Abstract
Powdery mildew resistance gene Pm21, located on the chromosome 6V short arm of Haynaldia villosa and transferred to wheat as a 6VS·6AL translocation (T6VS·6AL), confers durable and broad-spectrum resistance to wheat powdery mildew. Pm21 has become a key gene resource for powdery mildew resistance breeding all over the world. In China, 12 wheat varieties containing Pm21 have been planted on more than 3.4 million hectares since 2002. Pm21 has been intractable to molecular genetic mapping because the 6VS does not pair and recombine with the 6AS. Moreover, all known accessions of H. villosa are immune to powdery mildew fungus. Pm21 is still defined by cytogenetics as a locus. In the present study, a putative serine and threonine protein kinase gene Stpk-V was cloned and characterized with an integrative strategy of molecular and cytogenetic techniques. Stpk-V is located on the Pm21 locus. The results of a single cell transient expression assay showed that Stpk-V could decrease the haustorium index dramatically. After the Stpk-V was transformed into a susceptible wheat variety Yangmai158, the characterized transgenic plants showed high and broad-spectrum powdery mildew resistance similar to T6VS·6AL. Silencing of the Stpk-V by virus-induced gene silencing in both T6VS·6AL and H. villosa resulted in their increased susceptibility. Stpk-V could be induced by Bgt and exogenous H2O2, but it also mediated the increase of endogenous H2O2, leading to cell death and plant resistance when the plant was attacked by Bgt.
Keywords: gene cloning, microarray, transgenic wheat, disease resistance gene, hypersensitive response
Wheat is the most widely grown food crop in the world and ranks first in world crop production. It is the national staple food of 43 countries and feeds at least one third of the world's population (1). Currently, China is the biggest wheat producer and consumer in the world (2). Wheat powdery mildew, caused by Blumeria graminis f. sp. tritici (Bgt), is one of the most destructive diseases of wheat worldwide (3). Heavily infected seedlings gradually become yellow and dry, significantly affecting the efficiency of photosynthesis. The disease can usually lead to a 13% to 34% loss of yield, but loss can be as severe as 50% when the flag leaf becomes severely diseased during the heading and filling stages (4). To date, there have been 59 powdery mildew resistance alleles identified and designated at 43 loci (5), among which only the Pm3b and its alleles have been cloned (6, 7). Cloning of more genes which are highly resistant to the available powdery mildew pathogens is necessary for wheat breeding through biotechnological methods.
Race-specific resistance and broad-spectrum resistance (BSR) are two major types of disease resistance in plants. BSR refers to resistance against two or more types of pathogen species or the majority of races of the same pathogen species (8). Several BSR genes have been cloned, such as powdery mildew resistance gene mlo in barley (9) and leaf rust resistance gene Lr34 and stripe rust resistance gene Yr36 in wheat (10, 11). The mechanisms of action of these BSR genes are not characterized simply in a “gene-to-gene” model, and BSR, which cannot easily be overcome by newly developed pathogens, is usually correlated with durability. The broad spectrum and durability properties make BSR genes highly valuable in breeding programs.
Haynaldia villosa (Dasypyrum villosum, 2n = 2x = 14, VV) is a wild relative species of wheat. Pm21 from H. villosa has been identified to confer durable and BSR to Bgt worldwide since the late 1970s. The Pm21 gene was located to the short arm of chromosome 6V by the development of the wheat-H. villosa translocation line T6VS·6AL (12), and was then further localized to the 6VS bin [fraction length (FL) 0.45–0.58] with the construction of the resistant alien deletion line del6VS-1 (FL 0.58) (13) and susceptible alien deletion line del6VS-2 (FL 0.45) (14). It was reported in many other cases that the disease resistance was the integrated effect of a cluster of genes located tightly in one chromosome region or separately in different chromosome regions. It is still unclear whether Pm21 is a single gene functioning independently or if it is a cluster of genes working simultaneously; therefore, Pm21 is still defined as a locus by cytogenetics. T6VS·6AL has been released to 71 scientific research institutes in China and 23 other countries since 1995, and feedback from these researchers has revealed that Pm21 shows high resistance in all these regions of the world and still confers immunity to all of the analyzed Bgt strains. T6VS·6AL has been used widely as a parent in breeding programs, and 12 new resistant varieties have been developed and released since 2002. The newly developed varieties have been cultivated on more than 3.4 million hectares by farmers in China, and they have been rapidly expanding since 2007. Our previous study showed that the introduction of T6VS·6AL in the newly developed varieties had no obvious adverse effect on the other agronomic traits (15). With other Pm genes losing their resistance in China, it is expected that Pm21 will be widely used as a main resistance gene resource in future breeding programs.
Although Pm21 is a pivotal gene in wheat breeding for powdery mildew resistance, little is known about the nature of the gene and its mechanism of BSR. However, as a result of the low frequency of pairing and suppressed recombination between the 6VS of H. villosa and 6AS of wheat, it is extremely difficult to characterize the Pm21 locus through a map-based cloning strategy. In this study, an integrated strategy was conducted to clone a resistance gene from the Pm21 locus by using a GeneChip microarray combined with genetic mapping using a series of alien deletion and translocation lines. Stpk-V, a putative serine and threonine kinase gene cloned from the Pm21 locus of H. villosa, will potentially play an important role in wheat resistance breeding via genetic engineering. This study also provides a step toward understanding the nature of the high and BSR to powdery mildew of Pm21.
Results
A Serine/Threonine Protein Kinase Gene Is Induced by Bgt in H. villosa.
Cloning of the resistance gene in the Pm21 locus by map-based cloning failed because no recombination was found between 6VS and 6AS in our previous study. Microarray is a feasible method for cloning a target gene that is expressed differentially between different samples. In this study, a GeneChip microarray was used to identify Bgt-induced genes in H. villosa. The 196 genes, whose signal intensities in the Bgt-inoculated H. villosa were twofold higher than in the uninoculated H. villosa, were selected as differentially expressed genes. Among the 131 genes with putative functions, there were pathogenesis-related genes, defense response genes, signal transduction and transcription factor genes, and resistance gene analogues (RGAs). To select the candidate genes located in the Pm21 locus, we focused first on the four RGAs, including Contig17515 (serine and threonine protein kinase), Contig16386 (putative disease resistance protein), Contig7534 (serine and threonine protein kinase), and Contig13968 (Mlo-like seven-transmembrane protein). However, only Stpk-V, the homologue of Contig17515 in H. villosa, was chosen as the candidate gene based on its cytogenetic localization to 6VS and its expression pattern (as detailed later). A pair of primers, CINAU15-F and CINAU15-R, were designed based on Contig17515 to clone its homologue from H. villosa. Stpk-V (a putative serine and threonine protein kinase gene from H. villosa), a 448-bp fragment isolated from Bgt-inoculated H. villosa by RT-PCR, shared 96% similarity with Contig17515 (16). RT-PCR confirmed that Stpk-V was significantly up-regulated by Bgt (16). In the resistant T6VS·6AL, Stpk-V was induced by Bgt (Fig. S1A, 1), but not by Fusarium graminearum (Fig. S1A, 2) or abiotic stresses such as heat and salt treatments (Fig. S1A, 3 and 4).
The full-length cDNA of Stpk-V was obtained by RACE, and the ORF contains 401 amino acids (HM241655). To obtain the genomic sequence of Stpk-V, the transformation-competent artificial chromosome (TAC) library of T6VS·6AL was screened by pooled PCR using the CINAU15-F and CINAU15-R. A 30-kb TAC clone and a 5,160-bp subclone containing the whole genomic sequence of Stpk-V were obtained (HQ864471). Sequence comparisons indicated that the cDNA of Stpk-V matches the TAC subclone completely. Stpk-V contains six exons and five introns (Fig. S2A), and a putative serine/threonine protein kinase domain was found within Stpk-V. The HRDIKASNIL sequence is the putative catalytic loop, and DFGLAKLLPP, ISTRV, and GTLGYLAPE form the putative activation loop. The amino acid residues Y, N, L, and SDF form the putative ATP binding pocket, and K, TR, G, and LG form the putative substrate binding pocket (Fig. S2B). The comparisons of the protein (Figs. S3 and S4) and putative promoter sequence (Fig. S5) of the Stpk-V with its homologues from other species are described in SI Results.
Stpk-V Is Located in the 6VS Bin FL0.45–0.58.
The location of the Stpk-V gene was determined by PCR using CINAU15-F and CINAU15-R. The partial sequence of Stpk-V (902 bp in length) was amplified from materials containing 6VS, including H. villosa, T. durum–H. villosa amphiploid, T6VS·6AL, and wheat–H. villosa disomic addition line of chromosome 6V (DA6V), but not from Yangmai158 and other addition lines. These data localized Stpk-V to 6VS of H. villosa. Further amplification in the resistant alien deletion del6VS-1 (FL 0.58) and susceptible alien deletion del6VS-2 (FL 0.45) mapped Stpk-V to the bin FL 0.45–0.58 (Fig. S6A).
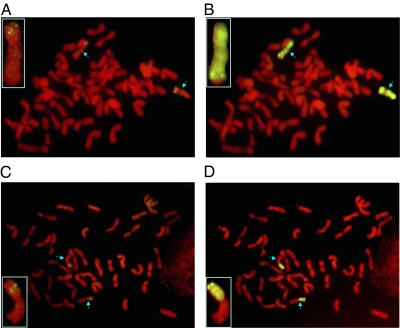
To precisely map Stpk-V, FISH using the 30-kb TAC clone with Stpk-V as the probe and the following sequential genomic in situ hybridization (GISH) using gDNA of H. villosa as the probe were conducted. A TAC signal was detected near the telomeres of 6VS in del6VS-1, and the signal was located from FL 0.45–0.58 of 6VS in T6VS·6AL (Fig. 1). Both the PCR and FISH results indicated that Stpk-V is located in the same region as Pm21, i.e., from FL 0.45–0.58 of 6VS.
Fig. 1.
Mapping of Stpk-V by FISH and sequential GISH on mitotic metaphase chromosomes of T6VS·6AL and del6VS-1. (A and C) FISH with 30-kb TAC clone as the probe, and the green signal shows the Stpk-V position. (B and D) Sequential GISH with the genomic DNA of H. villosa as the probe. The arrows in A and B show the deletion 6V chromosomes, and in C and D they indicate the translocation chromosomes.
Stpk-V Is Localized to the Membrane, Cytoplasm, and Nucleus of Epidermal Cells and Decreases the Haustorium Index of Bgt in Susceptible Yangmai 158.
A single-cell transient expression assay of Stpk-V-YFP in the epidermal cells of H. villosa localized Stpk-V to the membrane, cytoplasm, and nucleus (Fig. S6B).
Haustorium is a key structure for nutrient extraction during Bgt development. If the haustorium cannot form successfully, the host–Bgt interaction is considered to be incompatible. The haustorium index is usually used as a criterion to estimate the compatibility of interactions between the host and Bgt. The haustorium index was 54.8% in epidermal cells of susceptible Yangmai158 transformed with a single GUS gene, but it decreased to 13.6% in epidermal cells cotransformed with GUS and Stpk-V. In epidermal cells of T6VS·6AL transformed with GUS, the haustorium index was 14.1% (Fig. S7). The results indicate that Stpk-V acts by preventing the formation of the haustoria.
Stpk-V Shows High Resistance and BSR to Bgt.
The function of Stpk-V was further studied in transgenic wheat plants stably expressing Stpk-V from the T0 to T3 generation. Stpk-V transgenic plants were produced by transforming pAHC:Stpk-V into the susceptible variety Yangmai158 by particle bombardment. Southern blotting (T0 generation), BASTA resistance evaluation (T1 generation), Stpk-V expression analysis (T1 generation), and detection of the transformed genes (T3 generation) on the transgenic plants verified the stable incorporation and expression of Stpk-V (Fig. S8 A–D). RT-PCR analysis showed that Stpk-V was induced by Bgt in T6VS·6AL, and its expression was undetectable in the susceptible Yangmai158, but Stpk-V was constitutively expressed even in the uninoculated seedlings in the transgenic plants (Fig. S8E).
Leaves detached from the T0 plants were inoculated with mixed Bgt collected from eastern China, and HRs were observed (Fig. 2A). In the T1 generation, only plants with Stpk-V showed high resistance similar to that of T6VS·6AL (Fig. 2B). By the T3 generation, the resistance conferred by Stpk-V has been stably transmitted (Fig. 2C). The homozygous resistant lines of the T3 generation were used to evaluate the function of Stpk-V using eight different strains isolated from a population of Bgt from northern China. The transgenic plants showed the highest level of resistance—grade 0 or 0;—to all eight strains; however, the receptor Yangmai158 showed the highest level of susceptibility—grade 3 or 4—to all eight strains (Table S1). These results suggested that Stpk-V may be a BSR gene.
Fig. 2.
Functional analysis of Stpk-V by stable transformation into the susceptible wheat variety Yangmai158. (A) Detached leaves of the T0 plants showed HRs 7 d after inoculation with Bgt, whereas the leaves of Yangmai158 were covered with colonies. (B) T1 plant without Stpk-V (T1(S)) showed susceptibility, whereas the T1 plant with Stpk-V (T1(R)) showed high resistance in the greenhouse. (C) T3 lines showed a similar high level of resistance in the field as T6VS·6AL. T3-6, resistant line with hypersensitive mosaics, T3-2, resistant line without hypersensitive mosaics.
Resistance to Bgt Is Compromised by Stpk-V Silencing in H. villosa and T6VS·6AL.
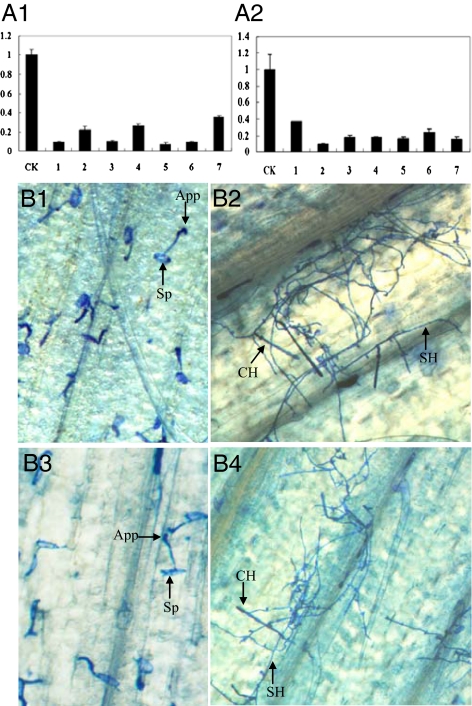
A virus-induced gene silencing (VIGS) system (17) established in H. villosa (18) was used to evaluate the function of Stpk-V. The expression levels of Stpk-V decreased by 2.5- to 10-fold in the fifth leaves of both resistant materials challenged with barley stripe mosaic virus (BSMV):Stpk-V (Fig. 3A).
Fig. 3.
Functional analysis of Stpk-V by VIGS. (A) Quantitative RT-PCR analysis of the expression level of Stpk-V in the plants inoculated with BSMV:Stpk-V using the plants inoculated with BSMV:GFP as the control (CK). In seven BSMV:Stpk-V–inoculated H. villosa plants (A1) and seven BSMV:Stpk-V–inoculated T6VS·6AL plants (A2), the expression level of Stpk-V was decreased. (B) Observations of the development of Bgt in the BSMV:Stpk-V–inoculated plants using the BSMV:GFP inoculated plants as the control. H. villosa (B1) and T6VS·6AL (B3) were infected with BSMV:GFP and then inoculated with Bgt. Infection with BSMV:GFP did not alter the resistance to Bgt. H. villosa (B2) and T6VS·6AL (B4) infected with BSMV:Stpk-V and then inoculated with Bgt. Stpk-V silencing resulted in increased susceptibility. Arrows indicate spore (Sp), appressorium (App), secondary hyphae (SH), and conidiophore (CH).
The fifth fully expanded leaves inoculated with BSMV:Stpk-V were detached from the plants for inoculation with fresh Bgt conidia, using plants infected with BSMV:GFP as a control. In T6VS·6AL and H. villosa inoculated with BSMV:GFP, most of the germinated spores formed only the appressorium penetration peg (App), and approximately 15% of the spores could form haustorium with no more than four branches of hyphae (Fig. 3B, 1 and 3). However, approximately 40% of the germinated spores could produce more branches of hyphae in BSMV:Stpk-V–treated leaves of H. villosa and T6VS·6AL, some of which even formed conidiophore (Fig. 3B, 2 and 4). Although gene silencing was not complete, the decreased expression of Stpk-V in the BSMV:Stpk-V–treated plants compromised the resistance of H. villosa and T6VS·6AL to Bgt.
The sequence homology between Stpk-V and the three orthologous genes (Stpk-A, Stpk-B, and Stpk-D) of wheat was greater than 95%. Therefore, in the homozygous wheat–H. villosa translocation line T6VS·6AL, besides the Stpk-V in the V genome, the other two copies in the B and D genomes (Stpk-B and Stpk-D) may also be silenced if they are expressed. As Stpk-B and Stpk-D made no contribution to the resistance in common wheat, we proposed that the phenotypic change of T6VS·6AL was most likely a result of the silencing of Stpk-V.
Resistance of Stpk-V Is Correlated with Hypersensitive Cell Death and H2O2 Accumulation.
Obvious hypersensitive cell death was observed in the leaves of T0 (Fig. 2A) and T3 plants (Fig. 2C and Table S1), indicating that the hypersensitive response (HR) was a crucial mechanism of resistance mediated by the over-expression of Stpk-V. HR is usually associated with a burst of reactive oxygen species, especially H2O2. To study whether the production of H2O2 is responsible for HR, the subcellular localization of H2O2 at the sites of interaction and development of Bgt were investigated.
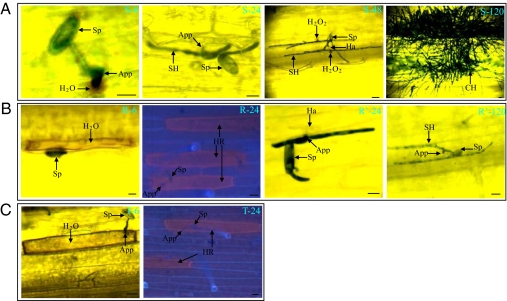
For the susceptible Yangmai158, a small area of brown staining around the App, indicative of H2O2 production, was observed at 6 h (Fig. 4, S6). At 24 h, the haustorium beneath the App and several branches of primary hyphae were formed (Fig. 4, S24). At 48 h, a small area of brown staining was again detected under the secondary penetration peg (Fig. 4, S48). At 120 h, the hyphae and conidiophores covered the leaf surface (Fig. 4, S120). In the whole process, no HR was observed.
Fig. 4.
Characterization of the resistance mechanism of Stpk-V by observing of H2O2 and HRs after Bgt inoculation in leaves of Yangmai158 (S), T6VS·6AL (R), and the transgenic plants (T). (A) In Yangmai158, Bgt developed normally. H2O2 was localized around the primary penetration peg at 6 h (S-6) and then appeared under the newly developed penetration pegs at 48 h (S-48). At 120 h, the leaves were covered with hyphae and conidiophores (S-120). (B) In the T6VS·6AL, there were two types of Bgt development patterns. For the first type, the development of Bgt stopped after App formation. H2O2 was detected in the interaction cell (R-6), and the HR was then observed at 24 h (R-24). For the second one, the haustoria could form (R′-24), and the hyphae elongated, but very slowly (R′-120). (C) In the transgenic plants, Bgt could not develop after App formation. H2O2 was detected in the interaction cell (T-6), and the HR was observed at 24 h (T-24). Arrows indicate spore (Sp), appressorium (App), haustoria (Ha), secondary hyphae (SH), HR cell (HR), and conidiophore (CH). Numbers indicate the hours of Bgt inoculation. (Scale bar, 25 μm.)
For the resistant T6VS·6AL, approximately 44% of the germinated spores induced a brown staining response throughout the whole cell at 6 h (Fig. 4, R6) and HR were observed at 24 h (Fig. 4, R24). Fifteen percent of the spores developed haustoria at 24 h (Fig. 4, R24′), but hyphae developed slowly and no conidiophore formed at 120 h (Fig. 4, R120′).
In the transgenic plants, 76% of the germinated spores induced brown staining in the whole infected cells at 6 h (Fig. 4, T6), and HR but no haustoria was observed at 24 h in the Bgt-infected cells (Fig. 4, T24). However, the haustorium index was 13.6% in the single-cell transient experiments, indicating that there was a low percentage of GUS expressing cells without Stpk-V expression.
H2O2, salicylic acid (SA), or jasmonic acid (JA) has proven to be closely correlated with disease resistance by inducing the pathogenesis related genes or acting as early signaling components. In this study, Stpk-V could be induced by H2O2 (Fig. S1B, 1), but not by SA (Fig. S1B, 2) and JA (Fig. S1B, 3). H2O2 can induce cell death (19), promote cross-linking of cell wall structural proteins (20), or act directly as the defense substance (21). Observations of the T6VS·6AL and transgenic plants revealed that H2O2 production was tightly correlated with the HR and the suppression of fungus growth, and the Stpk-V gene has a crucial role in mediating the accumulation of H2O2.
Discussion
In this study, the combined evidence from gene mapping, stable transformation, transient expression assay, and gene silencing strongly suggests that Stpk-V, a putative serine/threonine protein kinase gene, is a key gene conferring the durable BSR in the Pm21 locus. Serine/threonine protein kinases (EC 2.7.11.1) phosphorylate the OH group of serine or threonine residues leading to a functional change of the target protein, and protein phosphorylation has been identified as one of the most important events in the disease resistance pathway. The tomato bacterial speck disease resistance gene Pto encodes serine/threonine protein kinases (22); bacterial blight disease resistance gene Xa21 (23) in rice, flagellin perception gene FLS2 (24) and AvrPphB recognition gene PBS1 (25) in Arabidopsis, stem rust resistance gene Rpg5 (26) in barley, and stripe rust resistance gene Yr36 (11) in wheat also contain a serine/threonine protein kinase domain. However, the homology of Stpk-V with these genes was lower than 40%, and the homologous region was limited to 39 to 201 aa.
HR, involving the rapid development of cell death immediately surrounding infection sites, is a common weapon used by plants to limit development of the pathogen. Oxidative bursts occur at early stages after pathogen infection and induce HR later (27). There are two distinct phases of reactive oxygen species production during plant–pathogen interactions (19, 21). In this study, the production of H2O2 in resistant and susceptible plants was observed. The first H2O2 burst in the basal resistance stage in susceptible Yangmai158 made no contribution to resistance; however, the first H2O2 burst in the basal resistance stage in the resistant plant T6VS·6AL could induce the expression of Stpk-V, which then mediated the second round of H2O2 burst, leading to HR. The H2O2-induced expression of Stpk-V, which was revealed by RT-PCR, supported this hypothesis.
The formation of mature haustoria and the development of secondary hyphae are prerequisites for the establishment of a compatible interaction between the host and the parasite (28). In our study, some primary haustoria were formed in T6VS·6AL, but the hyphae developed slowly and no colony was observed. In the transgenic plants, no haustorium was observed. The difference in the development pattern of Bgt in both resistant plants may be a result of the different expression patterns of Stpk-V. In the transgenic plants, the constitutive overexpression of Stpk-V allowed the leaves to respond to Bgt very quickly, and the fungus could not produce even primary haustoria. However, in the T6VS·6AL plants, the Stpk-V was induced by Bgt, and the defense response mediated by Stpk-V was slower, thus leading to the formation of primary haustoria.
Wild species have been widely used as genetic resources for introgression of useful traits into cultivated species by wide hybridization (2). The cloning of genes from wild relatives and the use of these genes in transgenic studies is an efficient way for modern genetic improvement. However, because of the low frequency of pairing and recombination between the chromosomes from the wild and cultivated species, map-based cloning of these elite genes from wild species is a challenge. Our research is a successful example of cloning useful wild genes by integrative cytogenetic and molecular methods.
Stpk-V, a gene from a wild species conferring BSR against Bgt, can now be used in wheat breeding for powdery mildew resistance by genetic engineering. The gene cloning strategies used in this study will benefit other research aimed at cloning genes from wild species. In the follow-up research, we will evaluate the function of Stpk-V by using its endogenous promoter, and focus on the polymorphisms between Stpk-V with its orthologous genes in the susceptible wheat varieties. It will be interesting to identify the protein(s) interacting with Stpk-V to investigate the molecular mechanism of BSR mediated by Stpk-V. We will also create more cytogenetic stocks and mutants involving the 6VS to help us determine how many genes are involved in the Pm21 locus, and whether Stpk-V is the only one necessary to confer the resistance phenotype.
Materials and Methods
Plant Materials.
H. villosa (introduced from Cambridge Botanical Garden, United Kingdom), T. durum–H. villosa amphiploid [developed by the Cytogenetics Institute of Nanjing Agricultural University (CINAU); accession no. NAU201], wheat–H. villosa translocation line T6VS·6AL (developed by CINAU; accession no. NAU405), DA1V-DA7V (seven T. aestivum–H. villosa alien addition lines, each containing one pair of chromosomes of H. villosa from 1V to 7V in the T. aestivum cv. Chinese Spring background, developed by CINAU; accession nos. NAU307–NAU313), del.6VS-1 and del.6VS-2 (deletion addition lines that lack the distal 42% and 55% part of the 6VS chromosome respectively, developed by CINAU; seed accession nos. NAU453 and NAU454) were used in this study. All the NAU accession numbers were given by Cytogenetics Institute of Nanjing Agricultural University, China.
GeneChip Microarray.
GeneChip microarray was conducted only to screen genes for independent analysis in the later study. Leaves of 3-wk-old seedlings of resistant H. villosa were collected at 24, 48, and 72 h after inoculation with a mixture of Bgt, and total RNA isolated from three samples was pooled as the test. The uninoculated leaves were collected at the same three time points, and the total RNAs were pooled as the control. More information about data analysis is supplied in SI Materials and Methods.
Semiquantitative RT-PCR.
Stpk-V expression level was analyzed by semiquantitative RT-PCR under different abiotic and biotic stresses in H. villosa, T6VS·6AL, and transgenic plants. Details are provided in SI Materials and Methods.
TAC Library Screening and RACE.
To isolate the genomic sequence of Stpk-V, CINAU15-F and CINAU15-R (SI Materials and Methods) were used to screen a genomic TAC library of T6VS·6AL (29) by a pooled PCR screening procedure. The 30-kb positive TAC clone was obtained and digested with EcoRI, and then a 5,160-bp subclone containing the whole Stpk-V was further obtained. Details of RACE are provided in SI Materials and Methods.
FISH and Sequential GISH.
FISH using 30-kb TAC as probe in del.6VS-1 and T6VS·6AL was conducted as described (30). After TAC-FISH, the hybridization signals were removed for the following GISH, which was performed using DNA of H. villosa as probe. Details are provided in SI Materials and Methods.
Vector Construction and Wheat Transformation.
The vector pAHC:Stpk-V was constructed by replacing the GUS gene with ORF of Stpk-V in the plant expression vector pAHC 25, and the pAHC:Stpk-V transgenic wheat plants were produced by particle bombardment of calli cultured from immature embryos of susceptible variety Yangmai158. Details are provided in SI Materials and Methods.
Evaluation of Powdery Mildew Resistance of Transgenic Plants.
Powdery mildew resistance of the transgenic plants was evaluated via inoculation with Bgt using Yangmai158 and T6VS·6AL as the control. The detached leaves from T0 transgenic plants were inoculated with a mixture of Bgt collected from Eastern China. The adult T1- and T3-generation transgenic plants were inoculated with a mixture of Bgt in the natural field of Eastern China. Two lines (T3-6 and T3-2) of homozygous resistant T3 transgenic plants were also inoculated with eight different individual strains (Bgt11, Bgt18, Bgt311, Bgt411, Bgt401, Bgt413, Bgt611, and Bgt711) collected from Northern China to evaluate the BSR. The level of resistance was classified as grades 0 to 4 (0, 0;, 1, 2, 3, and 4) according to the standard of Sheng et al. (31). Grade 0 indicates the highest resistance level to Bgt at which leaves are immune, grade “0;” represents high resistance at which only hypersensitive mosaics are observed, and grade 4 is the highest susceptible level at which all leaves were covered with vast stretches of hyphae producing large amounts of spores.
Single-Cell Transient Expression Assay.
The single-cell transient expression assay was performed according to Shirasu et al. (32). Reporter plasmids containing β-glucuronidase (GUS) genes and the plasmids pAHC:Stpk-V were mixed before coating of the particles (molar ratio of 1:1; 1 μg of total DNA). The bombarded leaves were transferred to 1% agar plates supplemented with 85 μM benzimidazole and incubated at 18 °C for 8 h before high-density inoculation with single Bgt spores. Leaves were stained for GUS to identify Stpk-V transformed cells at 48 h after spore inoculation. The haustorium index (percentage of GUS-staining cells with haustoria in the total GUS-staining cells attacked by Bgt) is indicated by the mean of three independent experiments, each contributing at least 40 interactions.
Functional Analysis of Stpk-V by VIGS.
VIGS was mediated by BSMV (17). Vector construction, plasmid linearization, in vitro transcription, and virus infection were conducted as described by Wang et al. (18) and Zhou et al. (33). Details are provided in SI Materials and Methods.
Subcellular Localization of H2O2 and Cell Death at Interaction Sites in Leaves After Inoculation with Bgt.
The first leaves were cut from Yangmai158, T6VS·6AL, and pAHC:Stpk-V transgenic plants at 6, 24, 48, 72, 96, and 120 h after Bgt18 inoculation. Detection of H2O2 was performed by in situ histochemical staining using DAB as described by Thordal-Christensen et al. (34). The development of fungus and H2O2 production were observed under an Olympus microscope in bright field. For dead cells localization, the inoculated leaves were observed in the UV light after bleaching in 90% ethanol.
Supplementary Material
Acknowledgments
We thank Prof. Cao Yuanyin for kindly providing individual strains of Bgt, Prof. Liu Yaoguang for help in TAC library construction, Prof. Cheng Zhukuan for help in TAC-FISH, Prof. Wang Daowen for help in VIGS system establishment, Prof. Shen Qianhua for help in single cell transient expression assays, and Prof. Bikram S. Gill for helpful suggestions. This research was supported by the High-Tech Program of China Grants 2004AA222140 and 2006AA10A104, National Natural Science Foundation of China Grant 30700503, China Transgenic Research and Commercialization Key Special Project Grant 2008ZX08002-001, “111 Project” of Ministry of Education of China Grant B08025, and National Basic Research Program of China Grant 2009CB118304.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.D. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. HM241655, HQ864471, and JF439306–JF439310).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016981108/-/DCSupplemental.
See Commentary on page 7657.
References
- 1.Huang X, Röder MS. Molecular mapping of powdery mildew resistance genes in wheat: A review. Euphytica. 2004;137:203–223. [Google Scholar]
- 2.Li Z, Li B, Tong Y. The contribution of distant hybridization with decaploid Agropyron elongatum to wheat improvement in China. J Genet Genomics. 2008;35:451–456. doi: 10.1016/S1673-8527(08)60062-4. [DOI] [PubMed] [Google Scholar]
- 3.Bennett FGA. Resistance to powdery mildew in wheat: A review of its use in agriculture and breeding programmes. Plant Pathol. 1984;33:279–300. [Google Scholar]
- 4.Griffey CA, Das MK, Stromberg EL. Effectiveness of adult-plant resistance in reducing grain yield loss to powdery mildew in winter wheat. Plant Dis. 1993;77:618–622. [Google Scholar]
- 5.He R, et al. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet. 2009;118:1173–1180. doi: 10.1007/s00122-009-0971-z. [DOI] [PubMed] [Google Scholar]
- 6.Yahiaoui N, Srichumpa P, Dudler R, Keller B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004;37:528–538. doi: 10.1046/j.1365-313x.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhullar NK, Street K, Mackay M, Yahiaoui N, Keller B. Unlocking wheat genetic resources for the molecular identification of previously undescribed functional alleles at the Pm3 resistance locus. Proc Natl Acad Sci USA. 2009;106:9519–9524. doi: 10.1073/pnas.0904152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kou Y, Wang S. Broad-spectrum and durability: Understanding of quantitative disease resistance. Curr Opin Plant Biol. 2010;13:181–185. doi: 10.1016/j.pbi.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Büschges R, et al. The barley Mlo gene: A novel control element of plant pathogen resistance. Cell. 1997;88:695–705. doi: 10.1016/s0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- 10.Krattinger SG, et al. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science. 2009;323:1360–1363. doi: 10.1126/science.1166453. [DOI] [PubMed] [Google Scholar]
- 11.Fu D, et al. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science. 2009;323:1357–1360. doi: 10.1126/science.1166289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen PD, Qi LL, Zhou B, Zhang SZ, Liu DJ. Development and molecular cytogenetic analysis of wheat-Haynaldia villosa 6VS/6AL translocation lines specifying resistance to powdery mildew. Theor Appl Genet. 1995;91:1125–1128. doi: 10.1007/BF00223930. [DOI] [PubMed] [Google Scholar]
- 13.Qi LL, Wang SL, Chen PD, Liu DJ, Gill BS. Identification and physical mapping of three Haynaldia villosa chromosome-6V deletion lines. Theor Appl Genet. 1998;97:1042–1046. [Google Scholar]
- 14.Chen S, Chen P, Wang X. Inducement of chromosome translocation with small alien segments by irradiating mature female gametes of the whole arm translocation line. Sci China C Life Sci. 2008;51:346–352. doi: 10.1007/s11427-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 15.Li G, et al. Effects of the 6VS/6AL translocation on agronomic traits and dough properties of wheat. Euphytica. 2007;155:305–313. [Google Scholar]
- 16.Cao A, et al. A sequence-specific PCR marker linked with Pm21 distinguishes chromosomes 6AS, 6BS, 6DS of Triticum aestivum and 6VS of Haynaldia villosa. Plant Breed. 2006;125:201–205. [Google Scholar]
- 17.Holzberg S, Brosio P, Gross C, Pogue GP. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002;30:315–327. doi: 10.1046/j.1365-313x.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. Establishment of an effective virus induced gene silencing system with BSMV in Haynaldia villosa. Mol Biol Rep. 2010;37:967–972. doi: 10.1007/s11033-009-9766-1. [DOI] [PubMed] [Google Scholar]
- 19.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 20.Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- 22.Martin GB, et al. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993;262:1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- 23.Song WY, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Gómez L, Bauer Z, Boller T. Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell. 2001;13:1155–1163. [PMC free article] [PubMed] [Google Scholar]
- 25.Swiderski MR, Innes RW. The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J. 2001;26:101–112. doi: 10.1046/j.1365-313x.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 26.Brueggeman R, et al. The stem rust resistance gene Rpg5 encodes a protein with nucleotide-binding-site, leucine-rich, and protein kinase domains. Proc Natl Acad Sci USA. 2008;105:14970–14975. doi: 10.1073/pnas.0807270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellingboe AH. Genetics and physiology of primary infection by Erysiphe graminis f.sp. hordei. Phytopathology. 1972;62:401–406. [Google Scholar]
- 29.Fang YD, et al. Construction of a transformation-competent artificial chromosome (TAC) library of a wheat-Haynaldia villosa translocation line. Sheng Wu Gong Cheng Xue Bao. 2000;16:433–436. [PubMed] [Google Scholar]
- 30.Cheng Z, Presting GG, Buell CR, Wing RA, Jiang J. High-resolution pachytene chromosome mapping of bacterial artificial chromosomes anchored by genetic markers reveals the centromere location and the distribution of genetic recombination along chromosome 10 of rice. Genetics. 2001;157:1749–1757. doi: 10.1093/genetics/157.4.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng B. Grades of resistance to powdery mildew classified by different phenotypes of response in the seeding stage of wheat. Plant Protection. 1988;1:49. [Google Scholar]
- 32.Shirasu K, Nielsen K, Piffanelli P, Oliver R, Schulze-Lefert P. Cell-autonomous complementation of mlo resistance using a biolistic transient expression system. Plant J. 1999;17:293–299. [Google Scholar]
- 33.Zhou H, et al. Molecular analysis of three new receptor-like kinase genes from hexaploid wheat and evidence for their participation in the wheat hypersensitive response to stripe rust fungus infection. Plant J. 2007;52:420–434. doi: 10.1111/j.1365-313X.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 34.Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.