Abstract
It is well known that prokaryotic life can withstand extremes of temperature, pH, pressure, and radiation. Little is known about the proliferation of prokaryotic life under conditions of hyperacceleration attributable to extreme gravity, however. We found that living organisms can be surprisingly proliferative during hyperacceleration. In tests reported here, a variety of microorganisms, including Gram-negative Escherichia coli, Paracoccus denitrificans, and Shewanella amazonensis; Gram-positive Lactobacillus delbrueckii; and eukaryotic Saccharomyces cerevisiae, were cultured while being subjected to hyperaccelerative conditions. We observed and quantified robust cellular growth in these cultures across a wide range of hyperacceleration values. Most notably, the organisms P. denitrificans and E. coli were able to proliferate even at 403,627 × g. Analysis shows that the small size of prokaryotic cells is essential for their proliferation under conditions of hyperacceleration. Our results indicate that microorganisms cannot only survive during hyperacceleration but can display such robust proliferative behavior that the habitability of extraterrestrial environments must not be limited by gravity.
Keywords: astrobiology, extremophiles
The robustness of prokaryotic life to physical extremes of temperature, pH, pressure, and radiation is well known (1) and has led to their ubiquitous presence on Earth (2, 3). Resilience to physical extremes is also extremely likely to be required for the existence of life beyond this planet (1, 4). Finding extraterrestrial life is a major motivation driving searches for extrasolar planets (5); thus, understanding the physical limits for known organisms is crucial in evaluating the probability that such planets harbor life (1, 4). Assessing the habitability of extraterrestrial environments requires an expanded set of criteria involving factors that can be ignored for terrestrial environments. The effect that extremes of gravity have on organisms is one such factor to consider when exploring for life beyond Earth.
The effect of microgravity on biological processes has been an active area of research particularly because it is relevant to human health during space flight (6, 7). Microorganisms make ideal model life forms for microgravity research because they are lightweight, small, and relatively easy to handle in space and have short generation times (7). Consequently, numerous experiments have been performed on microorganisms both in orbit and in Earth-based clinostats that simulate microgravity. The results demonstrate that microgravity affects microorganisms in a wide variety of ways related to their growth, physiology, pathogenesis, stress resistance, and gene expression (7–23).
The majority of these studies indicate that microgravity stimulates the growth of microorganisms (e.g., Salmonella enterica serovar Typhimurium, Bacillus subtilis, Escherichia coli) compared with 1 × g controls (8–17). In the case of E. coli, for example, the lag phase was shortened, the duration of exponential growth was increased, and the final cell population density was approximately doubled during space flights (13). Simulated microgravity can also affect the secondary metabolism of microorganisms. For example, production of β-lactam antibiotics by Streptomyces clavuligerus, production of rapamycin by Streptomyces hygroscopicus, and production of microcin B17 by E. coli were suppressed during culturing in simulated microgravity, whereas production of gramicidin S by Bacillus brevis was unaffected (14, 18). S. enterica serovar Typhimurium showed enhanced virulence in a murine infection model (19, 20) conducted in space flight and under modeled microgravity compared with conditions of normal gravity (19, 20). These microorganisms also showed increased resistance to environmental stresses, increased survival in macrophages, and significant changes in protein expression levels (19). To elucidate the molecular mechanisms of microbial responses to microgravity, 2D gel electrophoresis and DNA microarray analysis have been used (19–23). Recent analysis of S. enterica serovar Typhimurium grown in space identified 167 transcripts and 73 proteins that changed expression compared with ground controls, and conserved RNA-binding protein Hfq was identified as a likely global regulator (20). Gene expression of eukaryotic Saccharomyces cerevisiae is also affected by simulated microgravity (22, 23).
Compared with the relatively active research on microbial responses to microgravity, there are fewer studies that report experiments on microorganisms exposed to gravities greater than 1 × g (11, 16, 24–28). Unlike in microgravity, experiments in hypergravity were performed exclusively in simulated environments and primarily by subjecting microorganisms to centrifugal acceleration in centrifuges. Bouloc and D'Ari (11) reported that hyperaccelerations of 3 and 5 × g did not affect the growth of E. coli, whereas Brown et al. (16) observed growth suppression at 50 × g. Similar observations were reported for Paramecium tetraurelia, which showed no effect at 10 × g but a significantly lower proliferation rate and a lower population density at 20 × g (24).
At hyperaccelerations much greater than ~102 × g, the effect of sedimentation on microbial cells becomes significant. In a typical example, cultures of bacterial cells subjected to centrifugation at 3,000–5,000 × g for 5–10 min yielded pellets of intact bacterial cells (29). If microbial growth had occurred under these (or similar) conditions, it must have happened within or on the pellet. In stark contrast, the effect of cellular sedimentation is not very significant at lower accelerations, where growth can occur planktonically. Studying microbial proliferation, and not simply survival, at such hyperaccelerations addresses the fundamental biological question of what are the physical limits of organismic viability (1) under a range of gravitational accelerations larger than those found on Earth. Understanding the gravity limits for microorganism growth has important implications in considering the emergence, transport, adaptation, and evolution of life in extraterrestrial habitats (4, 30).
Previous studies that dealt with microorganisms under accelerations much greater than ~102 × g focused mostly on survival, however. Spores of B. subtilis tolerate accelerations exceeding 10,000–15,000 × g for indefinite periods of time but were inactivated to a 10% survival rate when they were subjected for 65 h to 436,000 × g (25, 26). Inactivation of various microorganisms, including prokaryotic E. coli, Thiobacillus intermedius, Bacillus amyloliquefaciens, and Staphylococcus aureus as well as eukaryotic S. cerevisiae, was also studied after they were subjected to 450,000 × g (27). These studies were done in phosphate-buffered or physiological saline at 4 °C, wherein microbial proliferation was not possible even at 1 × g because of the lack of nutrients and low temperature.
To our knowledge, the only study that has dealt with the proliferation of microorganisms under hyperaccelerative conditions was that of Montgomery et al. (28). In their experiments, E. coli suspended in nutrient broth at 35 °C was subjected to centrifugation at 1,000 or 110,000 × g for 24 h (28). They reported that the growth pattern of E. coli was not altered at 1,000 × g. They found that growth was disturbed at 110,000 × g, however. This was characterized by an increased duration of the lag phase, prolonged generation time, and decreased maximal cell concentration compared with 1 × g controls. The study of proliferation of microorganisms under hyperaccelerations much greater than ~100 × g still remained largely unexplored, however (1). Here, we report that microorganisms can grow surprisingly well under hyperaccelerations and are able to proliferate even at 403,627 × g.
Results
Growth of Microorganisms Under Hyperaccelerations.
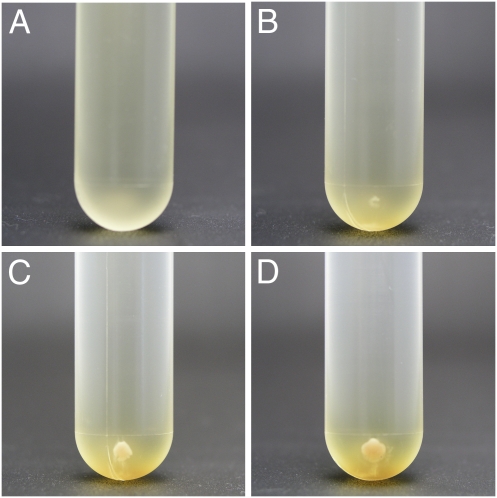
A variety of microorganisms were cultured in nutrient media under hyperaccelerations in centrifuges. The microorganisms studied here include Gram-negative Paracoccus denitrificans, E. coli, and Shewanella amazonensis; Gram-positive Lactobacillus delbrueckii subsp. delbrueckii; and eukaryotic S. cerevisiae. Fig. 1 A–D shows photographs of cultures of P. denitrificans in LB broth containing 25 mM KNO3 after spinning in an ultracentrifuge at 403,627 × g and at 30 °C. At this acceleration, P. denitrificans cells sedimented and formed a pellet at the bottom of a centrifuge tube soon after centrifugation began. Initially, a pellet was not visible (Fig. 1A) because the total number of P. denitrificans cells in the culture was small (~106 cells). A pellet of a visible size formed after spinning the culture for 6 h (Fig. 1B), however, and it increased in size with time (Fig. 1 C and D). The observation demonstrates unambiguously that P. denitrificans can proliferate even at 403,627 × g.
Fig. 1.
Growth of P. denitrificans at 403,627 × g. Photographs of pellet of P. denitrificans cells after incubation at 403,627 × g and 30 °C for 0 h (A), 6 h (B), 24 h (C), and 48 h (D). The outer diameter of the tube is 18 mm.
Growth Characteristics of Microorganisms at Hyperaccelerations.
To quantify the effect of hyperacceleration on growth characteristics of various microorganisms, growth curves were followed (Fig. 2). Pellets of microorganisms formed after incubation at hyperaccelerations were redispersed on a vortex mixer, and the number of live cells was counted by the spread plate technique.
Fig. 2.
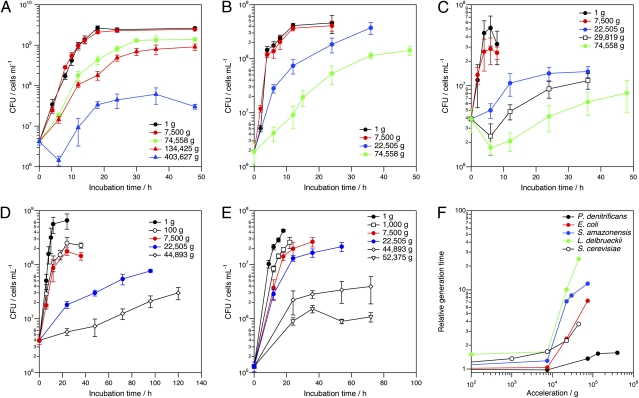
Growth of various microorganisms under hyperaccelerations. (A) Growth curves of P. denitrificans at 30 °C and hyperaccelerations up to 403,627 × g. (B) Growth of E. coli in LB broth at 37 °C and hyperaccelerations up to 74,558 × g. (C) Growth of S. amazonensis in LB broth at 37 °C and hyperaccelerations up to 74,558 × g. (D) Growth of L. delbrueckii subsp. delbrueckii in MRS broth at 37 °C and hyperaccelerations up to 30,000 × g. (E) Growth of S. cerevisiae in yeast extract-peptone-dextrose broth at 30 °C and hyperaccelerations up to 74,558 × g. (F) Change in grel of various microorganisms as a function of acceleration.
The growth curves of P. denitrificans at 1 and 7,500 × g were identical within experimental error, indicating that hyperacceleration up to 7,500 × g did not affect the growth of P. denitrificans at all. The growth was slightly retarded when the incubation acceleration was increased to 74,558 and 134,425 × g, as judged by the slight decreases in the respective final cell concentrations. At 403,627 × g, a distinct lag phase appeared and the growth was retarded significantly. The final cell concentration was significantly smaller at this acceleration compared with those at lower values. The results again confirm the growth of P. denitrificans under a range of hyperaccelerations, although growth is retarded above 74,558 × g.
Growth of E. coli under hyperaccelerations showed similar trends to that of P. denitrificans. The growth curve at 7,500 × g was identical to that at 1 × g. This observation is consistent with a previous study in which the authors reported no difference in the growth of E. coli at either 1 or 1,000 × g (28). We observed that growth was retarded at 22,505 × g but measured a final cell concentration identical to that at 1 × g. At 74,558 × g, the growth was further retarded and the final cell concentration was lower. Formation of a macroscopic pellet was observed after incubation at 134,425 × g for 48 h (Fig. S1), again confirming growth and also consistent with the previously reported growth of E. coli at 110,000 × g (28). The cells were packed so tightly in the pellet that we were not able to redisperse them and determine the growth curve, however, whereas no such observation was described in the previous work (28). At 403,627 × g, E. coli formed a pellet of barely visible size even after incubation for 60 h (Fig. S2), suggesting that growth at this acceleration is highly suppressed. The cells in the pellet could not be redispersed either.
An additional Gram-negative microorganism, S. amazonensis, showed identical growth curves when cultured at 1 and 7,500 × g. At 22,505 × g, however, a lag phase appeared, growth was retarded, and final cell concentrations became lower relative to those at 1 × g. These effects on growth became more significant as the incubation acceleration was increased beyond 22,505 × g.
We found that growth of Gram-positive L. delbrueckii subsp. delbrueckii was very sensitive to hyperacceleration. The final cell concentration became noticeably lower even at 100 × g, and the growth rate became slightly smaller. Growth at 7,500 × g was identical to that at 100 × g. Very significant growth retardation was observed at 22,505 and 44,893 × g, but we did not observe any distinct lag phase.
Growth of S. cerevisiae, the only eukaryote studied here, was also rather sensitive to hyperaccelerations, with evidence of growth retardation observed at 1,000 × g. For S. cerevisiae, the retardation effect on growth gradually magnified up to 22,505 × g. Very significant growth retardation was observed at 44,893 and 52,375 × g, including significantly lower final cell concentrations compared with 1 × g. A visible pellet did not form at 74,558 × g, indicating that growth was completely suppressed at this acceleration.
Generation Time of Microorganisms at Hyperaccelerations.
To analyze the growth characteristics of different microorganisms under hyperaccelerations further, we obtained generation times from the observed growth curves. To make the comparison between different microorganisms easier, we calculated relative generation time (grel), which is defined as
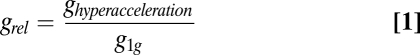 |
where ghyperacceleration and g1g are generation times of a given organism under hyperaccelerations and at 1 × g, respectively. The results are summarized in Fig. 2F.
For all prokaryotes tested here (i.e., both Gram-negative and Gram-positive bacteria), hyperacceleration had either no effect or a relatively small effect on growth below 7,500 × g, whereas growth was significantly retarded above ~2 × 104 × g. Among all prokaryotes tested here, the effect of hyperacceleration on growth was lowest for P. denitrificans. For the eukaryotic organism S. cerevisiae, the growth behavior observed here stands in contrast to that of prokaryotes in that the generation time became progressively longer with increased acceleration, particularly above 44,893 × g.
Possible Effect of Hyperacceleration on Microbial Growth.
To understand how hyperacceleration affects microbial growth, we considered three aspects that differentiate the growth of microorganisms under hyperacceleration from that at 1 × g. These are sedimentation, mechanical deformation, and hydrostatic pressure.
Effect of Sedimentation.
Under conditions of hyperacceleration, microbial cells grew in sedimented congested pellets. The density of cells within these pellets increased as gravity increased, leading to relatively smaller void volumes between cells in pellets formed at relatively high acceleration. Therefore, diffusion rates of small molecules in these pellets became progressively slower because of an increasing obstruction effect. Consequently, nutrient uptake and waste disposal by cells in the growing pellets were inhibited, thereby affecting the growth rates.
Conversely, differential sedimentation of subcellular moieties and the formation of other molecular concentration gradients within cells may also negatively affect growth under hyperaccelerative conditions. By analogy, centrifugation at 105 × g is routinely used for sedimentation, and thus separation of relatively large particulates like ribosomes from the molecular milieu of cell lysates (31). In this study, a distinct concentration gradient of nutrient components in LB broth was observed when the culture of P. denitrificans was spun at 403,627 × g for longer than 6 h (Fig. 1 B–D). Yellow-colored components formed a sediment at the bottom of the centrifuge tube, leaving a colorless supernatant. A similar concentration gradient of cytoplasmic components may form within microbial cells at hyperaccelerations.
The concentration gradient of a particle subject to gravitational force at a sedimentation equilibrium is described by (32)
where m and V are the mass and the volume of the particle, ρm is the density of the medium, kB is the Boltzmann constant, T is the absolute temperature, and z is the particle position relative to the bottom. We calculated concentration gradients of model proteins of various molecular masses (1 kDa, 10 kDa, 100 kDa, and 1 MDa) at 500,000 × g and 30 °C over a distance of 10 μm (Fig. 3A) within a model cytosol. Volumes of the respective proteins were calculated using an average value of the partial specific volumes of most proteins (0.73 cm3⋅g−1) (33). A value of 1.1 g⋅cm−3 was used as an average representative density of the model cytosol (34).
Fig. 3.
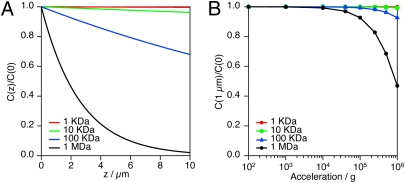
Concentration gradient of model proteins within a cell. (A) Calculated concentration gradients of model proteins of different molecular masses (1 kDa, 10 kDa, 100 kDa, and 1 MDa) at the sedimentation equilibrium at 500,000 × g and 30 °C over a distance of 10 μm. (B) Sedimentation of model proteins of different molecular masses over a distance of 1 μm as a function of acceleration.
If we consider sedimentation over 1 μm, which is a typical size for prokaryotic cells, the calculation shows that sedimentation is negligible for relatively small proteins having molecular masses of 1, 10, and 100 kDa. Significantly, even the 100-kDa protein had a concentration at the top of the cell (z = 1 μm) that was 96% of the value at the bottom (z = 0 μm). In contrast, a very profound effect of sedimentation was calculated for the 1-MDa protein, where the concentration at the top was 69% of that at the bottom. These analyses indicate that intracellular sedimentation of large molecular complexes [e.g., bacterial 70S ribosome, a large nucleoprotein complex with a molecular mass of ~2.5 MDa (35)] may take place at 500,000 × g, thereby affecting relevant cellular processes.
Very different results were obtained when we consider sedimentation over a distance of 10 μm, which is a characteristic size for eukaryotic cells. In this case, a profound sedimentation effect was calculated even for 100-kDa proteins. Thus, intracellular sedimentation effects may be more significant in the nearly spherical cells of S. cerevisiae (ca. 8 μm in diameter) (36) compared with smaller prokaryotes. These results strongly indicate that the size of cells must be small, like that of prokaryotes, to be resistant to the formation of intracellular concentration gradients and molecular sedimentation under hyperaccelerations.
Fig. 3B shows the ratio of the concentration of hypothetical proteins at z = 1 μm to that at z = 0 as a function of acceleration. Hyperaccelerations up to 106 × g appear not to have induced a sedimentation effect on model proteins with masses of 1 and 10 kDa. The sedimentation became noticeable at ~105 × g for a 100-kDa protein and at ~104 × g for a 1-MDa protein. It is interesting to point out that the very significant retardation of growth by hyperacceleration was also observed above ~104 × g for all the prokaryotic microorganisms studied (Fig. 2F). The similarity between these two results suggests that sedimentation of large molecular complexes, such as ribosomes, may be one reason for growth retardation under hyperaccelerations above ~104 × g.
The generation time of eukaryotic S. cerevisiae showed different acceleration dependence compared with the prokaryotes (Fig. 2F). The difference could be attributed, in part, to the larger cell size of S. cerevisiae, which affects the sedimentation of proteins (Fig. 3A). In eukaryotic cells, however, sedimentation of their organelles is highly likely because these are significantly larger than either proteins or ribosomal complexes. Nuclei and mitochondria of S. cerevisiae, for example, can be pelleted easily by centrifugation at 600 × g for 5 min and at 10,000 × g for 2 min, respectively (37). Thus, during growth of S. cerevisiae at hyperaccelerations above 10,000 × g, these organelles could not have been distributed homogeneously in the cytoplasm as they are at 1 × g. Rather, they should have separated and clustered at the bottom of the cell. Although the effect was not lethal enough to suppress the growth completely, organellar sedimentation and clustering should have affected and retarded the cell growth.
Mechanical Deformation of Microbial Cells Under Hyperaccelerations.
Macroorganisms easily collapse under accelerations of only a few times that of Earth's surface gravity (25). In contrast, microorganisms are likely to show higher resistance to mechanical deformation under hyperaccelerations because the gravitational potential is proportional to the size of an object. Indeed, Yoshida et al. (27) reported no change in the cell shape for E. coli and B. amyloliquefaciens after ultracentrifugation in physiological saline at 450,000 × g and 4 °C for 24 h. The effect of mechanical deformation on cells that replicate via binary fission at hyperacceleration was not known, however.
To evaluate the effect of hyperacceleration on cell morphology, P. denitrificans cells incubated at 30 °C for 48 h at both 1 and 134,425 × g were imaged by transmission electron microscopy. Distributions of cell length, width, and aspect ratio were then measured using image analysis software (Fig. 4).
Fig. 4.
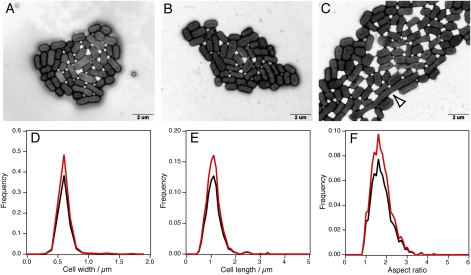
P. denitrificans cells after incubation at hyperaccelerations. Transmission electron microscopy images of P. denitrificans cells after incubation at 30 °C and at 1 × g for 4 h (A) and 134,425 × g for 48 h (B and C). (C) Occasionally, highly elongated cells were observed when incubated at 134,425 × g (white arrowhead). (Scale bar: 2 μm.) (D–F) Size distribution of P. denitrificans cells after incubation at 1 × g (black) and 134,425 × g (red).
Comparison of the images (Fig. 4 A and B) and histograms (Fig. 4 D–I) shows no significant differences in the morphology of both sets of cells. Occasionally, highly elongated cells were observed when incubated at 134,425 × g (indicated by a white arrowhead in Fig. 4C), but the population of such cells was minor, comprising less than 1% of the cells examined. These results suggest that prokaryotic cells are small enough (~1 μm) that irreversible mechanical deformation attributable to gravity is not significant.
Effect of Hydrostatic Pressure on Growth of Microbial Cells Under Hyperaccelerations.
Microbial cells in the pellet at the bottom of the centrifuge tube were subjected to a hydrostatic pressure, Pcentrifuge, because of the weight of the water column above the pellet. Pcentrifuge is negligibly small at 1 × g but increases linearly with acceleration and becomes significant at hyperaccelerations. Pcentrifuge is estimated to be 0.1 MPa at 100 × g, 0.6 MPa at 1,000 × g, 3.6 MPa at 7,500 × g, 10.3 MPa at 22,505 × g, 13.6 MPa at 29,819 × g, 20.4 MPa at 44,893 × g, 23.7 MPa at 52,375 × g, 33.8 MPa at 74,558 × g, 42.2 MPa at 134,425 × g, and 126.5 MPa at 403,627 × g (SI Materials and Methods, Fig. S3, Table S1).
For most mesophilic microorganisms, cell division is not affected at hydrostatic pressures below ~20 MPa (38). For example, the colony-forming ability of E. coli is suppressed only when it is cultured under hydrostatic pressures above 40 MPa (39). Thus, it is unlikely that hydrostatic pressure at hyperaccelerations up to 52,375 × g plays a dominant role in the growth retardation under hyperaccelerations above ~104 × g (Fig. 2F).
Hydrostatic pressure may have had an impact on the growth of P. denitrificans above 74,558 × g, where hydrostatic pressure is estimated to be higher than 33.8 MPa. Very interestingly, we found that the growth of P. denitrificans was inhibited completely above 40 MPa (Fig. S4), suggesting that the observed growth here should not have occurred above 134,425 × g. Although the reason for this discrepancy is not clear at present, one possibility is attributable to the compositional gradient in the medium induced during sedimentation. As can be seen in Fig. 1 B–D, high-molecular-weight protein molecules may be advantageously accumulated at the bottom of the centrifuge tube along with P. denitrificans, providing local nutrients for growth. The discrepancy may also be ascribed to the difference in cell density during growth at elevated pressures compared with hyperaccelerations. P. denitrificans grew planktonically when it was cultured at elevated pressures, whereas the growth occurred in a densely packed pellet at hyperaccelerations. The pressure effect on cellular processes depends on cell density in some cases (40). Human dermal fibroblasts undergo a significant morphological change and become rounded when they are subjected to 70 MPa at a low cell density (subconfluence). No such change is observed when they are pressurized at a high cell density (full confluence), however. Higher cell density at hyperacceleration may therefore offset the pressure effect to some extent.
Discussion
Our results clearly demonstrate that microorganisms not only survive at hyperacceleration but can grow by binary fission, producing viable cells. All microorganisms studied here displayed growth in culture at hyperaccelerations up to ~2 × 104 × g. Because of the instrumental limitation of our ultracentrifuge, we did not determine the upper limit of acceleration for the growth of the most tolerant organisms. We did observe the proliferation of P. denitrificans and E. coli even at 403,627 × g, however. We argue that this latter finding is significant when trying to understand the limits of hypergravity tolerance for our version of carbon-based life. Because the list of bacteria and yeast here is a short one, we anticipate that further accumulation of experimental data on various microorganisms will result in discoveries of previously undescribed species that expand the range of habitable accelerations.
In an attempt to understand our various observations of microbial growth under hyperaccelerative conditions, we considered three most obvious effects, namely, sedimentation of cells, mechanical deformation of membranes, and hydrostatic pressure. Most notably, our analysis did not explain why some species are more tolerant. For example, we do not explain why only P. denitrificans and E. coli showed growth at 403,627 × g, whereas other species did not. This likely implies that species-specific biochemical processes led to the differential sensitivities that were observed. Our experiments were aimed at gaining an improved understanding of the physical effects of hyperaccelerative conditions on microbial cells rather than at elucidating subtle details of the biological processes likely affected by gravity, however (6, 41, 42).
Nonetheless, we briefly consider some biological processes in the hypergravity environment. Previous studies reported that the physical effects of microgravity can be sensed by cell surfaces and transformed into biochemical responses (7). Cytoskeletal proteins and their polymerized superstructures, for example, may play important roles in gravity sensing of mammalian cells (43), and possibly of microbial cells (7). It has also been suggested that mechanical changes of cell membranes in microgravity conditions are sensed by mechanosensitive channels (7). Under conditions of hyperacceleration, congestive packing and jamming of cells within microbial pellets would likely induce critical changes in the curvature and/or internal bilayer stress within the microbial cell membranes. Consequently, changes in the conformation of various sensor proteins could then lead to direct cellular and quorum responses (of various kinds) to the hypergravity field.
Our findings compliment previous studies that primarily focused on hypogravity and relatively mild hypergravity by extending the range of studies well into the hypergravity regime. Especially relevant in this regard are our findings on the hypergravity tolerance of E. coli and several other microorganisms. Here, the growth of E. coli was not affected at all by hyperaccelerations up to 7,500 × g and we even observed growth at 403,627 × g, although it was significantly retarded. This range is three orders of magnitude wider than most of the previous studies (10–16).
From a practical perspective, it has been suggested that altering the accelerative environment could be used to manipulate bacterial fermentation processes (44) via production and localization of microbial metabolites known to be affected by microgravity (14, 18). It is likely that hyperaccelerative conditions may also be used to induce unique metabolite production in growing cultures. For example, the suppressed biosynthesis of antibiotics reported under microgravity conditions compared with 1 × g controls (14, 18) may be enhanced at elevated gravities.
Exploring the physical limits of organismic viability is crucial in the search for life in extraterrestrial habitats, because the knowledge effectively helps in narrowing down possible targets to search (4). This present study expands the limits for life into the hypergravity regime, where this had not been seriously considered before (1). We propose that this has significant implications for astrobiology. For example, microorganisms subjected to hyperaccelerations on the order of 105 × g have attracted scientific attention in terms of bacterial transport between planets (panspermia). The hypothesized process begins with an asteroidal impact on a donating planet followed by consequent ejection of bacteria-bearing rocks (30). Under impact conditions, ejected rocks typically experience maximum accelerations of 3 × 105 × g and rise times of 0.5 ms in the case of (ejection from) Mars (26). Bacteria have to survive extremes in both acceleration and rate of change of acceleration (25). Dormant spores of B. subtilis are inactivated when they are subjected to ~105 × g (26). The inactivation follows first-order kinetics and decreases exponentially with exposure time, however (26). Our results show that hyperacceleration of ~105 × g is within a habitable range for some microorganisms. This significantly enhances the evidence that bacteria can remain robustly viable after asteroidal impact-style ejection.
Most significantly, our finding also extends the possibility of life beyond planets to massive substellar objects like brown dwarfs, the coldest of which has an effective surface temperature of ~400 K (45), which is extremely close in value to the known upper temperature limit for life (395 K) (46). The relatively strong gravitational fields associated with brown dwarfs are one of several limiting factors in considering existence of life on brown dwarfs (47). Our results unambiguously show that the ~10–102 × g gravitational fields existent on relatively cold (~600 K) brown dwarfs (48) must not be a primary limiting factor in assessing their potential for harboring life as we know it.
Materials and Methods
Microbial Culture Under Hyperaccelerations.
P. denitrificans ATCC17741T (American Type Culture Collection) was incubated in LB broth containing 25 mM KNO3 at 30 °C. E. coli W3110 and S. amazonensis ATCC 700329T (American Type Culture Collection) were incubated in LB broth at 37 °C. L. delbrueckii subsp. delbrueckii was incubated in de Man, Rogosa, and Sharpe (MRS) broth (Difco) at 37 °C. S. cerevisiae YPH499 was incubated in yeast extract-peptone-dextrose broth at 30 °C. Detailed culture conditions are available in SI Materials and Methods.
Incubation of microorganisms under hyperaccelerative conditions was performed using either an EIX-135 centrifuge (TOMY Seiko) for incubation below 7,500 × g or an XL-80 ultracentrifuge (Beckman Instruments) for incubation above 22,505 × g. The accelerations reported are the maximum values achieved at the bottom of the respective centrifuge tubes in which microbial growth occurred. Control experiments at 1 × g were performed in stationary culture.
Determination of Growth Curves.
Immediately after incubation, the centrifuge tubes were taken out and cooled in an ice bath. The microbial cells were dispersed on a vortex mixer. The culture was diluted with physiological saline to ~105 cells/mL and spread on agar plates containing the same culture media used for culture under hyperaccelerative conditions, with the exception of P. denitrificans, for which KNO3-free LB broth was used. The number of colonies formed was enumerated after incubation at 1 × g and at the same temperature used for incubation at hyperaccelerations.
Cell Size Measurements.
P. denitrificans was cultured at 30 °C and 134,425 × g for 48 h. After incubation, P. denitrificans cells were fixed with glutaraldehyde, negatively stained with phosphotungstic acid, and examined on a JEOL JEM-1210 (JEOL, Ltd.). Cell dimensions were measured using “analySIS” (Soft Imaging System, GmbH). Measurements were performed on 1,460 and 1,155 cells for 1 × g and 134,425 × g, respectively.
Supplementary Material
Acknowledgments
Katsuyuki Uematsu and Tomoko Takahashi are acknowledged for assistance in transmission electron microscopy observations. We thank Kanya Kusano and Yasushi Suto for discussions and Sandra Carpenter for editing assistance. S.M. is supported by the Program for Improvement of Research Environment for Young Researchers from Special Coordination Funds for Promoting Science and Technology, Japan. H.S. acknowledges Ron Usami, Toyo University, for support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
2On leave from: Graduate School of Interdisciplinary New Science, Toyo University, 2100 Kujirai, Kawagoe 350-0815, Japan.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018027108/-/DCSupplemental.
References
- 1.Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- 2.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: The unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schleifer K-H. Microbial diversity: Facts, problems and prospects. Syst Appl Microbiol. 2004;27:3–9. doi: 10.1078/0723-2020-00245. [DOI] [PubMed] [Google Scholar]
- 4.Des Marais DJ, et al. The NASA astrobiology roadmap. Astrobiology. 2008;8:715–730. doi: 10.1089/ast.2008.0819. [DOI] [PubMed] [Google Scholar]
- 5.Wolszczan A. Confirmation of earth-mass planets orbiting the millisecond pulsar PSR B1257+12. Science. 1994;264:538–542. doi: 10.1126/science.264.5158.538. [DOI] [PubMed] [Google Scholar]
- 6.Todd P. Gravity-dependent phenomena at the scale of the single cell. ASGSB Bull. 1989;2:95–113. [PubMed] [Google Scholar]
- 7.Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, Pierson DL. Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev. 2004;68:345–361. doi: 10.1128/MMBR.68.2.345-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor GR. Space microbiology. Annu Rev Microbiol. 1974;28:121–137. doi: 10.1146/annurev.mi.28.100174.001005. [DOI] [PubMed] [Google Scholar]
- 9.Mennigmann HD, Lange M. Growth and differentiation of Bacillus subtilis under microgravity. Naturwissenschaften. 1986;73:415–417. doi: 10.1007/BF00367283. [DOI] [PubMed] [Google Scholar]
- 10.Ciferri O, Tiboni O, Di Pasquale G, Orlandoni AM, Marchesi ML. Effects of microgravity on genetic recombination in Escherichia coli. Naturwissenschaften. 1986;73:418–421. doi: 10.1007/BF00367284. [DOI] [PubMed] [Google Scholar]
- 11.Bouloc P, D'Ari R. Escherichia coli metabolism in space. J Gen Microbiol. 1991;137:2839–2843. doi: 10.1099/00221287-137-12-2839. [DOI] [PubMed] [Google Scholar]
- 12.Gasset G, et al. Growth and division of Escherichia coli under microgravity conditions. Res Microbiol. 1994;145:111–120. doi: 10.1016/0923-2508(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 13.Klaus D, Simske S, Todd P, Stodieck L. Investigation of space flight effects on Escherichia coli and a proposed model of underlying physical mechanisms. Microbiology. 1997;143:449–455. doi: 10.1099/00221287-143-2-449. [DOI] [PubMed] [Google Scholar]
- 14.Fang A, Pierson DL, Koenig DW, Mishra SK, Demain AL. Effect of simulated microgravity and shear stress on microcin B17 production by Escherichia coli and on its excretion into the medium. Appl Environ Microbiol. 1997;63:4090–4092. doi: 10.1128/aem.63.10.4090-4092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kacena MA, et al. Bacterial growth in space flight: Logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl Microbiol Biotechnol. 1999;51:229–234. doi: 10.1007/s002530051386. [DOI] [PubMed] [Google Scholar]
- 16.Brown RB, Klaus D, Todd P. Effects of space flight, clinorotation, and centrifugation on the substrate utilization efficiency of E. coli. Microgravity Sci Technol. 2002;13:24–29. doi: 10.1007/BF02881678. [DOI] [PubMed] [Google Scholar]
- 17.Wilson JW, et al. Low-Shear modeled microgravity alters the Salmonella enterica serovar typhimurium stress response in an RpoS-independent manner. Appl Environ Microbiol. 2002;68:5408–5416. doi: 10.1128/AEM.68.11.5408-5416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demain AL, Fang A. Secondary metabolism in simulated microgravity. Chem Rec. 2001;1:333–346. doi: 10.1002/tcr.1018. [DOI] [PubMed] [Google Scholar]
- 19.Nickerson CA, et al. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect Immun. 2000;68:3147–3152. doi: 10.1128/iai.68.6.3147-3152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson JW, et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc Natl Acad Sci USA. 2007;104:16299–16304. doi: 10.1073/pnas.0707155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JW, et al. Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. Proc Natl Acad Sci USA. 2002;99:13807–13812. doi: 10.1073/pnas.212387899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johanson K, et al. Saccharomyces cerevisiae gene expression changes during rotating wall vessel suspension culture. J Appl Physiol. 2002;93:2171–2180. doi: 10.1152/japplphysiol.01087.2001. [DOI] [PubMed] [Google Scholar]
- 23.Purevdorj-Gage B, Sheehan KB, Hyman LE. Effects of low-shear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl Environ Microbiol. 2006;72:4569–4575. doi: 10.1128/AEM.03050-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y, Mogami Y, Baba SA. Responses to hypergravity in proliferation of Paramecium tetraurelia. Zoolog Sci. 2003;20:1373–1380. doi: 10.2108/zsj.20.1373. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastrapa RME, Glanzberg H, Head JN, Melosh HJ, Nicholson WL. Survival of bacteria exposed to extreme acceleration: Implications for panspermia. Earth Planet Sci Lett. 2001;189:1–8. [Google Scholar]
- 27.Yoshida N, Minamimura T, Yoshida T, Ogawa K. Effect of hypergravitational stress on microbial cell viability. J Biosci Bioeng. 1999;88:342–344. doi: 10.1016/s1389-1723(00)80023-7. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery POB, Orden FV, Rosenblum E. A relationship between growth and gravity in bacteria. Aerosp Med. 1963;34:352–354. [Google Scholar]
- 29.Koval SF, Sprott GD. Cell fractionation. In: Reddy CA, et al., editors. Methods for General and Molecular Microbiology. Washington, DC.: ASM Press; 2007. pp. 108–137. [Google Scholar]
- 30.Fajardo-Cavazos P, Schuerger AC, Nicholson WL. Testing interplanetary transfer of bacteria between Earth and Mars as a result of natural impact phenomena and human spaceflight activities. Acta Astronaut. 2006;60:534–540. [Google Scholar]
- 31.Gold LM, Schweiger M. Synthesis of phage-specific alpha- and β-glucosyl transferases directed by T-even DNA in vitro. Proc Natl Acad Sci USA. 1969;62:892–898. doi: 10.1073/pnas.62.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson P. Biological Physics: Energy, Information, Life. New York: Freeman; 2004. [Google Scholar]
- 33.Voet D, Voet JG. Biochemistry. Hoboken, NJ: John Wiley & Sons; 2004. [DOI] [PubMed] [Google Scholar]
- 34.Loferer-Krössbacher M, Klima J, Psenner R. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl Environ Microbiol. 1998;64:688–694. doi: 10.1128/aem.64.2.688-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cate JH, Yusupov MM, Yusupova GZ, Earnest TN, Noller HF. X-ray crystal structures of 70S ribosome functional complexes. Science. 1999;285:2095–2104. doi: 10.1126/science.285.5436.2095. [DOI] [PubMed] [Google Scholar]
- 36.Madigan MT, Martinko JM, Parker J. Brock Biology of Microorganisms. 8th Ed. Upper Saddle River, NJ: Prentice Hall; 1997. [Google Scholar]
- 37.Diekert K, de Kroon AIPM, Kispal G, Lill R. Isolation and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Mitochondria, Methods in Cell Biology. In: Schon EA, Pon LA, editors. Vol. 65. San Diego: Academic; 2001. pp. 37–51. [DOI] [PubMed] [Google Scholar]
- 38.Abe F. Exploration of the effects of high hydrostatic pressure on microbial growth, physiology and survival: Perspectives from piezophysiology. Biosci Biotechnol Biochem. 2007;71:2347–2357. doi: 10.1271/bbb.70015. [DOI] [PubMed] [Google Scholar]
- 39.Ishii A, Sato T, Wachi M, Nagai K, Kato C. Effects of high hydrostatic pressure on bacterial cytoskeleton FtsZ polymers in vivo and in vitro. Microbiology. 2004;150:1965–1972. doi: 10.1099/mic.0.26962-0. [DOI] [PubMed] [Google Scholar]
- 40.Koyama S, Fujii S, Aizawa M. Post-transcriptional regulation of immunomodulatory cytokines production in human skin fibroblasts by intense mechanical stresses. J Biosci Bioeng. 2002;93:234–239. doi: 10.1263/jbb.93.234. [DOI] [PubMed] [Google Scholar]
- 41.Todd P. Gravity dependent processes and intracellular motion. ASGSB Bull. 1991;4:35–39. [PubMed] [Google Scholar]
- 42.Todd P, Klaus DM. Theories and models on the biology of cells in space. Adv Space Res. 1996;17:3–10. doi: 10.1016/0273-1177(95)00606-f. [DOI] [PubMed] [Google Scholar]
- 43.Ingber D. How cells (might) sense microgravity. FASEB J. 1999;13(Suppl):S3–S15. doi: 10.1096/fasebj.13.9001.s3. [DOI] [PubMed] [Google Scholar]
- 44.Klaus DM. Microgravity and its implication for fermentation biotechnology. Trends Biotechnol. 1998;16:369–373. doi: 10.1016/s0167-7799(98)01197-4. [DOI] [PubMed] [Google Scholar]
- 45.Eisenhardt PRM, et al. Ultracool field brown dwarf candidates selected at 4.5 μm. Astron J. 2010;139:2455–2464. [Google Scholar]
- 46.Takai K, et al. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulze-Makuch D, Irwin LN. Life in the Universe: Expectations and Constraints. Berlin: Springer; 2008. [Google Scholar]
- 48.Leggett SK, et al. The physical properties of four ~600 K T dwarfs. Astrophys J. 2009;695:1517–1526. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.