prepared per a previously described procedure (32).
PS II particles from spinach.
PS II membrane fragments from spinach chloroplasts were isolated as reported previously (33). QB was removed by Triton X-100 treatment, as described previously (34). The oxygen-evolving activity was ~600 μmol O2·(mg Chl)−1·hr−1.
Preparation of biochemically treated samples: Mn depletion.
To obtain Mn-depleted samples, Tris, CHES, and NH2OH treatments were performed on PS II complexes isolated from Synechocystis (17–19, 35–37).
Reconstitution of Mn-depleted PS II complexes.
Photo-activation of the Mn cluster was achieved as described previously on PS II complexes isolated from Synechocystis and spinach PS II particles (17, 19–21, 35, 37). The oxygen-evolving activities were recovered to ~40–55% of that of the untreated PS II complexes. The experimental details for the reconstitution procedures are given in SI Materials and Methods.
Titration.
All experiments were performed in the presence of betaine, a natural osmolyte, at pH 7.0, because it has previously been shown that PS II preparations are more stable in the presence of 1 M glycine betaine (12, 38–43). Details of the physiological conditions used for various species are summarized in SI Materials and Methods, Figs. 1 and 2, and Figs S1−S3).
Phe titration.
We adopted and performed a titration procedure as described in our previous report (12). The experimental details for the redox titration are given in SI Materials and Methods.
QA titration.
The Em(QA/QA−) was titrated at 25 °C by Chl fluorescence that directly correlated with the amount of QA−; when the fluorescence intensity was plotted as a function of the redox potential, it gave a Nernst curve. We used a double-modulation fluorometer (model FL 3500; Photon System Instruments) and took measurements using only the measuring light (very low intensity). The redox titration was performed under anaerobic conditions (under argon) in a reaction mixture (5 mL; ~8 μg of Chl·mL−1) containing 50 mM Mes (pH 7.0), 10 mM NaCl, 2 mM MgCl2, 5 mM CaCl2, 0.04% β-d-dodecyl maltoside, and 1 M betaine in the presence of sodium dithionite [50–100 μM; Em at Em = −660 mV at pH 7 (44)] and/or potassium ferricyanide (up to 100 μM; Em = 430 mV at pH 7). Reversible redox titrations were obtained easily at low concentration of Chl (8 μg of Chl·mL−1). After titrations in the reducing direction, reoxidation was performed two to three times on the same samples. Each sample was used for two to four different measurements of potential in the reducing or oxidizing direction. Reductive and oxidative titrations were performed by gradual addition of sodium dithionite and potassium ferricyanide, respectively. The titration was performed without redox mediators.
Supplementary Material
Acknowledgments
We dedicate this paper to the memory of our co-author, Prof. Mamoru Mimuro, who passed away on February 8, 2011 while this paper was in review. This work was supported by Grant-in-Aid 17GS0314 for Creative Research from the Japanese Society for the Promotion of Science (to M.M.) and in part by Grants-in-Aid 21570038 (to T. Tomo) and 22370017 (to M.M.) for Scientific Research from the Ministry of Education of Japan, by a grant from the Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology (PRESTO) program (to T. Tomo), by Grant 11-04-01389a and 11-04-92690a from the Russian Foundation for Basic Research, by Grant 16.740.11.0176 from the Russian Ministry of Science and Education, and by grants from the Molecular and Cell Biology Programs of the Russian Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
2Deceased February 8, 2011.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100173108/-/DCSupplemental.
References
- 1.Partensky F, Hoepffner N, Li WKW, Ulloa O, Vaulot D. Photoacclimation of Prochlorococcus sp. (Prochlorophyta) strains isolated from the North Atlantic and the Mediterranean Sea. Plant Physiol. 1993;101:285–296. doi: 10.1104/pp.101.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mimuro M, et al. Identification of the primary electron donor in PS II of the Chl d-dominated cyanobacterium Acaryochloris marina. FEBS Lett. 2004;556:95–98. doi: 10.1016/s0014-5793(03)01383-8. [DOI] [PubMed] [Google Scholar]
- 3.Miyashita H, et al. Chlorophyll d as a major pigment. Nature. 1996;383:402. [Google Scholar]
- 4.Murakami A, Miyashita H, Iseki M, Adachi K, Mimuro M. Chlorophyll d in an epiphytic cyanobacterium of red algae. Science. 2004;303:1633. doi: 10.1126/science.1095459. [DOI] [PubMed] [Google Scholar]
- 5.Tomo T, et al. Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium. Proc Natl Acad Sci USA. 2007;104:7283–7288. doi: 10.1073/pnas.0701847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomo T, et al. Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017. J Biol Chem. 2008;283:18198–18209. doi: 10.1074/jbc.M801805200. [DOI] [PubMed] [Google Scholar]
- 7.Jursinic P, Govindjee Temperature dependence of delayed light emission in the 6 to 340 microsecond range after a single flash in chloroplasts. Photochem Photobiol. 1977;26:617–628. [Google Scholar]
- 8.Wydrzynski TJ. Water splitting by Photosystem II—Where do we go from here? Photosynth Res. 2008;98:43–51. doi: 10.1007/s11120-008-9391-1. [DOI] [PubMed] [Google Scholar]
- 9.Klimov VV, Allakhverdiev SI, Demeter S, Krasnovsky AA. Photoreduction of pheophytin in the photosystem II of chloroplasts depending on the oxidation-reduction potential of the medium. Dokl Akad Nauk SSSR. 1979;249:227–230. [Google Scholar]
- 10.Renger G. Functional pattern of photosystem II. In: Renger G, editor. Primary Processes of Photosynthesis—Part 2. Cambridge, UK: RSC Publishing; 2008. pp. 237–292. [Google Scholar]
- 11.Kato Y, Sugiura M, Oda A, Watanabe T. Spectroelectrochemical determination of the redox potential of pheophytin a, the primary electron acceptor in photosystem II. Proc Natl Acad Sci USA. 2009;106:17365–17370. doi: 10.1073/pnas.0905388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allakhverdiev SI, et al. Redox potential of pheophytin a in photosystem II of two cyanobacteria having the different special pair chlorophylls. Proc Natl Acad Sci USA. 2010;107:3924–3929. doi: 10.1073/pnas.0913460107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenderlein M, Cetin M, Barber J, Telfer A, Schlodder E. Spectroscopic studies of the chlorophyll d containing photosystem I from the cyanobacterium, Acaryochloris marina. Biochim Biophys Acta. 2008;1777:1400–1408. doi: 10.1016/j.bbabio.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Krieger A, Rutherford AW, Johnson GN. On the determination of redox midpoint potential of the primary quinone electron acceptor, QA, in photosystem II. Biochim Biophys Acta. 1995;1229:193–201. [Google Scholar]
- 15.Krieger-Liszkay A, Rutherford AW. Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: Relevance to photodamage and phytotoxicity. Biochemistry. 1998;37:17339–17344. doi: 10.1021/bi9822628. [DOI] [PubMed] [Google Scholar]
- 16.Shibamoto T, et al. Species-dependence of the redox potential of the primary quinone electron acceptor QA in photosystem II verified by spectroelectrochemistry. FEBS Lett. 2010;584:1526–1530. doi: 10.1016/j.febslet.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Baranov SV, et al. Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry. 2004;43:2070–2079. doi: 10.1021/bi034858n. [DOI] [PubMed] [Google Scholar]
- 18.Nagata T, Zharmukhamedov SK, Khorobrykh AA, Klimov VV, Allakhverdiev SI. Reconstitution of the water-oxidizing complex in manganese-depleted photosystem II preparations using synthetic Mn complexes: A fluorine-19 NMR study of the reconstitution process. Photosynth Res. 2008;98:277–284. doi: 10.1007/s11120-008-9319-9. [DOI] [PubMed] [Google Scholar]
- 19.Allakhverdiev SI, Yruela I, Picorel R, Klimov VV. Bicarbonate is an essential constituent of the water-oxidizing complex of photosystem II. Proc Natl Acad Sci USA. 1997;94:5050–5054. doi: 10.1073/pnas.94.10.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananyev GM, Murphy A, Abe Y, Dismukes GC. Remarkable affinity and selectivity for Cs+ and uranyl (UO22+) binding to the manganese site of the apo-water oxidation complex of photosystem II. Biochemistry. 1999;38:7200–7209. doi: 10.1021/bi990023u. [DOI] [PubMed] [Google Scholar]
- 21.Baranov SV, Ananyev GM, Klimov VV, Dismukes GC. Bicarbonate accelerates assembly of the inorganic core of the water-oxidizing complex in manganese-depleted photosystem II: A proposed biogeochemical role for atmospheric carbon dioxide in oxygenic photosynthesis. Biochemistry. 2000;39:6060–6065. doi: 10.1021/bi992682c. [DOI] [PubMed] [Google Scholar]
- 22.Kozlov YN, et al. Oxidation potentials and electron donation to photosystem II of manganese complexes containing bicarbonate and carboxylate ligands. Phys Chem Chem Phys. 2004;6:4905–4911. [Google Scholar]
- 23.Shutova T, et al. The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J. 2008;27:782–791. doi: 10.1038/emboj.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klimov VV, Baranov SV, Allakhverdiev SI. Bicarbonate protects the donor side of photosystem II against photoinhibition and thermoinactivation. FEBS Lett. 1997;418:243–246. doi: 10.1016/s0014-5793(97)01392-6. [DOI] [PubMed] [Google Scholar]
- 25.Shibamoto T, Kato Y, Sugiura M, Watanabe T. Redox potential of the primary plastoquinone electron acceptor Q(A) in photosystem II from Thermosynechococcus elongatus determined by spectroelectrochemistry. Biochemistry. 2009;48:10682–10684. doi: 10.1021/bi901691j. [DOI] [PubMed] [Google Scholar]
- 26.Rappaport F, Guergova-Kuras M, Nixon PJ, Diner BA, Lavergne J. Kinetics and pathways of charge recombination in photosystem II. Biochemistry. 2002;41:8518–8527. doi: 10.1021/bi025725p. [DOI] [PubMed] [Google Scholar]
- 27.Grabolle M, Dau H. Energetics of primary and secondary electron transfer in Photosystem II membrane particles of spinach revisited on basis of recombination-fluorescence measurements. Biochim Biophys Acta. 2005;1708:209–218. doi: 10.1016/j.bbabio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, et al. The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina. Photochem Photobiol Sci. 2005;4:1060–1064. doi: 10.1039/b507057k. [DOI] [PubMed] [Google Scholar]
- 29.Itoh S, et al. Function of chlorophyll d in reaction centers of photosystems I and II of the oxygenic photosynthesis of Acaryochloris marina. Biochemistry. 2007;46:12473–12481. doi: 10.1021/bi7008085. [DOI] [PubMed] [Google Scholar]
- 30.Schlodder E, et al. Both chlorophylls a and d are essential for the photochemistry in photosystem II of the cyanobacteria, Acaryochloris marina. Biochim Biophys Acta. 2007;1767:589–595. doi: 10.1016/j.bbabio.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Shevela D, Nöring B, Eckert H-J, Messinger J, Renger G. Characterization of the water oxidizing complex of photosystem II of the Chl d-containing cyanobacterium Acaryochloris marina via its reactivity towards endogenous electron donors and acceptors. Phys Chem Chem Phys. 2006;8:3460–3466. doi: 10.1039/b604389e. [DOI] [PubMed] [Google Scholar]
- 32.Tomo T, et al. Replacement of chlorophyll with di-vinyl chlorophyll in the antenna and reaction center complexes of the cyanobacterium Synechocystis sp. PCC 6803: Characterization of spectral and photochemical properties. Biochim Biophys Acta. 2009;1787:191–200. doi: 10.1016/j.bbabio.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Enami I, et al. Nearest neighbor relationships among constituent proteins of oxygen-evolving photosystem II membranes: Binding and function of the extrinsic 33 kDa protein. Biochim Biophys Acta. 1989;973:35–40. [Google Scholar]
- 34.Johnson GN, Boussac A, Rutherford AW. The origin of 40–50°C thermoluminescence bands in photosystem II. Biochim Biophys Acta. 1994;1184:85–92. [Google Scholar]
- 35.Dasgupta J, Tyryshkin AM, Baranov SV, Dismukes GC. Bicarbonate coordinates to Mn3+ during photo-assembly of the catalytic Mn4Ca core of photosynthetic water oxidation: EPR characterization. Appl Magn Reson. 2010;37:137–150. [Google Scholar]
- 36.Hays A-MA, Vassiliev IR, Golbeck JH, Debus RJ. Role of D1-His190 in proton-coupled electron transfer reactions in photosystem II: A chemical complementation study. Biochemistry. 1998;37:11352–11365. doi: 10.1021/bi980510u. [DOI] [PubMed] [Google Scholar]
- 37.Klimov VV, Allakhverdiev SI, Shuvalov VA, Krasnovsky AA. Effect of extraction and re-addition of manganese on light reactions of photosystem-II preparations. FEBS Lett. 1982;148:307–312. doi: 10.1016/0014-5793(82)80830-2. [DOI] [PubMed] [Google Scholar]
- 38.Papageorgiou GC, Fujimura Y, Murata N. Protection of the oxygen-evolving photosystem II complex by glycinebetaine. Biochim Biophys Acta. 1991;1057:361–366. [Google Scholar]
- 39.Stamatakis C, Papageorgiou GC. Stabilization of photosystem II particles isolated from the thermophilic cyanobacterium Phormidium laminosum with glycinebetaine and glycerol. Biochim Biophys Acta. 1993;1183:333–338. [Google Scholar]
- 40.Allakhverdiev SI, et al. Stabilization of oxygen evolution and primary electron transport reactions in photosystem II against heat stress with glycinebetaine and sucrose. J Photochem Photobiol B. 1996;34:149–157. doi: 10.1016/1011-1344(95)07276-4. [DOI] [PubMed] [Google Scholar]
- 41.Allakhverdiev SI, et al. Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J Plant Physiol. 2003;160:41–49. doi: 10.1078/0176-1617-00845. [DOI] [PubMed] [Google Scholar]
- 42.Park E-J, et al. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 2004;40:474–487. doi: 10.1111/j.1365-313X.2004.02237.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen THH, Murata N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011;34:1–20. doi: 10.1111/j.1365-3040.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- 44.Mayhew SG. The redox potential of dithionite and SO−2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem. 1978;85:535–547. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.