Abstract
Plant glandular trichomes exude secondary metabolites with defensive functions, but these epidermal protuberances are surprisingly the first meal of Lepidopteran herbivores on Nicotiana attenuata. O-acyl sugars, the most abundant metabolite of glandular trichomes, impart a distinct volatile profile to the body and frass of larvae that feed on them. The headspace composition of Manduca sexta larvae is dominated by the branched chain aliphatic acids hydrolyzed from ingested O-acyl sugars, which waxes and wanes rapidly with trichome ingestion. In native habitats a ground-hunting predator, the omnivorous ant Pogonomyrmex rugosus, but not the big-eyed bug Geocoris spp., use these volatile aliphatic acids to locate their prey.
Keywords: indirect defenses, dangerous body odors
Trichomes, or leaf hairs, have long been considered a plant's first line of defense. These epidermal protuberances are known to function as physical and chemical barriers against attacking herbivores and pathogens (1, 2), as well as protecting plants against UV-B radiation, water loss, and heat stress (3). Glandular trichomes can be found on ≈30% of all vascular plants. These secretory hairs contribute to a plant's resistance in numerous ways: as obstacles that thwart movement across plant surfaces (4), as entrapment devices (5), and sources of volatile and nonvolatile secondary metabolites (6, 7) or proteins (8) that poison or repel herbivores.
The native tobacco, Nicotiana attenuata, as it is common among Solanaceous plants, produces glandular trichomes on both adaxial and abaxial leaf surfaces (Fig. 1A and Fig. S1 A and B). These trichomes contain as a minor constituent, nicotine (Fig. S1C), a secondary metabolite known to inhibit growth even of adapted larvae (6, 9). O-acyl sugars (AS), viscous liquids that consist of aliphatic acids of different chain lengths esterified to sucrose, are the most abundant secondary metabolites in the glandular trichomes of Solanaceous plants (10, 11). They dominate aqueous extracts of N. attenuata leaf surfaces and can be readily detected after Rhodamine B staining (Fig. 1 A and B) and by HPLC-ToFMS (Fig. S1). AS, particularly those esterified to aliphatic acids with alkyl chains longer than eight carbons, are effective defenses against psyllids (12), aphids (13), white flies, and spider mites (14), likely by speeding the desiccation of entrapped or anointed herbivores (15). AS from Datura wrightii, although differing in their sugar moiety and aliphatic acid residues, decrease growth of Manduca sexta larvae when applied to artificial diets (16).
Fig. 1.
Trichomes and their exudates are commonly the first meal of neonate larvae of the three main Lepidopteran species that feed on N. attenuata. (A) HPLC-ToFMS analysis of an N. attenuata leaf surface wash. Inset shows a single trichome with the O-acyl sugars stained by Rhodamine B dye. (B) Structures of the two classes of O-acyl sugars (ASII, III; ref. 10) found in N. attenuata trichomes and the four most abundant BCAA substituents. (C) Feeding choices of neonate larvae of the three most abundant Lepidopteran herbivores of N. attenuata in nature: M. sexta, S. littoralis, and S. exigua. Choice is expressed as the mean (±SE) percent of feeding choices of larvae in four (M. sexta, n = 55), three (S. littoralis, n = 43), and one (S. exigua, n = 20) experiments. Asterisks indicate significant differences by χ2 analysis: *P < 0.05, **P < 0.01, ***P < 0.001. (D) Headspace of N. attenuata-fed M. sexta larvae trapped on Super-Q traps (Inset) is dominated by the four BCAAs esterified to the sucrose moiety within AS-2 and AS-3. Dashed line represents ion-trace m/z 74 and the solid line m/z 60 (Fig. S3).
Results and Discussion
Neonate Lepidopteran Larvae Consume Trichomes as a First Meal.
Given the established defensive value of trichomes and their chemistry, we were surprised to observe that these trichomes and their exudates were the first feeding choices of both generalist (Spodoptera littoralis and S. exigua) and specialist (M. sexta) Lepidopteran herbivores on N. attenuata (Fig. 1C, Fig. S2A, and Movie S1). More than 70% of all larvae chose to feed on trichomes for their first meal after hatching. Moreover, larvae did not suffer from this choice. When placed on N. attenuata sepals, which are entirely covered in glandular trichomes, larvae feeding on sepals survived and grew as well as larvae fed on previously washed and AS-free wild-type (WT) sepals as well as sepals from plants transformed to silence nicotine production (Fig. S2 C and D). Observations of neonate M. quinquemaculata and S. littoralis feeding behavior on native N. attenuata growing in natural habitats in Utah confirmed these laboratory observations and led us to conclude that trichomes and their exudates, rather than being directly defensive, provide a sugary first meal for these Lepidopteran herbivores. Interestingly, trichome feeding has been reported for other Lepidopteran species and may be common (4, 17).
Ingestion of O-Acyl Sugars Dramatically Alters M. sexta Body Headspace.
Although the ingestion of trichomes and their AS contents may not be directly deleterious for larvae, it dramatically alters larval body and frass volatile profiles. Trapping and GC-MS analysis of the headspace surrounding larvae that had fed on N. attenuata leaves and trichomes revealed that four branched chain aliphatic acids (BCAA), namely 2-methyl butanoic acid (I), 3-methyl butanoic acid (II), 3-methyl pentanoic acid (III), and 4-methyl pentanoic acid (IV) were the most abundant constituents (Fig. 1 B and D). These 4 BCAAs found in larval body headspace are exactly those found from an alkaline hydrolysis of N. attenuata AS (Fig. S3), and we hypothesized that BCAAs in the larval headspace originate from AS hydrolysis in the alkaline midgets (18) of these larvae. AS are readily removed by washing leaves with water and do not occur in a species of Nicotiana that lacks trichomes, N. glauca (Fig. S4A). When fed on water-washed N. attenuata or N. glauca leaves, larvae lose their characteristic body or frass volatile profile. Similarly, when fed on an artificial diet amended with AS from N. attenuata leaves, larvae produce a body headspace similar to that of larvae feeding on WT plants (Fig. 2 A and B). The effect of AS ingestion on larval body headspace rapidly waxes and wanes with diet; when switched from the AS-free N. glauca to N. attenuata diets, larvae rapidly emit the characteristic BCAAs, and these volatile acids disappear again when larvae are returned to AS-free N. glauca leaves (Fig. 2C). We conclude that consuming AS from trichomes has a similar effect for larvae as the consumption of asparagus has for humans: It conveys a strongly dynamic and distinct olfactory signature to bodies and excretions. Although only a potential social annoyance for humans, these emissions, which approach 5 μg of single BCAAs from larval bodies and 30 μg from frass over 2 h (Fig. 2), may have more serious consequences for a caterpillar.
Fig. 2.
Ingestion of O-acyl sugars determines the body and frass headspace of M. sexta larvae. (A) Mean ± SE of four BCAAs trapped from M. sexta body headspace from caterpillars feeding on plants with AS (CTRL) or without AS (washed) as well as artificial diets supplemented with N. attenuata AS (+AS) or without AS (CTRL). (B) Mean ± SE of four BCAAs trapped from M. sexta frass headspace from larvae feeding on plants with (CTRL) or without AS (washed) as well as AS-containing N. attenuata plants and AS-free N. glauca plants. Values are expressed as micrograms per 2-h trapping interval. (C) Mean ± SE of BCAA III (3-methyl pentanoic acid) from M. sexta body headspace from larvae feeding sequentially on AS-free N. glauca, AS-containing N. attenuata, and AS-free N. glauca. Inset shows mean ± SE of BCAA III released from larvae reared on AS-free N. glauca and switched to AS-containing N. attenuata leaves at time 0.
The natural enemies of caterpillars are known to use fecal odors to locate their prey. For example, parasitic wasps can learn to associate frass volatiles with prey location under laboratory conditions (19, 20), and many insects have developed sophisticated means of distancing themselves from their potentially traitorous excrements. The larvae of the silver-spotted skipper (Epargyreus clarus), a shelter-building insect, for example, goes to lengths to remove its frass from its hideouts to avoid detection by predators (21). Plants have exploited the proclivity of carnivorous insects to use plant volatiles to locate their prey and have evolved means of amplifying and modifying these emissions in response to herbivore attack to provide reliable information about the location of herbivorous prey to hunting carnivores (22).
Hemipteran Predator Geocoris spp. Is Not Attracted by Larval Body Headspace.
Herbivory-induced plant volatiles released from N. attenuata plants have been shown to attract and optimize the foraging behavior of Geocoris hemipteran predators in nature (23, 24), and we hypothesized that this predator might also use the volatile BCAAs released from AS-feeding larvae. Because the release of BCAAs occurs rapidly from AS-feeding larvae (Fig. 2C), we imagined that the BCAAs might complement the rapid isomerization of green leaf volatiles (GLVs) that is elicited by M. sexta oral secretions to provide Geocoris predators with rapid and spatially explicit information about the whereabouts of their prey on plants (23). This however, was not the case. Although the native Geocoris predators at our field station in Utah responded to the perfuming of plants with GLV blends produced by M. sexta-attacked plants in an egg predation assay, they did not respond to perfuming with either BCAA III or a mixture of four BCAAs in amounts equivalent to that released from frass (Fig. S5 and Table S1). However, when early instar larvae were perfumed with BCAAs in either lanolin pastes or water sprays, perfumed larvae tended to be preyed on more frequently than control larvae reared on AS-free N. glauca leaves. These larvae were placed on D. wrightii plants that differ from N. attenuata in AS composition, on AS-free plants (N. glauca), and on N. attenuata plants transformed to silence their ability to release herbivore-induced volatiles (irlox2/3) (Fig. S6). Although none of the differences in predation rates were statistically significant (lanolin paste: χ2 = 3.2, P = 0.073; χ2 = 2.57, P = 0.109; spray: χ2 = 2.67, P = 0.102), the tendency toward higher predation rates of perfumed larvae motivated us to examine the responses of other ground-hunting predators.
Pogonomyrmex rugosus Is Attracted by Larval Body Headspace.
Ants are ground-hunting insects that are common in N. attenuata's habitat and are frequently observed carrying larvae of various species, including M. sexta and M. quinquemaculata, back to their nests. These social insects are capable of associating particular volatiles with potential prey (25) and might therefore be able to associate BCAAs from M. sexta larvae or frass with the presence of larvae on a plant. To determine which of the many different ant species that occur in N. attenuata’s native habitat might respond to larval BCAAs, we scented cooked rice grains with frass quantities of the BCAA mixture, and counted the number of scented and unscented rice grains removed in 15 min from each of 24 nests. With this rice pickup assay, we identified five BCAA-responsive ant nests (Fig. S7E, colony A–E) that all belonged to the same species: P. rugosus, the rough harvester ant.
P. rugosus is a seed-harvesting ant that is also known to be an opportunistic predator of cicadas during outbreaks of this prey species (26). P. rugosus were occasionally observed climbing elongated N. attenuata plants in the field and carrying cutworm larvae back to their nests. They were also found to readily climb 20-cm wooden sticks to retrieve M. sexta larvae from pieces of leaves affixed to the top (Fig. S7A). To exclude the possibility that the ants climbed the sticks in response to visual cues from the caterpillars at the top of the sticks, we conducted trials without larvae or leaves and placed ≈3 cm3 of either oven dried, and hence BCAA free, M. sexta frass or fresh frass from M. sexta larvae-fed N. attenuata leaves at the base of the sticks. Ants showed a strong preference to climb sticks with fresh, BCAA redolent frass, at the base compared with sticks with oven-dried frass at their base (Fig. S7F).
BCAA are rapidly volatilized from frass excreted by the larvae, particularly when it falls onto the hot desert soil from larvae that commonly feed on the undersides of leaves, as Manduca larvae tend to do. To determine how long BCAAs would be retained in the headspace, we measured the recovery of the BCAA mixture from a 45 °C surface (Fig. S8A). Frass quantities of aqueous BCAA mixtures were found to completely dissipate within 15 min. We monitored the tendencies of P. rugosus ants from one nest to climb sticks scented at their base either with a BCAA mixture or a detergent control. At both morning and evening activity periods of the ants, 73–82% of all ants that climbed sticks within 15 min of treatment, climbed sticks that were scented at their base with BCAAs (Fig. S7 G and H).
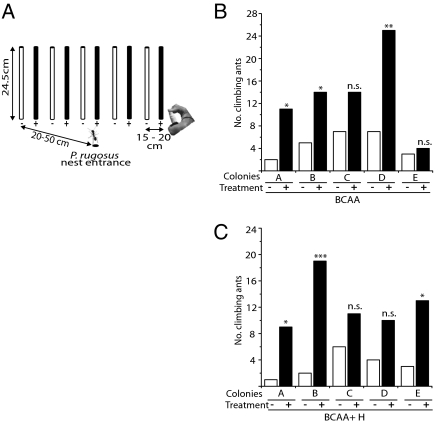
To determine the consistency of P. rugosus responses to BCAA from different nests, we repeated the assay with five nests that differed in their proximity to Manduca-infested N. attenuata and D. wrightii plants (Fig. 3). The AS of D. wrightii trichomes include sucrose esterified with hexanoic acid (H) and Manduca larvae that feed on Datura leaves have body and frass volatile profiles that are dominated by H (Fig. S8B). We conducted two sequential tests of each of the five colonies: with the BCAA mixture characteristic of the ingestion of N. attenuata AS followed by a test of the same BCAA mixture amended with H. Colonies A–C were located in an area with Manduca-infested N. attenuata and D. wrightii plants, and in both trials, ants from two of three colonies significantly preferred to climb sticks scented with BCAA or BCAA+H. Colony D, which likely only foraged within N. attenuata plants, showed a strong response to the BCAA treatment, but responded less strongly when H was added to the mixture. In contrast, colony D, which foraged in an area in which only D. wrightii plants grew, did not respond to the BCAA treatment, but responded strongly to the BCAA+H treatment (Fig. 3). These results demonstrate that P. rugosus foragers respond strongly to BCAA from larval and frass volatile emissions and may even adjust their responsiveness to the particular BCAA produced by locally available prey. Ants are known to use cuticular hydrocarbons (27, 28) and aliphatic acids, albeit with different chain lengths and structures, in nest mate recognition. To our knowledge, there is only one report of a free carboxylic acid that acts as a ant trail pheromone, namely nerolic acid, found in the hindgut of Camponotus floridanus (29). P. rugosus might learn to associate prey availability with BCAAs, but this hypothesis will require additional work to place on solid experimental footing.
Fig. 3.
P. rugosus foragers are attracted by larval body headspace BCAA. (A) Experimental setup for the multiple colony assays. The base of bamboo sticks were sprayed with either 100 μL of an aqueous BCAA mixture (+) or a Tween-20 control (-), which did not differ from a water control. In a second experiment, hexanoic acid (H) (B) was added to the BCAA mixture (C). Ten sticks with alternating treatments were placed in a line 20–50 cm from the nest entrance. Shown are the number of climbing ants 15 min after treatment at 5 different ant nests (A–E). Asterisks indicate significant differences between choices by χ2 analysis: *P < 0.05, **P < 0.01, ***P < 0.001. (B) A: χ2 = 6.23, P = 0.013; B: χ2 = 4.26, P = 0.039; D: χ2 = 10.12, P = 0.002. Colony C did not show a significant difference but a clear trend toward BCAAs (χ2= 2.33, P = 0.127). Colony E did not show a preference to any of the aliphatic acids present in N. attenuata-fed headspace (χ2= 0.143, P = 0.706). (C): A: χ2= 6.4, P = 0.011; B:χ2= 13.76, P = 0.0001; Colony C and D did not show a significant difference but a clear trend toward aliphatic acids (C: χ2= 1.47, P = 0.225; D: χ2= 2.57, P = 0.109). Colony E, when hexanoic acid, present in D. wrightii-fed headspace, was added to the BCAA mixture, responded significantly (χ2 = 6.25, P = 0.012).
Trichomes Function as “Dangerous Lollipops.”
Despite their well-documented function as direct defenses in other Solanaceous plants, the trichome-associated AS of N. attenuata combine aspects of nutritional- and information-based indirect resistance traits. These abundant trichome exudates do not directly defend the plant against attack by M. sexta larvae but rather are the first feeding choice of neonate larvae. As an exuded sugar-rich compound, AS have similarities with the production of extra floral nectars (EFNs) that function as indirect defenses by providing nutritional rewards for carnivorous insects (30). In contrast to EFNs, however, AS function as a dangerous lollipop that tags caterpillars with a distinctive odor that complements the information-based indirect defenses of plants that provide spatially and temporally explicit information about the location of feeding larvae to predators. To determine whether AS increase plant fitness and, thereby, can be justifiably considered an indirect “defense” will require the engineering of plants that do not esterify aliphatic acids to their trichome-sugar exudates, a potentially manageable task given the recent advances in our molecular understanding of acyl sugar biosynthesis (11).
Materials and Methods
Plant Material and Insect Rearing.
We used an isogenic line, obtained after 30 generations of inbreeding, of N. attenuata Torr. ex Watson derived from field-collected seeds. Seeds of N. glauca also originated from a field collection from the campus of the University of California, Davis. Additionally, we used the following N. attenuata RNAi lines that have been characterized: irPMT (31) and irLOX2/3 (32, 33). irPMT plants are impaired in nicotine production because of silencing in the putrescine-methyltransferase (PMT) activity. irLOX2/3 plants are silenced in both LOX2 and LOX3 and have dramatically lower levels of GLVs (LOX2) (32) and of the signaling molecule, JA, and its conjugates (LOX3) (32, 33).
Seed germination was performed as described in Krügel et al. (34). All plants were grown in the glasshouse in 1-L individual pots at 26–28 °C under 16 h of light supplied by Philips Sun-T Agro 400- or 600-W sodium lights. Artificial diet for feeding experiments was prepared according to Waldbauer et al. (35). M. sexta larvae were derived from an in-house colony. Larvae of S. littoralis and S. exigua (Lepidoptera, Noctuidae) were supplied as egg clutches by BayerCropScience (40789). M. sexta frass was collected daily from fourth to fifth instar caterpillars feeding on either of the Nicotiana species and stored at −80 °C until used for experiments.
Trichome Density Determinations.
Trichome density (Fig. S1 A and B) was measured, as described by Boughton et al. (36), by counting trichomes on the adaxial and abaxial sides of leaf discs of three different laminar positions that span the length of the leaf. Trichomes were stained with a 0.5% m/v solution of Rhodamine B in water as described in Lin and Wagner (37), and subsequently counted under a Zeiss Stereomicroscope Discovery.V8 (Carl Zeiss). Leaf areas were calculated by using the SigmaScanPro 5 (SPSS Inc.) software, and trichome densities were calculated as the number of trichomes per leaf area.
Glasshouse Bioassays.
Freshly hatched neonates of M. sexta, S. littoralis, and S. exigua were transferred to the abaxial side of freshly excised leaves of N. attenuata and observed under a Zeiss stereomicroscope SV11 (Carl Zeiss). The caterpillars were allowed to move freely, their first feeding choice within 60 s was monitored, and the first feeding choice was recorded (Fig. 1C).
To evaluate the effects of N. attenuata O-acyl sugars on caterpillar performance, we used sepals subtending immature capsules (Fig. S2). These tissues are characterized by their high trichome densities so that larvae cannot consume the lamina without first consuming the trichomes that cover the lamina and, hence, are ideally suited for trichome feeding assays. To dissect the roles of the trichome specific metabolites, we used N. attenuata wild-type plants and irPMT plants, which do not contain nicotine in their trichomes. Additionally, O-acyl sugars were removed from sepals by gently shaking leaves or sepals three times in 30 mL of distilled water for 10 s. Freshly hatched M. sexta larvae were placed on excised sepals or unripe seed capsules of either washed or unwashed irPMT and WT plants. Caterpillars were placed in single plastic containers with one bud and caterpillar and allowed to feed freely for 3 h. Afterward, the plant tissue was removed, larvae were starved for 18 h, and the survival rate was recorded.
O-Acyl Sugar Analysis.
Rosette leaves were harvested by cutting the petiole with a scalpel, washed with 10 mL of methanol (VWR International), and spiked with 10 μg of sucrose monolaureate as an internal standard. The washing solution was filtered through paper filter (Whatman) and gently evaporated to dryness under a stream of nitrogen. Before HPLC-ToFMS analysis, the residue was resolved in 1 mL of methanol and transferred to a 2-mL LC-vial.
One-microliter aliquots of AS extract were separated by using an Agilent HPLC 1100 Series system, combined with Gemini NX C18 column (150 × 2 mm). Eluted compounds were detected by a Bruker MicroToF mass spectrometer (Bruker Daltronik) equipped with an ion spray source in positive-ion mode. The instrument was operated with parameters according to Heiling et al. (38).
The composition of the aliphatic acids moieties was studied by analysis of aliphatic acid methyl esters. The dichloromethane phase was collected, the remaining water was removed by filtering through a Pasteur pipette filled with anhydrous sodium sulfate, and 1 μL was analyzed by GCxGC-ToFMS according to Gaquerel et al. (39). Identification was performed by comparison of spectra and retention times with those of authentic standards.
For the alkaline hydrolysis, 500 μL of KOH (0.2 M) were added to 500 μL of the methanolic extract and sealed in a vial for 24 h. The mixture was neutralized by addition of HCL (0.2 M), and the aliphatic acids were extracted and measured as described above (Fig. S3). For additional information, see SI Materials and Methods.
M. sexta Body and Feces Headspace Analysis.
An alkaloid-free mixture of N. attenuata and D. wrightii AS was extracted by following the method described in ref. 16. A small portion was dissolved in MeOH and analyzed by HPLC-ToFMS to verify the extraction.
The alkaloid-free mixture of AS obtained from leaves of N. attenuata was dissolved in diethylether and applied to artificial diet (1 mg/g diet). The diethlylether was allowed to evaporate, and afterward, caterpillars were allowed to feed on either AS-free or AS-containing diet. AS were removed from excised leaves of N. attenuata by gently soaking leaves in MeOH for 30 s and carefully drying them with paper tissue (Fig. S4 B and C).
Caterpillars of second to third instar were enclosed in two 50-mL food-quality plastic containers (Huhtamaki) containing either artificial diet (±AS) or leaf tissue (washed/unwashed N. attenuata, N. glauca, or D. wrightii). Volatile emissions from the enclosed caterpillars were trapped for 2 h. Immediately after collection, traps were eluted by spiking each with 400 ng of tetralin (Sigma Aldrich) as an internal standard and flushing the trap with 150 μL of dichloromethane into a GC vial containing a glass insert. The sample was subjected to GC-MS analysis on a Varian 3800 system (Varian) equipped with a 30-m DB-Wax column (ID 0.25 mm, df 0.25 μm; Supelco) connected to a Saturn 2000 ion trap. One microliter was injected into a 230 °C injector. The flow was constant and kept at 1 mL/min throughout the following temperature gradient: 3 min at 40 °C, 4 °C/min until 180 °C and 10 °C/min until 240 °C. Transfer line was maintained at 250 °C and ionization was performed in EI-mode.
Field Bioassays.
We choose five colonies of which two were spatially separated from the remaining three (Fig. S7E). We placed wooden sticks near to the nest entrance but without leaflets or caterpillars attached. BCAA in water (0.05% Tween-20) or water controls were sprayed at the bases of the sticks. We used five sticks for each treatment and counted the number of climbing ants within a 15-min interval (Fig. 3). Additionally we added hexanoic acid (H), which is the major aliphatic acid within the body odor of D. wrightii-fed caterpillars (Fig. S8 B and C), to the previously used BCAA mixtures (BCAA+H). The same assays as before were performed on the previously described colonies spraying BCAA+H to the base of the sticks and using water +0.05% Tween-20 as a control.
Statistical Analysis.
All statistical analyses were performed with Excel (Microsoft Corporation) or StatView (SAS Institute). Data were transformed, if necessary, to meet the requirements for homogeneity of variance.
Supplementary Material
Acknowledgments
We thank D. Heckel, M. Knaden, J. Gershenzon, B. Hansson, and anonymous reviewers for comments on the manuscript; P. Frandsen and B. Hölldobler for determining the ant species; J. Baldwin, J. Jochens, and C. Diezel for help with the field experiments; E. Gaquerel for analytical help; Animal and Plant Health Inspection Service for constructive regulatory oversight; and Brigham Young University for the use of their Lytle Ranch Preserve field station. Dedicated to Thomas Eisner (1929–2011), who taught his students to think like insects and perceive the world from their perspective.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101306108/-/DCSupplemental.
References
- 1.Kennedy GG. Tomato, pests, parasitoids, and predators: Tritrophic interactions involving the genus Lycopersicon. Annu Rev Entomol. 2003;48:51–72. doi: 10.1146/annurev.ento.48.091801.112733. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd RW, Bass WT, Houtz RL, Wagner GJ. Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell. 2005;17:1851–1861. doi: 10.1105/tpc.105.031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot (Lond) 2004;93:3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso MZ. Herbivore handling of a Plants trichome: The case of Heliconius charithonia (L.) (Lepidoptera: Nymphalidae) and Passiflora lobata (Killip) Hutch. (Passifloraceae) Neotrop Entomol. 2008;37:247–252. doi: 10.1590/s1519-566x2008000300002. [DOI] [PubMed] [Google Scholar]
- 5.Simmons AT, Gurr GM, McGrath D, Martin PM, Nicol HI. Entrapment of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on glandular trichomes of Lycopersicon species. Aust J Entomol. 2004;43:196–200. [Google Scholar]
- 6.Laue G, Preston CA, Baldwin IT. Fast track to the trichome: Induction of N-acyl nornicotines precedes nicotine induction in Nicotiana repanda. Planta. 2000;210:510–514. doi: 10.1007/s004250050038. [DOI] [PubMed] [Google Scholar]
- 7.Schilmiller A, et al. Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J. 2010;62:391–403. doi: 10.1111/j.1365-313X.2010.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd RW, Wagner GJ. Phylloplane proteins: Emerging defenses at the aerial frontline? Trends Plant Sci. 2007;12:51–56. doi: 10.1016/j.tplants.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine's defensive function in nature. PLOS-Biology. 2004;8:1074–1080. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrendale RF, et al. Characterization of the sucrose ester fraction from Nicotiana Glutinosa. J Agric Food Chem. 1990;38:75–85. [Google Scholar]
- 11.Slocombe SP, et al. Transcriptomic and reverse genetic analyses of branched-chain fatty acid and acyl sugar production in Solanum pennellii and Nicotiana benthamiana. Plant Physiol. 2008;148:1830–1846. doi: 10.1104/pp.108.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie CL, Puterka GJ. Effect of sucrose octanoate on survival of nymphal and adult Diaphorina citri (Homoptera: Psyllidae) J Econ Entomol. 2004;97:970–975. doi: 10.1603/0022-0493(2004)097[0970:eosoos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez AE, Tingey WM, Mutschler MA. Acylsugars of Lycopersicon pennellii deter settling and feeding of the green peach aphid (Homoptera, Aphididae) J Econ Entomol. 1993;86:34–39. [Google Scholar]
- 14.Chortyk OT, Pomonis JG, Johnson AW. Syntheses and characterizations of insecticidal sucrose esters. J Agric Food Chem. 1996;44:1551–1557. [Google Scholar]
- 15.Puterka GJ, Farone W, Palmer T, Barrington A. Structure-function relationships affecting the insecticidal and miticidal activity of sugar esters. J Econ Entomol. 2003;96:636–644. doi: 10.1603/0022-0493-96.3.636. [DOI] [PubMed] [Google Scholar]
- 16.Van Dam NM, Hare JD. Biological activity of Datura wrightii glandular trichome exudate against Manduca sexta larvae. J Chem Ecol. 1998;24:1529–1549. [Google Scholar]
- 17.Eisner T, Shepherd J. Caterpillar feeding on a sundew plant. Science. 1965;150:1608–1609. doi: 10.1126/science.150.3703.1608. [DOI] [PubMed] [Google Scholar]
- 18.Martin JS, Martin MM, Bernays EA. Failure of tannic acid to inhibit digestion or reduce digestibility of plant protein in gut fluids of insect herbivores. J Chem Ecol. 1987;13:605–621. doi: 10.1007/BF01880103. [DOI] [PubMed] [Google Scholar]
- 19.Agelopoulos NG, Dicke M, Posthumus MA. Role of volatile infochemicals emitted by feces of larvae in host-searching behavior of parasitoid Cotesia rubecula (Hymenoptera: Braconidae): A behavioral and chemical study. J Chem Ecol. 1995;21:1789–1811. doi: 10.1007/BF02033677. [DOI] [PubMed] [Google Scholar]
- 20.Herard F, Keller MA, Lewis WJ, Tumlinson JH. Beneficial arthropod behavior mediated by airborne semiochemicals. 4. Influence of host diet on host-oriented flight chamber responses of Microplitis demolitor Wilkinson (Hymenoptera: Braconidae) J Chem Ecol. 1988;14:1597–1606. doi: 10.1007/BF01012525. [DOI] [PubMed] [Google Scholar]
- 21.Weiss MR. Good housekeeping: Why do shelter-dwelling caterpillars fling their frass? Ecol Lett. 2003;6:361–370. [Google Scholar]
- 22.Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- 23.Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329:1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- 24.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 25.Guerrieri FJ, d'Ettorre P. Associative learning in ants: Conditioning of the maxilla-labium extension response in Camponotus aethiops. J Insect Physiol. 2010;56:88–92. doi: 10.1016/j.jinsphys.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Whitford WG, Jackson E. Seed harvester ants (Pogonomyrmex rugosus) as “pulse” predators. J Arid Environ. 2007;70:549–552. [Google Scholar]
- 27.Wagner D, Tissot M, Cuevas W, Gordon DM. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J Chem Ecol. 2000;26:2245–2257. [Google Scholar]
- 28.Hölldobler B, David Morgan E, Oldham N, Liebig J, Liu Y. Dufour gland secretion in the harvester ant genus Pogonomyrmex. Chemoecology. 2004;14:101–106. [Google Scholar]
- 29.Haak U, Hölldobler B, Bestmann HJ, Kern F. Species-specificity in trail pheromones and Dufour's gland contents of Camponotus atriceps and C. floridanus (Hymenoptera: Formicidae) Chemoecology. 1996;7:85–93. [Google Scholar]
- 30.Kessler A, Heil M. The multiple faces of indirect defences and their agents of natural selection. Funct Ecol. 2011;25:348–357. [Google Scholar]
- 31.Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine's defensive function in nature. PLoS Biol. 2004;2:E217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allmann S, Halitschke R, Schuurink RC, Baldwin IT. Oxylipin channelling in Nicotiana attenuata: Lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ. 2010;33:2028–2040. doi: 10.1111/j.1365-3040.2010.02203.x. [DOI] [PubMed] [Google Scholar]
- 33.Halitschke R, Baldwin IT. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003;36:794–807. doi: 10.1046/j.1365-313x.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 34.Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12:177–183. [Google Scholar]
- 35.Waldbauer GP, Yamamoto RT, Bowers WS. Laboratory rearing of tobacco hornworm Protoparce sexta (Lepidoptera: Sphingidae) J Econ Entomol. 1964;57:93. [Google Scholar]
- 36.Boughton AJ, Hoover K, Felton GW. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol. 2005;31:2211–2216. doi: 10.1007/s10886-005-6228-7. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y, Wagner GJ. Rapid and simple method for estimation of sugar esters. J Agric Food Chem. 1994;42:1709–1712. [Google Scholar]
- 38.Heiling S, et al. Jasmonate and ppHsystemin regulate key Malonylation steps in the biosynthesis of 17-Hydroxygeranyllinalool Diterpene Glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell. 2010;22:273–292. doi: 10.1105/tpc.109.071449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaquerel E, Weinhold A, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphigidae) and its natural host Nicotiana attenuata. VIII. An unbiased GCxGC-ToFMS analysis of the plant's elicited volatile emissions. Plant Physiol. 2009;149:1408–1423. doi: 10.1104/pp.108.130799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.