Abstract
The maintenance of energy homeostasis is essential for life, and its dysregulation leads to a variety of metabolic disorders. Under a fed condition, mammals use glucose as the main metabolic fuel, and short-chain fatty acids (SCFAs) produced by the colonic bacterial fermentation of dietary fiber also contribute a significant proportion of daily energy requirement. Under ketogenic conditions such as starvation and diabetes, ketone bodies produced in the liver from fatty acids are used as the main energy sources. To balance energy intake, dietary excess and starvation trigger an increase or a decrease in energy expenditure, respectively, by regulating the activity of the sympathetic nervous system (SNS). The regulation of metabolic homeostasis by glucose is well recognized; however, the roles of SCFAs and ketone bodies in maintaining energy balance remain unclear. Here, we show that SCFAs and ketone bodies directly regulate SNS activity via GPR41, a Gi/o protein-coupled receptor for SCFAs, at the level of the sympathetic ganglion. GPR41 was most abundantly expressed in sympathetic ganglia in mouse and humans. SCFA propionate promoted sympathetic outflow via GPR41. On the other hand, a ketone body, β-hydroxybutyrate, produced during starvation or diabetes, suppressed SNS activity by antagonizing GPR41. Pharmacological and siRNA experiments indicated that GPR41-mediated activation of sympathetic neurons involves Gβγ-PLCβ-MAPK signaling. Sympathetic regulation by SCFAs and ketone bodies correlated well with their respective effects on energy consumption. These findings establish that SCFAs and ketone bodies directly regulate GPR41-mediated SNS activity and thereby control body energy expenditure in maintaining metabolic homeostasis.
Keywords: microbiota, superior cervical ganglion, FFAR3, probiotics, fasting
To balance energy intake, dietary excess and fasting triggers an increase or a decrease in energy expenditure, respectively, by regulating activity of the sympathetic nervous system (SNS) (1–3), and its dysregulation leads to metabolic disorders such as obesity and diabetes (4, 5). In feeding, excessive energy is consumed by the enhancement of sympathetic function, resulting in increases in heart rate and diet-induced thermogenesis (2, 6), whereas in fasting, energy use is saved by the suppression of the sympathetic function as a survival mechanism, resulting in the reduction in heart rate and activity (3, 6). Under a fed condition, mammals use glucose as the main metabolic fuel, and short-chain fatty acids (SCFAs) produced by the colonic bacterial fermentation of dietary fiber also contribute a significant proportion of the daily energy requirement (7, 8). Under ketogenic conditions such as fasting and diabetes, ketone bodies produced in the liver from fatty acids are used as the main energy sources (9, 10). However, the effect of monocarboxylic metabolites, as SCFAs and ketone bodies, on the regulation of SNS activity remains unclear.
Free fatty acids (FFA) not only are essential nutrients but also act as signaling molecules in various cellular processes. Recently, several groups reported that five orphan G protein-coupled (GPR) receptors—GPR40, GPR41, GPR43, GPR84, and GPR120—can be activated by FFAs. Long-chain fatty acids are specific agonists for GPR40 and GPR120 (11, 12) and medium-chain fatty acids are for GPR84 (13). Short-chain fatty acids can activate GPR41 (14) and GPR43 (15). Stimulation of GPR41 by FFAs resulted in inhibiting cAMP production and activation of the ERK cascade, which suggests interactions with the Gα(i/o) family of G proteins (16, 17). GPR41 has been reported to be expressed in adipose tissue and to promote secretion of leptin (18, 19). Other physiological functions of GPR41 still remain to be explored.
In the present study we show that SCFAs and ketone bodies, major energy sources in the body, directly regulate sympathetic activity via GPR41. By examining the cardiac and metabolic sympathetic responses in vitro as well as in mice lacking GPR41, we found that propionate (a major SCFA) and β-hydroxybutyrate (a major ketone body) promotes or depresses GPR41-mediated SNS activation, respectively.
Results
Abundant Gpr41 Expression in Sympathetic Ganglia and Reduced Sympathetic Nerve Activity in Gpr41−/− Mice.
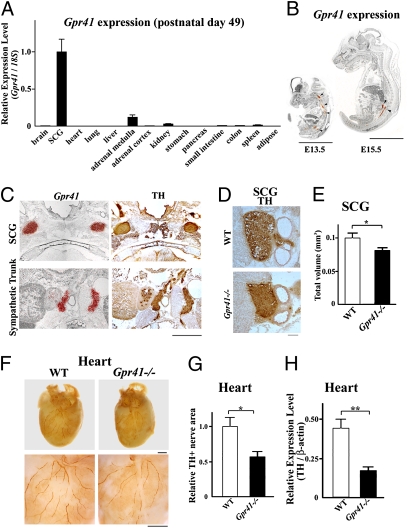
Examining Gpr41 and Gpr43 expression in tissues, we found that Gpr41 was most abundantly expressed in the sympathetic ganglia such as the superior cervical ganglion (SCG) in adult mouse (Fig. 1A). SCG is an extension of the cervical sympathetic chain and a mass of sympathetic neurons. Compared with Gpr41, Gpr43 was scarcely expressed in the SCG of either wild-type or Gpr41−/− mice (Fig. S1 A and B). In situ hybridization revealed that Gpr41 was most abundantly expressed in sympathetic ganglia and trunks during embryonic (E13.5 and E15.5) and postnatal (P1) stages (Fig. 1 B and C) to adulthood in mice and also in the sympathetic ganglia of human adults (Fig. S1 C and D).
Fig. 1.
Abundant Gpr41 expression in sympathetic ganglia and reduced sympathetic nerve activity in GPR41-deficient mice. (A) Gpr41 expression in postnatal mouse tissues (P49) measured by qRT-PCR (n = 3). SCG, superior cervical ganglion. Internal control: 18S rRNA expression. (B) Gpr41 mRNA localization in mouse embryos (E13.5 and E15.5) as determined by in situ hybridization using an 35S-labeled antisense Gpr41 RNA probe. Red grains (arrowheads) superimposed on a hematoxylin−eosin-stained section indicate Gpr41 mRNA localization. (Scale bar: 5 mm.) (C) Gpr41 mRNA localization in postnatal day 1 (P1) mice (Left). Anti-tyrosine hydroxylase (TH) immunostaining (brown) (Right). (Scale bar: 1 mm.) (D and E) Anti-TH antibody immunostaining (brown) in SCG at P1. Total volume was measured by quantifying the TH-positive area (n = 6). (F) (Upper) Whole-mount immunostaining of the heart with anti-TH antibodies (brown). (Lower) High magnification view. (Scale bar: 1 mm.) (G) Quantitative analysis of TH+ nerve areas (n = 5). (H) TH protein expression (n = 6). β-Actin (loading control). Mice were analyzed at 12 wk of age (F–H). *P < 0.05; **P < 0.005.
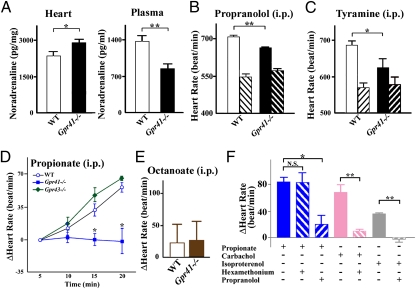
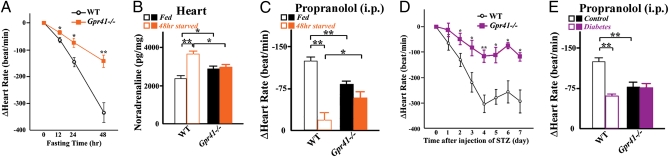
To determine the effect of GPR41 on the SNS, we generated Gpr41−/− mice (Fig. S2). Gpr41−/− mice exhibited normal growth and no major morphological abnormalities. Body weight, heart-weight/body-weight ratio and metabolic parameters and hormones (plasma concentrations of glucose, triglycerides, free fatty acids, leptin, and insulin) were comparable between wild-type and Gpr41−/− mice (Fig. S3 A–G). During development (P1), the SCG volume was significantly smaller in Gpr41−/− than in wild-type mice (Fig. 1 D and E). Gpr41−/− mice exhibited significantly reduced density of sympathetic innervations and tyrosine hydroxylase (TH) protein in the heart (Fig.1 F–H), indicating that GPR41 may be involved in sympathetic nerve growth. TH is the rate-limiting enzyme for catecholamine biosynthesis and plays an important role in the sympathetic regulation of the heart function. Corresponding with the retarded growth of sympathetic innervations, resting heart rate was significantly reduced in Gpr41−/− mice (682 ± 10 beat/min and 610 ± 16 beat/min in wild-type and Gpr41−/−; n = 12 each, respectively) (Fig. 2B). In Gpr41−/− mice, on the other hand, cardiac noradrenaline (NA) content was significantly increased, whereas plasma NA level was decreased (Fig. 2A). The reduction in heart rate associated with treatment using the β-adrenoreceptor blocker propranolol was more marked in wild-type than in Gpr41−/− mice, and the resultant heart rates were similar to those of both wild-type and Gpr41−/− mice (Fig. 2B). Treatment with tyramine, an agent causing release of stored NA from sympathetic nerve terminals, reduced heart rate and plasma and cardiac NA concentrations in both groups of mice, and the between-group differences in the parameters before tyramine treatment were not found (Fig. 2C and Fig. S3H). Results suggested that the lower resting heart rate in Gpr41−/− mice is due to the reduced SNS activity. Furthermore, enhanced cardiac NA stores and the reduced circulating NA levels in Gpr41−/− mice indicated that GPR41 may contribute to NA release from SNS.
Fig. 2.
Effects of SCFA on sympathetic activity via GPR41 in GPR41-deficient mice. (A) Noradrenaline (NA) concentrations in hearts (n = 10) and plasma (n = 6). (B) Effects of propranolol on heart rate in Gpr41−/− mice. Measurement of heart rate at 10 min after propranolol administration (i.p.; n = 7–9). Both hatched bars, propranolol-treated. (C) Effects of tyramine on heart rate. Measurement of heart rate (n = 9) at 24 h following tyramine injection (i.p.). Both hatched bars, tyramine-treated. (D) Effects of propionate on heart rate in Gpr41−/− mice (i.p.; n = 5–7). (E) Effects of octanoate on heart rate in Gpr41−/− mice. Measurement of heart rate at 20 min after octanoate administration (i.p.; n = 4–5). (F) After pretreatment with hexamethonium (20 mg/kg) or propranolol (4 mg/kg) for 10 min, at time 0, a bolus of propionate (1 g/kg), carbachol (50 μg/kg), or isoproterenol (3 μg/kg) was administered intraperitoneally (n = 4–6). Data of was measured at 20 min (propionate) and at 3 min (carbachol and isoproterenol). Mice were analyzed at 12 wk of age. *P < 0.05; **P < 0.005.
Effects of SCFA on Sympathetic Activity.
To demonstrate that SCFAs are relevant for the effects of GPR41 on SNS, we examined the effect of propionate, an SCFA found to have the most potent agonistic effect in the heterologous expression system (Fig. S4). Administration of propionate (1 g/kg, i.p.), but not of a middle-chain fatty acid octanoate (1 g/kg, i.p.), caused a significant increase in heart rate in wild-type and Gpr43−/− mice, whereas neither propionate nor octanoate caused any change in heart rate in Gpr41−/− mice (Fig. 2 D and E and Fig. S5 A and B). Also, metabolic parameters and hormones (plasma concentrations of glucose, triglycerides, free fatty acids, and leptin) following administration of propionate were comparable between wild-type and Gpr41−/− mice (Fig. S5C). Treatment with a ganglion blocker hexamethonium (20 mg/kg i.p.) had little effect on this propionate-induced increase in heart rate, whereas propranolol (4 mg/kg i.p.) abolished the response (Fig. 2F). To confirm that the treatments with hexamethonium and propranolol have respective ganglion blocking and β-blocking effects, we examined their effects on heart rate responses to a cholinergic agonist carbachol and β-adrenoreceptor agonist isoproterenol. Carbachol- and isoproterenol-induced increases in heart rate were suppressed by hexamethonium and by propranolol, respectively (Fig. 2F). Thus, propionate may modulate the SNS activity at the level of sympathetic ganglion via GPR41 but not via GPR43.
SCFA Propionate Induced Sympathetic Activation via GPR41-Gβγ in Sympathetic Neurons.
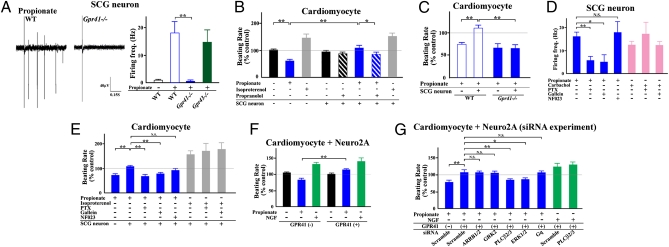
We further examined whether this propionate-induced positive chronotropism is due to the GPR41-mediated SNS activation or not, by using coculture of primary-cultured fetal isolated cardiomyocytes and sympathetic neurons. RT-PCR assays confirmed that primary-cultured sympathetic neurons obtained from fetal SCG highly express Gpr41, but that fetal isolated cardiomyocytes lack Gpr41 expression (Fig. S6A). In primary-cultured sympathetic neurons from wild-type and Gpr43−/− mice, propionate evoked extracellular action potentials (Fig. 3A and Fig. S6B). However, this propionate-activated response was not observed in sympathetic neurons obtained from Gpr41−/− mice (Fig. 3A). Propionate reduced intracellular cAMP concentrations and promoted ERK1/2 phosphorylation in primary-cultured sympathetic neurons (Fig. S6 C and D). These propionate-induced responses were not observed in sympathetic neurons from Gpr41−/− mice, and they were abolished by pertussis toxin (PTX) (Fig. S6 C–E), indicating that propionate-activated GPR41 signaling is required for Gi/o. We further examined the effects of SCFAs on cardiac SNS activity by using the coculture of fetal isolated cardiomyocytes with primary-cultured sympathetic neurons. Isoproterenol significantly increased beat rate in either monocultured or cocultured cardiomyocytes. Propionate, on the other hand, reduced beat rate in monocultured cardiomyocytes, whereas in cocultured cardiomyocytes propionate did not reduce, but rather increased, beat rate, which was abolished by propranolol (Fig. 3B). Therefore, propionate significantly (P < 0.005) increased the beat rate of the cardiomyocytes cocultured with SCG neurons compared with that of the monocultured myocytes (Fig. 3B). As with wild-type mice, isoproterenol increased and propionate reduced beat rate, respectively, in monocultured cardiomyocytes isolated from Gpr41−/− mice (Fig. S6F). However, in coculture of isolated cardiomyocytes with sympathetic neurons obtained from Gpr41−/− mice, propionate did not elicit a rise in beat rate (Fig. 3C). Rather, propionate reduced beat rate, as was observed in experiments with monocultured cardiomyocytes (Fig. 3C). Results showed that an SCFA propionate evokes action potentials in sympathetic neurons via GPR41 and thereby directly enhances SNS outflow. We further examined the GPR41-mediated signaling in SCG neurons. As G protein signaling has been recently well characterized by using selective pharmacological tools (such as inhibitors for Gα and Gβγ proteins), we adopted NF023 [Gα(i/o) blocker] and Gallein (Gβγ blocker) for the purpose (20, 21). GPR41-mediated generation of action potential and rise in beat rate was effectively blocked by Gallein and PTX treatment, whereas NF023 had no inhibitory effects (Fig. 3 D and E). We confirmed that PTX and NF023 successfully inhibited GPR41-mediated cAMP inhibition, whereas Gallein had no such an inhibitory effect (Fig. S6G). These series of pharmacological studies showed that GPR41-mediated excitatory responses in SCG are mediated by Gβγ, but not by Gα(i/o), coupled to cAMP inhibition. We further examined the intracellular signaling for GPR41-induced sympathetic activation by using RNA interference. As it was difficult to transfer small interfering RNA (siRNA) into the primary-cultured sympathetic neurons, we adopted mouse neuroblastoma cell line Neuro2A cells as a surrogate of sympathetic neurons (22, 23). Neuro2A cells express barely Gpr41 or Gpr43 (Fig. S6H). Propionate significantly increased beat rate when cardiomyocytes were cocultured with Neuro2A cells expressing GPR41 (Fig. 3F and Fig. S6I). NGF, known to stimulate noradrenaline release from sympathetic neurons (24), increased beat rate when cocultured with Neuro2A cells transfected either with or without Gpr41 (Fig. 3F). Treatment of GPR41-expressing Neuro2A cells with siRNA for PLCβ2/3, but not those for β-arrestin1/2 and GRK2 (25, 26), significantly inhibited the propionate-induced rise in beat rate of cardiomyocytes when cocultured (Fig. 3G and Fig. S6 J and K). Moreover, siRNA for ERK1/2, but not Gαq, showed a significant inhibitory effect (Fig. 3G). These results indicate that GPR41 activation of sympathetic neurons may involve Gβγ, PLCβ, and MAPK, but not Gα(i/o), β-arrestin, and GRKs.
Fig. 3.
SCFA-GPR41 signaling in sympathetic neurons. (A) Action potentials and firing frequency in Gpr41−/− sympathetic neurons following stimulation with propionate (10 mM) (n = 3–8). (B) Change in myocyte beating rate in sympathetic neurons with and without propionate (1 mM) treatment and effects of propranolol (0.2 μM) (n = 5–6). Isoproterenol (10 μM) was used as a positive control. (C) Effects of propionate on change in myocyte beating rate in Gpr41−/− cardiomyocytes and sympathetic neurons (n = 6–8). (D) Firing frequency in sympathetic neurons (n = 4–9) by propionate (10 mM) stimulation after pretreatment with or without PTX (100 ng/mL), Gallein (10 μM), or NF023 (10 μM) for 2 h. Carbachol (1 μM), a cholinergic agonist, was used as a positive control. (E) Effects of propionate on change in myocyte beat rate in cardiomyocytes and sympathetic neurons (n = 6–11). Cells were stimulated by propionate (1 mM) after pretreatment with or without PTX (100 ng/mL), Gallein (10 μM), or NF023 (10 μM) for 1 h. Isoproterenol (10 μM) was used as a positive control. (F) Effect of propionate (1 mM) and NGF (50 ng/mL) on the beating rate of cardiomyocytes when cocultured with Neuro2A cells either with or without transfection of Gpr41 (n = 6). (G) Effects of siRNA on propionate-induced increase in the beating rate of cardiomyocytes when cocultured with Neuro2A cells expressing GPR41 (n = 6). *P < 0.05; **P < 0.005.
Ketone Body β-Hydroxybutyrate Inhibited Sympathetic Activity by Antagonizing GPR41.
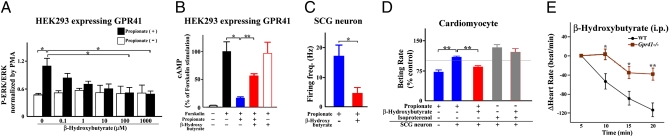
Under ketogenic conditions such as fasting, low-carbohydrate diet feeding, and diabetes, fatty acids and ketone bodies are used as the main energy sources (27). In assessing the effects of SCFAs and ketone bodies in GPR41-expressing HEK293 cells (16), we found that β-hydroxybutyrate has a potent antagonistic effect on GPR41, whereas acetoacetate, another major ketone body, has no significant effect (Fig. S7A). β-Hydroxybutyrate suppressed propionate-induced ERK1/2 activation in a dose-dependent manner (Fig. 4A) and inhibited the propionate-induced reduction in cAMP production in GPR41-expressing HEK293 cells (Fig. 4B). β-Hydroxybutyrate also inhibited both propionate-evoked firing frequency in primary-cultured sympathetic neurons (Fig. 4C), and propionate-induced rise in beat rate of cardiomyocytes cocultured with sympathetic neurons (Fig. 4D). Administration of β-hydroxybutyrate (500 mg/kg, i.p.), but not of another major ketone body, acetone (0.5 g/kg, i.p.), caused a significant decrease in heart rate in wild-type mice, whereas either β-hydroxybutyrate or acetone caused less change in heart rate in Gpr41−/− mice (Fig. 4E and Fig. S7 B–E). Moreover, β-hydroxybutyrate also inhibited the propionate-induced increase in heart rate in wild-type mice but not in Gpr41−/− mice (Fig. S7F). Treatment with hexamethonium did not affect the β-hydroxybutyrate-induced decrease in heart rate, whereas propranolol completely suppressed the response (Fig. S7G). The results show that β-hydroxybutyrate may also inhibit SNS activity at the level of the sympathetic ganglion. Hence, it may be possible for β-hydroxybutyrate to suppress the propionate-induced SNS activation as an antagonist for GPR41.
Fig. 4.
Inhibitory effects of β-hydroxybutyrate on GPR41 sympathetic activity. (A) Antagonistic effects of β-hydroxybutyrate on ERK1/2 phosphorylation by propionate (1 mM) in GPR41-expressing HEK293 cells (n = 3). (B) Reduction in cAMP levels in response to propionate (0.1 mM) treatment in GPR41-expressing HEK293 cells and inhibitory effects of β-hydroxybutyrate (100 mM) (n = 3). (C) Firing frequency in sympathetic neurons (n = 5) after propionate (10 mM) stimulation with or without β-hydroxybutyrate (10 mM). (D) Inhibitory effects of β-hydroxybutyrate (500 μM) on change in myocyte beat rate in cardiomyocytes and sympathetic neurons with propionate (1 mM) treatment (n = 5–8). (E) Effects of β-hydroxybutyrate on heart rate of Gpr41−/− mice (500 mg/kg i.p.; n = 8). Mice were analyzed at 12 wk of age. *P < 0.05; **P < 0.005.
Reduced Sympathetic Activity Under Ketogenic Conditions Is Partly Due to GPR41 Antagonism by β-Hydroxybutyrate.
We further assessed the effect of β-hydroxybutyrate, which can be endogenously produced under ketogenic conditions, on SNS activity. Following 48-h starvation, metabolic parameters (body weight and plasma levels of glucose, triglycerides, and free fatty acids) were comparable between wild-type and Gpr41−/− mice (Fig. S8 A–D). Plasma concentration of β-hydroxybutyrate was significantly higher in Gpr41−/− mice compared with wild-type mice (Fig. S8E). During fasting, heart rate can decline due to sympathetic depression (6, 10). The fasting-associated decline in heart rate was significantly lower in Gpr41−/− compared with wild-type mice (Fig. 5A). At 48-h starvation, an excessive increase in NA stores in the heart was observed in wild-type mice, which may reflect the decreased SNS activity; however, such an excessive increase in NA stores was not observed in Gpr41−/− mice (Fig. 5B). Furthermore, the reduction in heart rate by propranolol was markedly smaller during starvation compared with fed conditions in wild-type mice (Fig. 5C). On the other hand, in Gpr41−/− mice, the reduction in heart rate by propranolol was not affected by 48-h starvation (Fig. 5C). Taken together, the results showed that starvation-associated sympathetic depression appears to be lacking in Gpr41−/− mice.
Fig. 5.
Inhibitory effects of ketone bodies on GPR41 sympathetic activity during fasting or diabetes. (A) Heart rate following fasting in Gpr41−/− mice (n = 7–8). (B) NA content in the heart after 48-h starvation (n = 9−10). (C) Effects of sympathetic nerve blocking on heart rate of Gpr41−/− starved mice (n = 9–11). (D) Change in heart rate following the induction of diabetes (n = 4−10). (E) Effects of sympathetic nerve blocking on heart rate in Gpr41−/− diabetic mice (n = 4–5). Mice were analyzed at 12–14 wk of age. *P < 0.05; **P < 0.005.
Ketone bodies increase dramatically in diabetes (10). In streptozotocin (STZ)-induced diabetes in mice, heart rate declined during the progression of diabetes (28). Reduction of heart rate in the diabetic condition was significantly smaller in Gpr41−/− compared with wild-type mice (Fig. 5D), although the STZ-induced diabetic condition had similar effects on body weight and plasma levels of glucose and β-hydroxybutyrate in both groups (Fig. S8F). Furthermore, propranolol induced a significantly smaller reduction in heart rate in diabetic mice compared with control wild-type mice (Fig. 5E). In Gpr41−/− mice, on the other hand, propranolol did not cause any effect on heart rate, either with or without STZ treatment (Fig. 5E).
Collectively, our results indicate that GPR41 is required to induce sympathetic depression under ketogenic conditions, caused by either starvation or diabetes. However, the heart rate progressively decreased with time elapsed under ketogenic conditions in both wild-type and Gpr41−/− mice. Also, propranolol treatment decreased heart rate by ∼130 in wild-type mice (Fig. 5 C and E), whereas under ketogenic conditions it decreased heart rate by ∼300 (Fig. 5 A and D), respectively, indicating that ketone bodies not only inhibit sympathetic activity, but also may have a direct inhibitory effect on the heart rate.
Effects of GPR41-Mediated Regulation of Sympathetic Activity on Energy Expenditure.
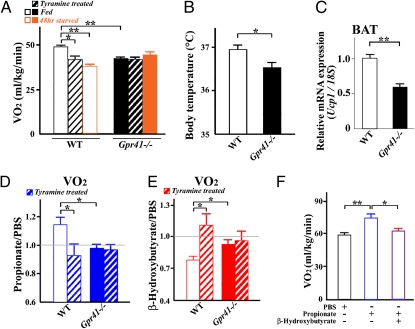
We further assessed to what extent the observed effects of SCFAs and ketone bodies on SNS activity affect energy expenditure. Oxygen consumption during feeding was significantly higher in wild-type mice than in Gpr41−/− mice (Fig. 6A). The oxygen consumption was significantly decreased by treatment with tyramine and during starvation in wild-type mice, whereas these effects were not exhibited in Gpr41−/− mice (Fig. 6A). As total activity was comparable between wild-type and Gpr41−/− mice during feeding and starvation (Fig. S9A), the change in oxygen consumption of Gpr41−/− mice may be due to the metabolic effect but not to physical activity. Also, the respiratory exchange ratio was comparable between wild-type and Gpr41−/− mice during feeding and starvation (Fig. S9B). Furthermore, body temperature and Ucp1 expression in brown adipose tissue were significantly lower in Gpr41−/− compared with wild-type mice (Fig. 6 B and C). As observed with changes in heart rate, propionate (1 g/kg, i.p.) increased and β-hydroxybutyrate (500 mg/kg, i.p.) decreased the oxygen consumption in wild-type mice, respectively. These responses were abolished by tyramine treatment (Fig. 6 D and E). In contrast, propionate, β-hydroxybutyrate, and tyramine treatment did not cause any change in the oxygen consumption of Gpr41−/− mice. Moreover, β-hydroxybutyrate inhibited the propionate-induced increase in oxygen consumption (Fig. 6F). Our results indicate that the effects of SCFAs and ketone bodies on oxygen consumption, which reflects energy expenditure, were well correlated with changes in heart rate, indicating that both physiological responses are controlled by GPR41-mediated SNS activation.
Fig. 6.
Effects of GPR41-mediated regulation of sympathetic activity on energy expenditure. (A) Effects on oxygen consumption in Gpr41−/− mice during feeding and at 48-h starvation. Measurement of oxygen consumption at 24 h after tyramine administration (100 mg/kg i.p.) (n = 5–7). (B) Body temperature of Gpr41−/− mice during feeding (n = 6). (C) Ucp1 expression in brown adipose tissue (BAT) in Gpr41−/− mice during feeding (n = 6). Internal control: 18S rRNA expression. (D) Rate of oxygen consumption in propionate and PBS administration. Oxygen consumption was measured at 40 min after propionate administration (1 g/kg i.p.) (n = 4–8). (E) Rate of oxygen consumption in β-hydroxybutyrate and PBS administration. Oxygen consumption was measured at 50 min after β-hydroxybutyrate administration (500 mg/kg i.p.) (n = 5–7). Propionate and β-hydroxybutyrate were administrated after 24 h treatment of tyramine. Hatched bars, tyramine-treated (A, D, and E). (F) Inhibitory effects of β-hydroxybutyrate on oxygen consumption. After pretreatment with β-hydroxybutyrate (500 mg/kg) for 10 min, at time 0, a bolus of propionate (1 g/kg) with or without β-hydroxybutyrate (500 mg/kg) was administered intraperitoneally (n = 8). Data were measured at time 40 min. Mice were analyzed at 14–16 wk of age. *P < 0.05; **P < 0.005.
Discussion
The high expression level of Gpr41 in sympathetic ganglia indicates that GPR41 might play an important role in these cells. A series of in vitro and in vivo studies with Gpr41−/− mice showed that an SCFA propionate potently activates SNS at sympathetic ganglia. The mechanism of GPR41-mediated activation of sympathetic ganglion neurons is not mediated by cAMP inhibition, but rather involves Gβγ and MAPK signaling. Furthermore, the major ketone body β-hydroxybutyrate antagonizes SCFA-GPR41 signaling and thereby inhibits SNS. As SCFAs and ketone bodies reflect the nutrient conditions, these monocarboxylic metabolites appear to control energy balance by directly regulating GPR41-mediated sympathetic activation. The GPR41-SNS activation pathway may work as one of the important physiological mechanisms for these metabolic fuels to regulate body energy balance.
Gpr41 had been reported to be expressed in adipose tissue in mice (19) and humans (14) and to stimulate leptin secretion (18, 19). Recent studies, however, have reported that Gpr41 is not expressed in mouse adipose tissues (29–32). Our quantitative RT-PCR (qRT-PCR) analysis also found that Gpr41 is not detected in mouse adipose tissues (Fig. S9C). In contrast to Gpr41, we found that Gpr43 is highly expressed in mouse adipose tissues by qRT-PCR (Fig. S9), confirming previous reports (28). Because propionate can activate GPR43 (14), it is possible that propionate can affect the SNS by GPR43-mediated effects on adipocytes, and especially by secretion of leptin that can potently activate the SNS (33). However, administration of propionate induced a similar extent of alterations in metabolic parameters and leptin levels in wild-type and Gpr41−/− mice (Fig. S5C), showing that propionate-activated GPR43 responses in Gpr41−/− mice were similar to wild-type mice. The results further confirmed the previous reports that SCFAs can promote leptin secretion from adipose and not via GPR41 (29, 30). Taken together, the results showed that the effects of SCFAs on SNS activity may not be mediated by their effects on adipocytes from which adipocytokines such as leptin that can change sympathetic nerve activity can be secreted.
GPR41 is a Gi/o-coupled G protein-coupled receptor (GPCR) related to the inhibition of cAMP production (17). In general, Gi/o-coupled GPCRs work as an inhibitory system. However, our study shows that activation of GPR41 by SCFA excites the SCG neurons by generating action potential and thereby releases noradrenaline from sympathetic nerve terminals, showing that GPR41 mediates an excitatory signaling. Recently, however, many excitatory responses in a variety of cell systems have been reported to be mediated by Gi/o-coupled GPCRs, and those responses were mediated via Gβγ but not via Gα(i/o) (34). Our study with selective antagonists for Gα(i/o) and Gβγ also showed that GPR41-mediated excitation of SCG neurons is mediated by Gβγ signaling but cAMP inhibition by Gα(i/o) is not. Furthermore, our siRNA experiments showed that GPR41-mediated activation of sympathetic neurons involves PLCβ and MAPK signaling, but not β-arrestin and GRK signaling. Taken together, because the catecholamine release in neurons is regulated by MAPK signaling (35, 36) and because Gβγ signaling activates MAPK signaling via PLCβ in Gi/o-coupled GPCRs (34), our results may indicate that GPR41-induced sympathetic activation involves Gβγ-PLCβ-MAPK signaling.
Under fed conditions, a substantial proportion of total dietary energy intake derives from SCFAs produced via the colonic fermentation of dietary fibers by gut microbiota (5–10%) (37). Levels of SCFAs in the gastrointestinal tract vary significantly, depending on the amount of nondigestible fiber in the diet and also relate to the composition of the gut microbiota. Change in the amount and composition of gut microbiota has been implicated in cardiovascular diseases and in obesity in humans (38, 39). Ketogenic conditions have been suggested as a reason for the increased incidence of cardiac disorders (40, 41). Furthermore, SCFAs have recently been reported to profoundly affect inflammatory responses via the chemoattractant receptor GPR43 (15). Because a plethora of physiological processes are regulated by SNS, SCFA–GPR41 interactions that regulate SNS could represent a central mechanism to account for the effects of diet, prebiotics, and probiotics on body homeostasis and may represent avenues for understanding and potentially manipulating physiology and disease.
Materials and Methods
RNA extraction, qRT-PCR, in situ hybridization, immunohistochemistry, animal and diabetic models, generation of HEK293 cells expressing mouse GPR41, cultures of sympathetic neurons, Neuro2A and cardiomyocytes, transfection and knockdown by siRNA, Western blotting and cAMP determination, cardiographic recording, biochemical analyses, electrophysiological recording, indirect calorimetry, thermometry, and statistical analyses are described in SI Materials and Methods. The sequences of primers for qRT-PCR and of siRNAs for knockdown are shown in Tables S1 and S2, respectively.
Supplementary Material
Acknowledgments
We thank Dr. T. Niidome for electrophysiological recording, Dr. N. Itoh and Dr. M. Konishi for in situ hybridization, Dr. K. Ohinata for measuring oxygen consumption, T. Takahashi and K. Suehiro for Western blotting, and Dr. H. Takeshima and Dr. D. Yamazaki for valuable discussion. This study was supported in part by research grants from the Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Science and Technology Agency. This work was supported by research grants from the Astellas Foundation for Research on Metabolic Disorders, Kowa Life Science Foundation, Takeda Science Foundation, and Tanabe Mitsubishi Pharma Research Foundation. S.M. and A.I are fellows supported by the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016088108/-/DCSupplemental.
References
- 1.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachman ES, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 3.Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977;196:1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- 4.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–610. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 5.Cottrell EC, Ozanne SE. Developmental programming of energy balance and the metabolic syndrome. Proc Nutr Soc. 2007;66:198–206. doi: 10.1017/S0029665107005447. [DOI] [PubMed] [Google Scholar]
- 6.Landsberg L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26:497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 8.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 9.Balasse EO, Féry F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247–270. doi: 10.1002/dmr.5610050304. [DOI] [PubMed] [Google Scholar]
- 10.Cahill GF., Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 11.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa A, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem. 2006;281:34457–34464. doi: 10.1074/jbc.M608019200. [DOI] [PubMed] [Google Scholar]
- 14.Brown AJ, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 15.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J Biol Chem. 2008;283:32913–32924. doi: 10.1074/jbc.M805601200. [DOI] [PubMed] [Google Scholar]
- 17.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 18.Al-Lahham SH, et al. Regulation of adipokine production in human adipose tissue by propionic acid. Eur J Clin Invest. 2010;40:401–407. doi: 10.1111/j.1365-2362.2010.02278.x. [DOI] [PubMed] [Google Scholar]
- 19.Xiong Y, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beindl W, et al. Inhibition of receptor/G protein coupling by suramin analogues. Mol Pharmacol. 1996;50:415–423. [PubMed] [Google Scholar]
- 21.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremblay RG, et al. Differentiation of mouse Neuro 2A cells into dopamine neurons. J Neurosci Methods. 2010;186:60–67. doi: 10.1016/j.jneumeth.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Bräutigam M, Dreesen R, Herken H. Tetrahydrobiopterin and total biopterin content of neuroblastoma (N1E-115, N2A) and pheochromocytoma (PC-12) clones and the dependence of catecholamine synthesis on tetrahydrobiopterin concentration in PC-12 cells. J Neurochem. 1984;42:390–396. doi: 10.1111/j.1471-4159.1984.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 24.Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci. 1997;17:9573–9582. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 27.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243–251. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Maeda CY, Fernandes TG, Timm HB, Irigoyen MC. Autonomic dysfunction in short-term experimental diabetes. Hypertension. 1995;26:1100–1104. doi: 10.1161/01.hyp.26.6.1100. [DOI] [PubMed] [Google Scholar]
- 29.Zaibi MS, et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010;584:2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Hong YH, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 31.Samuel BS, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers MG, Jr., Münzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: More complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert PR, Robillard L. G protein specificity: Traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 35.Rosmaninho-Salgado J, Araújo IM, Alvaro AR, Duarte EP, Cavadas C. Intracellular signaling mechanisms mediating catecholamine release upon activation of NPY Y1 receptors in mouse chromaffin cells. J Neurochem. 2007;103:896–903. doi: 10.1111/j.1471-4159.2007.04899.x. [DOI] [PubMed] [Google Scholar]
- 36.Park YS, et al. Activity-dependent potentiation of large dense-core vesicle release modulated by mitogen-activated protein kinase/extracellularly regulated kinase signaling. Endocrinology. 2006;147:1349–1356. doi: 10.1210/en.2005-0959. [DOI] [PubMed] [Google Scholar]
- 37.Owira PM, Winter TA. Colonic energy salvage in chronic pancreatic exocrine insufficiently. J Parenter Enteral Nutr. 2008;32:63–71. doi: 10.1177/014860710803200163. [DOI] [PubMed] [Google Scholar]
- 38.Vijay-Kumar M, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 40.Kuppermann N, Park J, Glatter K, Marcin JP, Glaser NS. Prolonged QT interval corrected for heart rate during diabetic ketoacidosis in children. Arch Pediatr Adolesc Med. 2008;162:544–549. doi: 10.1001/archpedi.162.6.544. [DOI] [PubMed] [Google Scholar]
- 41.Bank IM, Shemie SD, Rosenblatt B, Bernard C, Mackie AS. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol. 2008;39:429–431. doi: 10.1016/j.pediatrneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.