Abstract
Transporters at the hepatic canalicular membrane are essential for the formation of bile and the prevention of cholestatic liver disease. One such example is ATP8B1, a P4-type ATPase disrupted in three inherited forms of intrahepatic cholestasis. Mutation of the X-linked mouse gene Atp11c, which encodes a paralogous P4-type ATPase, precludes B-cell development in the adult bone marrow, but also causes hyperbilirubinemia. Here we explore this hyperbilirubinemia in two independent Atp11c mutant mouse lines, and find that it originates from an effect on nonhematopoietic cells. Liver function tests and histology revealed only minor pathology, although cholic acid was elevated in the serum of mutant mice, and became toxic to mutant mice when given as a dietary supplement. The majority of homozygous mutant females also died of dystocia in a maternal genotype-specific manner. ATP11C therefore represents a multifunctional transporter, essential for adult B-cell development, the prevention of intrahepatic cholestasis, and parturition, and is a new candidate for genetically undiagnosed cases of cholestasis and dystocia in humans.
Keywords: gallbladder, uterus, enterohepatic circulation, jaundice, bile acid
Bile is essential for the emulsification of dietary lipids and fat-soluble vitamins, for the catabolism of cholesterol, and for the expulsion of toxic metabolites such as bilirubin. The detergent properties of bile are conferred by bile acids, which are synthesized in the liver from cholesterol (1). Following their synthesis in liver, bile acids (along with other constituents of bile) are transported across the canalicular membrane of hepatocytes, drain into the gallbladder via bile ducts, and are expelled into the duodenum in response to cholecystokinin, secreted by epithelial cells of the duodenum.
Cholestasis is an acquired or inherited syndrome associated with the retention of bile constituents in the circulation. Among the inherited forms, progressive familial intrahepatic cholestasis (PFIC) is a degenerative liver disease caused by mutations affecting canalicular hepatocyte membrane transporters (2). PFIC1 is caused by mutation of ATP8B1 (3), encoding a P4-type ATPase involved in the translocation of phosphatidylserine (PS) from the exoplasmic to cytoplasmic membrane leaflets (4). PFIC2 is caused by mutations of ABCB11, encoding the bile salt export pump (BSEP) (5). BSEP is the primary transporter of hydrophobic bile acids, and as a consequence, patients with PFIC2 have extremely low concentrations of bile salts in bile (6).
Finally, mutation of ABCB4 leads to PFIC3 (7), which can be clinically distinguished from PFIC1 and PFIC2 by elevated quantities of serum γ-glutamyl transferase (GGT). ABCB4 encodes the MDR3 protein (MDR2 in mouse), which is essential for the translocation of phosphatidylcholine from the inner to outer leaflet of the canalicular membrane (8), as highlighted by the near absence of phosphatidylcholine from the bile of PFIC3 patients and Abcb4 mutant mice (9).
Dubin–Johnson syndrome, caused by mutations of ABCC2, is also caused by inactivation of a canalicular transporter (MRP2) (2). Hyperbilirubinemia is common to patients with PFIC and Dubin–Johnson syndrome, but for different reasons. In the case of PFIC, a failure of transport leads to liver damage, which in turn prevents the expulsion of bile acids and bilirubin, leading to hyperbilirubinemia. MRP2, on the contrary, is a key transporter of conjugated bilirubin, and patients with Dubin–Johnson syndrome therefore develop hyperbilirubinemia without excessive retention of bile acids.
The similar clinical outcomes of ATP8B1 and ABCB4 mutations, and ABCB11 and ABCC2 mutations, all highlight the importance of phospholipid asymmetry and its role in canalicular transport. Phospholipid asymmetry is known to be influenced by the P4-type ATPases, a group of hypothetical aminophospholipid flippases (to which ATP8B1 belongs), which are thought to enrich phosphatidylserine and/or phosphatidylethanolamine at the cytoplasmic membrane leaflet (10). However, beyond ATP8B1, little is known of the functions of the 13 other mammalian P4-type ATPases (11).
We and others have recently discovered that mutation of the P4-type ATPase ATP11C in mouse leads to a syndrome of B-cell lymphopenia associated with hyperbilirubinemia (12, 13), although the origins of hyperbilirubinemia were uncertain. Here we detail a key nonhematopoietic role for ATP11C, specifically in the prevention of intrahepatic cholestasis.
Results
Intrahepatic Hyperbilirubinemia in Atp11c Mutant Mice.
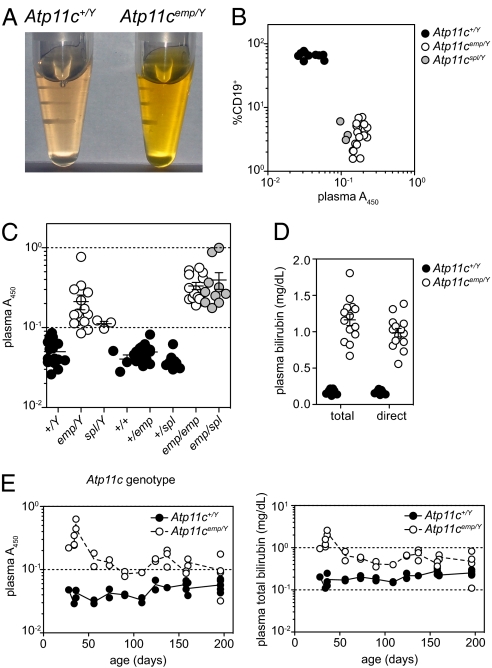
While investigating B-cell lymphopenia in Atp11c mutant mice (12), we observed strong yellow pigmentation in the plasma of mutant mice (Fig. 1A). Yellow plasma pigmentation segregated exclusively with B-cell lymphopenia in the Atp11cemptyhive pedigree (Fig. 1B), and was also apparent in mice with an independent chemically induced allele, Atp11cspelling (Fig. 1B). Compound heterozygosity for both alleles also led to plasma pigmentation (Fig. 1C), indicating that it was not caused by a genetically linked incidental mutation, but by mutation of the Atp11c locus itself. Measurement of plasma bilirubin revealed a 10-fold increase in both total and direct bilirubin (Fig. 1D). Hyperbilirubinemia was most dramatic in young mice, and declined progressively with age (Fig. 1E).
Fig. 1.
Hyperbilirubinemia caused by mutations of Atp11c. Plasma pigmentation (A) and its relationship with B-cell lymphopenia (B) in Atp11c mutant mice. (C) Allelism testing of emptyhive and spelling alleles of Atp11c. (D) Quantitation of total and direct bilirubin in plasma, and the severity of plasma hyperbilirubinemia and pigmentation across various ages (E). Error bars in C and D represent SEM. Data are representative of at least three independent experiments.
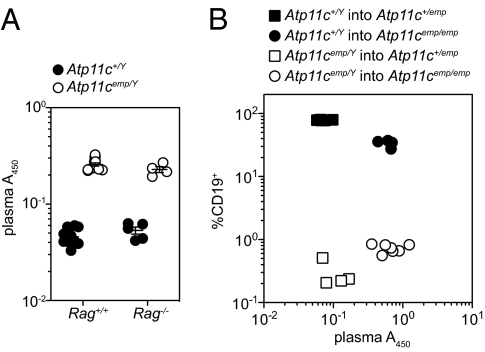
Hyperbilirubinemia may be a consequence of several physiological defects, including increased hemolysis (prehepatic), autoimmune hepatitis or hepatocyte death (intrahepatic), or biliary obstruction (posthepatic). Combining the Atp11c mutation with a null allele of Rag1 allowed us to exclude autoreactive lymphocytes as a cause (Fig. 2A), as did bone marrow transplantation experiments, which further established that plasma pigmentation was caused by radioresistant cell lineages (Fig. 2B), rather than increased hemolysis. These findings were consistent with the increase of direct (conjugated) bilirubin (Fig. 1D), which is not expected to occur in prehepatic hyperbilirubinemia.
Fig. 2.
Plasma hyperpigmentation is independent of B and T lymphocytes. Plasma pigmentation of Rag1/Atp11c double mutant mice (A) and reciprocal bone marrow chimeric mice (B). Error bars in A represent SEM. Data are representative of three (A) or two (B) independent experiments.
Histological and Biochemical Anomalies.
Histological analysis of H&E-stained liver sections from 3-mo-old mice indicated occasional portal triad-associated inflammation in mutant livers (Fig. S1A). Both Sirius red and trichrome blue staining were slightly more pronounced in mutant portal regions compared with littermate controls, which indicated an increased deposition of extracellular matrix (ECM), and suggested chronic, albeit mild, damage (Fig. S1B). Oil red O staining did not reveal an increase in lipid deposition, although apoptotic cells, as revealed by TUNEL staining, were more prevalent in 6-mo-old mutant livers (but not in 3-mo-old mutants; Fig. S1B). Variation in nuclear volume suggested that TUNEL-positive cells were of both parenchymal and nonparenchymal origin. Plasma pigmentation was nonetheless independent of the death domain-containing receptor Fas and the proapoptotic Bcl2 family member Bim (14, 15), as combined mutation of Fas or Bcl2l11 and Atp11c did not affect it (Fig. S2).
Quantities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), GGT, and alkaline phosphatase in plasma were also equivalent between 3-mo-old Atp11c mutants and controls, although total cholesterol was approximately 30% higher in mutant plasma, predominantly because of an increase in the LDL form (Table S1).
The collective implications of these data were that hyperbilirubinemia in Atp11c mutant mice appeared to be of hepatocellular origin. This appears consistent with the predominant expression of ATP11C-encoding mRNA in the liver (Fig. S3A), primarily in hepatocytes and liver endothelial cells (Fig. S3B).
High Incidence of Dystocia in Homozygous Atp11c Mutant Females.
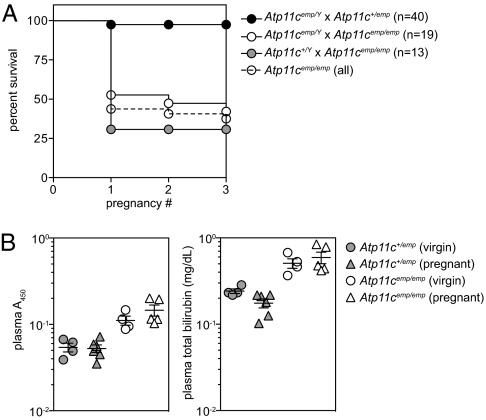
An additional pathological effect of the Atp11c mutation was a high frequency of dystocia in homozygous mutant females (Fig. 3A). More than 50% of Atp11cemp/emp females suffered dystocia in their first pregnancy, compared with fewer than 5% in Atp11c+/emp females. This phenomenon depended upon the genotype of the mother, rather than that of the pups, as Atp11c+/Y × Atp11cemp/emp matings had as many (if not more) cases of dystocia than Atp11cemp/Y × Atp11cemp/emp. Surviving Atp11cemp/emp females almost always gave additional litters without dystocia, with an average litter size of 4.05 (n = 19, SD = 4.55 from 13 Atp11cemp/emp females) compared with 4.79 for Atp11c+/emp females (n = 78, SD = 4.79 from 35 females). Neither plasma pigmentation nor hyperbilirubinemia was more severe in Atp11cemp/emp females at 18.5 d post coitum, compared with their virgin counterparts (Fig. 3B).
Fig. 3.
High frequency of dystocia in Atp11cemp/emp females. (A) Survival curve of homozygous and heterozygous Atp11c mutant female littermates following pregnancies to hemizygous mutant or WT males. (B) Plasma absorbance (A450) and total bilirubin measurements from the plasma of age-matched virgin or 18.5 d post coitum pregnant females. Error bars in B represent SEM.
Atp11c Mutant Mice Are Cholestastic.
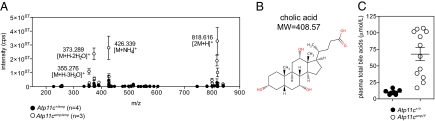
As a result of the absence of severe liver pathology, we screened for further metabolic abnormalities (adapted from ref. 16), subjecting mutant and WT serum to liquid chromatography/mass spectrometry (LC/MS). Ion peaks were sorted into groups based on ion retention time, and groups containing ions with the greatest difference in mean peak intensity were scrutinized further (Table 1). Ion molecular weights were cross-referenced with METLIN database and Human Metabolome Database, and one candidate molecule (cholic acid) was selected for validation by tandem MS (MS/MS; Fig. S4). LC/MS confirmed that cholic acid was present in higher quantities in Atp11c mutant serum (Fig. 4 A and B), with an independent assay confirming that total bile acid concentrations were also increased (Fig. 4C).
Table 1.
Ions on LC/MS with the greatest difference between WT and Atp11c mutant serum
| Atp11c+/emp (n = 4) |
Atp11cemp/emp (n = 3) |
|||||||||
| No. | m/z | Retention time | Group | Mean | SD | Mean | SD | Mean difference | Fold change | P value |
| 1 | 373.289 | 1,039.625 | 152 | 2.30 × 106 | 3.70 × 106 | 2.36 × 107 | 7.29 × 106 | 2.13 × 107 | 10 | 3.6 × 10−3 |
| 2 | 817.603 | 1,004.24 | 290 | 2.73 × 105 | 4.20 × 105 | 9.69 × 106 | 5.67 × 106 | 9.42 × 106 | 36 | 1.9 × 10−2 |
| 3 | 546.309 | 1,136.01 | 20 | 3.81 × 105 | 1.10 × 105 | 8.59 × 106 | 1.07 × 106 | 8.21 × 106 | 23 | 1.9 × 10−5 |
| 4 | 761.323 | 1,098.94 | 203 | 3.25 × 104 | 1.71 × 104 | 7.71 × 106 | 1.82 × 106 | 7.67 × 106 | 237 | 3.3 × 10−4 |
| 5 | 1031.612 | 882.73 | 192 | 4.32 × 105 | 6.62 × 105 | 6.68 × 106 | 4.14 × 106 | 6.25 × 106 | 15 | 2.8 × 10−2 |
| 6 | 584.269 | 1,680.245 | 331 | 4.66 × 105 | 6.49 × 105 | 4.68 × 106 | 1.11 × 106 | 4.22 × 106 | 10 | 1.4 × 10−3 |
| 7 | 257.156 | 56.77 | 18 | 4.19 × 105 | 2.38 × 105 | 4.24 × 106 | 1.23 × 106 | 3.82 × 106 | 10 | 1.5 × 10−3 |
| 8 | 761.326 | 1,077.81 | 343 | 1.13 × 104 | 7.38 × 103 | 2.55 × 106 | 9.61 × 105 | 2.54 × 106 | 225 | 2.8 × 10−3 |
| 9 | 373.285 | 927.82 | 100 | 1.20 × 104 | 1.32 × 104 | 2.05 × 106 | 6.72 × 105 | 2.04 × 106 | 171 | 1.5 × 10−3 |
| 10 | 599.307 | 1,313.93 | 229 | 1.76 × 103 | 3.08 × 103 | 2.03 × 106 | 8.50 × 105 | 2.03 × 106 | 1,155 | 4.3 × 10−3 |
Ions with a fold change greater than or equal to 10 and a mean peak intensity difference greater than 1 million were ranked by mean peak intensity difference. Multiple ions from a common retention group (and therefore likely to originate from the same molecule) are represented only once.
Fig. 4.
Atp11c mutant mice are cholestatic. (A) LC/MS ion intensity profile for the 1039.625s retention group. The ion at 373.289 (m/z) showed the greatest mean difference between Atp11c mutant (n = 3) and littermate control (n = 4) serum. Hypothetical ion identities are shown in parentheses below each m/z value, which are all consistent with the chemical structure and molecular weight of cholic acid (B). (C) Total bile acids as measured from the plasma of Atp11c mutant and littermate control mice. Error bars in A and C represent SEM.
Exogenous Bile Acid Toxicity in Atp11c Mutant Mice.
These findings, along with earlier data, were indicative of intrahepatic cholestasis, and led us to suspect that feeding mutant mice with a cholic acid-supplemented diet might exacerbate the phenotype, as is observed in mice with mutation of Atp8b1 (17) or Abcb11 (18). Indeed, Atp11c mutants lost weight more rapidly than their WT littermates (Fig. 5A), culminating in uniform lethality in mutant males by day 15 (Fig. 5B). Weight loss was also associated with dramatic increases in plasma bilirubin (Fig. 5C).
Fig. 5.
Severe pathology in Atp11c mutant mice induced by a cholic acid-supplemented diet. Atp11c mutant and control males were fed with a cholic acid-supplemented (n = 7 and n = 5, respectively) or control diet (n = 7 and n = 5, respectively), and monitored for weight loss (A), mortality (B), and changes in plasma constituents (C). Gallbladder distention (D) and jaundice (E) as observed in littermates on a cholic acid diet for 13 d. (F) Relative expression of Cyp7a1 and Atp11c mRNA in the livers of mice after 7 d of a cholic acid-supplemented or control diet (normalized to Actb). (G) Sirius red staining of liver sections after 13 d of a cholic acid-supplemented diet. Error bars in A, C, and F represent SEM.
Two striking observations made in cholic acid-fed mutant mice at necropsy were a pronounced enlargement of the gallbladder (Fig. 5D) and jaundiced skin (Fig. 5E), neither of which was observed in Atp11c mutants on a normal diet. Sirius red staining was also more apparent in mutant livers (Fig. 5G). Gallbladder enlargement did not appear to be caused by biliary obstruction, as no precipitates were apparent within the gallbladder, nor was there any histological evidence of ductular proliferation.
This potential disruption of enterohepatic bile circulation seen in Atp11c mutant mice could be explained by ATP11C acting in bile acid transport, either as a pump, channel, or sensor. If this were the case, one would expect that dietary supplementation of cholic acid would fail to suppress expression of cholesterol 7α-hydroxylase (encoded by Cyp7a1), a rate-limiting enzyme in bile acid synthesis from cholesterol, which occurs in a farnesoid X receptor-dependent manner (19). This was not the case, however, as Cyp7a1 expression was equally low in mutants as in WT littermates after cholic acid feeding (Fig. 5F). Atp11c mRNA was nonetheless increased in Atp11cemp/Y mutant mice and in mice fed a cholic acid-supplemented diet (Fig. 5F), suggesting that Atp11c is transcriptionally induced in response to cholestasis.
Discussion
The present findings reveal an additional, extrahematopoietic function for the P4-type ATPase ATP11C, namely in the prevention of cholestasis. This mild cholestatic phenotype of Atp11c mutant mice resembles other mouse models of PFIC, but with notable distinctions. Chief among them is hyperbilirubinemia, which was greatest in younger mutant mice, and declined progressively with age. The reason for this is yet to be determined, but may reflect an increased dependence on ATP11C-facilitated bilirubin excretion in younger mice, perhaps because of behavioral differences (20) or competition between substrates of ATP11C. Hyperbilirubinemia is observed in Atp11c and Abcb4 mutants (9), but not in Abcb11 or Atp8b1 mutants (17, 21), although, unlike in Abcb4 mutants, plasma ALT and AST were not elevated in 3-mo-old Atp11c mutants. TUNEL staining, however, was more prevalent in 6-mo-old Atp11c mutants, suggesting that liver damage is a mild but progressive consequence of the mutation. Ductular proliferation was also not apparent in Atp11c mutant sections, indicating that hepatocyte death and biliary obstruction are more prevalent in Abcb4 mutant mice (9).
More pathological distinctions emerged after dietary supplement of cholic acid, notably an enlargement of the gallbladder in Atp11c, but not Atp8b1 or Abcb11, mutant strains (17, 22). This does not seem to be a result of a physical obstruction such as gallstones or acalculous cholecystitis (“sludge”), as bile duct proliferation is not observed (as it is following bile duct ligation) (23). Other explanations could include a failure of cholestokinin signaling (with failure of gallbladder contraction or relaxation of the sphincter of Oddi), or an inability to control FGF15-mediated gallbladder filling (24).
Similar to Abcb11 mutants, cholestatic disease is very mild in Atp11c mutant mice, yet mice that consume cholic acid as a dietary supplement die within a matter of days (22). BSEP is known to be a major canalicular transporter of hydrophobic bile acids, including cholic acid (21), and this sensitivity to dietary cholic acid can be attributed to the strong cholestatic effects of cholic acid, but not the primary murine bile acids (α- and β-muricholic acid). This raises the possibility that ATP11C is involved in the transport of cholestatic bile acids, perhaps in concert with BSEP, that are not normally prevalent in mice. Other transporters are likely to be affected also, such as MRP2 (encoded by Abcc2), which transports conjugated bilirubin (but not cholic acid) (25).
We have excluded the possibility that ATP11C acts as a nonredundant sensor or transporter of cholic acid between the portal circulation and the hepatocyte, as farnesoid X receptor-mediated repression of Cyp7a1 in the liver remains intact after cholic acid feeding (19). We cannot, however, exclude that ATP11C acts as a transporter between the hepatocyte and the bile canaliculi, and it will be critical to determine the subcellular localization of ATP11C, and how mutation of it affects the flow of bile constituents between the hepatocyte and bile ducts.
Why is there such a high frequency of dystocia in Atp11c mutant females? Liver pathology did not appear to be more severe during late pregnancy, although the profile of bile acids in Atp11c mutant plasma might somehow be toxic to late-term embryos, or preclude their delivery. Another possibility is that smooth muscle function is compromised both in the uterus (where Atp11c transcript is detected; Fig. S3A) and in the gallbladder, during parturition and cholic acid feeding, respectively. Although dystocia has not been reported in other mouse models of PFIC, and is not characteristic of intrahepatic cholestasis of pregnancy in the clinic (26), Atp11c mutants may represent a unique model to understand the pathology of dystocia and other defects of parturition.
An important difference between our Atp11cemptyhive mutant strain (12) and an independent strain [Atp11cambrosius (13)] is the observation of hepatocellular carcinoma (HCC) in 6 mo-old ambrosius mutant mice. We have not observed this in our strain, even in mice beyond 1 y of age. The inbred genetic background is consistent in each case (C57BL/6J), although modifying effects of incidental mutations induced in either stock have not been excluded. As HCC is apparent in older Abcb4 mutant mice (27), perhaps a more likely explanation is that HCC in ambrosius mutant mice has an environmental cue (of microbial or dietary origin) present in one facility but not the other.
How might ATP11C function in both hepatocytes and adult B-cell progenitors? Canalicular membrane asymmetry is known to be critical for the formation of bile (9) and for hepatocytes to resist the detergent effects of bile (28), and ATP11C is presumably involved in one or both of these processes. Another conceivable possibility is that ATP11C mediates trafficking of other canalicular transporters. As much less is known of the consequences of altered membrane asymmetry in B-cell progenitors, understanding the hepatic function of ATP11C could provide important insights into lymphocyte development. Studies of canalicular membrane structure and function, by EM and functional analysis of other known canalicular transporters, will be important in this respect. Bile acid metabolites are also known to affect B-cell function. For instance, 25-hydroxycholesterol, an oxysterol metabolite of cholesterol and precursor for bile acid synthesis, can suppress secretion of IgA (29). The observation that Atp11cemp/Y mutant mice also have a significant deficiency of IgA (12) might reflect a surplus of 25-hydroxycholesterol, although whether this also accounts for the development defects remains to be determined.
What about the substrate of ATP11C? These mutant strains establish a previously unappreciated biochemical link between hepatocytes and B-cell progenitors, and identification of the substrate(s) of ATP11C will be of central importance to resolving its function. Yabas et al. have shown that mutant B-cell precursors are less efficient at internalizing exogenous PS at the cell membrane (13), but whether PS is the critical substrate remains to be seen. PS asymmetry is known to important not just at the cell membrane, but also at other organellar membranes (30), any number of which could affect pathways required for B-cell development. ATP8B1 also appears to be vital for inward translocation of PS at the cell surface, although other substrates have been proposed (31). Whatever their substrate, these mutants promise to provide new insight into disorders of lymphopoiesis, parturition, and cholestatic disease.
Materials and Methods
Mice.
All animal procedures were in accordance with institutional guidelines. Atp11cemptyhive, Atp11cspelling, and Rag1maladaptive (Mouse Genome Informatics database no. 3851764) alleles were generated on a pure C57BL/6J background by N-ethyl-N-nitrosourea mutagenesis as previously described (12), and are described at http://mutagenetix.scripps.edu. Faslpr mice were obtained from Jackson Laboratories, and Bcl2l11tm1.1Ast mice have been described previously (32). The emptyhive strain was maintained by hemizygote–to-heterozygote breeding, with male littermates used for experiments unless otherwise indicated. Mice were maintained on a PicoLab Mouse Diet 20 (LabDiet 5058), and sentinels present in the same room were routinely negative for serum antibodies against Mycoplasma pulmonis, mouse rotavirus, mouse hepatitis virus, murine norovirus, mouse parvovirus, minute virus of mice, Sendai virus, and Theiler murine encephalomyelitis virus.
Bone Marrow Chimeras.
Recipient mice were γ-irradiated with a dose of 10 Gy (137Cs source). The following day, mice received 5 × 106 bone marrow cells pooled from two donors via tail vein injection, and were maintained on trimethoprim/sulfamethoxazole antibiotic water until they were killed for analysis 8 wk later.
Cholate-Supplemented Diet.
Mice were fed a basal 14% protein diet (TD.00357; Harlan) with or without a supplement of 0.5% sodium cholate. Mice were weighed at biweekly intervals, bled weekly for serum analysis, and monitored daily for survival.
Quantitative PCR.
Total RNA was prepared from liver (RNeasy kit; Qiagen) and reverse-transcribed into cDNA (Retroscript; Ambion). Quantitative PCR reactions (SYBR Master Mix; BioPioneer) were analyzed on a 7300 Real-Time PCR System (Applied Biosystems). Relative quantities of Cyp7a1 cDNA were calculated by reference to Actb using the comparative CT method. Isolation of cDNA from MACS-sorted liver cell fractions has been described previously (23). Primer sequences were as follows: Actb, GGCTGTATTCCCCTCCATCG (forward); Actb, CCAGTTGGTAACAATGCCATGT (reverse); Atp11c, CTCGTTTCTCTTCTCCAGCA (forward); Atp11c, AGTGTGTTTTGTGGACGGC (reverse); Cyp7a1, GGGATTGCTGTGGTAGTGAGC (forward); and Cyp7a1, GGTATGGAATCAACCCGTTGTC (reverse).
Histology.
Livers were fixed in 10% neutral buffered formalin and embedded in paraffin wax, and 5-μm sections were stained with H&E, Sirius red, or trichrome blue. Oil red O staining was performed on frozen sections. TUNEL staining was performed on paraffin sections according to the manufacturer's instructions (Roche).
Plasma Analysis.
Blood from the retroorbital plexus of isoflurane-anesthetized mutant mice was collected in cluster tubes (Costar) containing 20 μL of 6% EDTA solution (wt/vol in water) or allowed to clot in serum separator tubes and centrifuged for 5 min at 10,000 × g. Mice were fasted overnight before bleeding where indicated. Serum absorbance was measured at 450 nm (subtracting absorbance at 540 nm) on a MAXline Emax Microplate Reader (Molecular Devices). Total and direct bilirubin (StanBio) and total bile acids (Diazyme) were measured according the manufacturer's instructions. All other plasma chemistry parameters, including ALT, alkaline phosphatase, and GGT were measured by standard clinical chemistry (University of California, San Diego, Murine Hematology Core).
Serum MS.
Mutant and WT littermates were fasted overnight, and the following morning blood was collected from the retroorbital plexus into serum separator tubes and allowed to clot. Serum was then subjected to positive ion-mode LC/MS on an Agilent 6520 Q-TOF mass spectrometer, with the output annotated by XCMS software (33). Ion peak intensities for mutant and WT groups were ranked by mean peak difference, and those with a peak difference greater than 1 million were ranked according to fold difference. Ions with the largest peak differences were then compared with the METLIN database (http://metlin.scripps.edu/) and Human Metabolome Database (http://www.hmdb.ca/) for potential candidate metabolites. For candidate validation, standards (including cholic acid, C1129; Sigma) were run in parallel with mutant serum on an Agilent 6520 Q-TOF in tandem mode (MS/MS).
Supplementary Material
Acknowledgments
We thank A. Knisely A. Hofmann, and M. Katsumi for valuable comments; E. Moresco for help preparing the manuscript; M. Gutierrez for animal care; D. Ditto and the University of California at San Diego, Murine Hematology Core for clinical chemistry; P. Krebs and M. Chadwell for help with histology; and S. Won for first noticing plasma pigmentation. This work was supported by the National Institute of Allergy and Infectious Diseases Broad Agency Announcement Contract HHSN272200700038C (to B.B.), with fellowship support from the General Sir John Monash Foundation (O.M.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104631108/-/DCSupplemental.
References
- 1.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 2.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 3.Bull LN, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 4.Ujhazy P, et al. Familial intrahepatic cholestasis 1: Studies of localization and function. Hepatology. 2001;34:768–775. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- 5.Strautnieks SS, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 6.Jansen PL, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 7.de Vree JM, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruetz S, Gros P. Phosphatidylcholine translocase: A physiological role for the mdr2 gene. Cell. 1994;77:1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 9.Smit JJ, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 10.Holthuis JCM, Levine TP. Lipid traffic: Floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 11.Folmer DE, Elferink RPJO, Paulusma CC. P4 ATPases - lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791:628–635. doi: 10.1016/j.bbalip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Siggs OM, et al. The P4-type ATPase ATP11C is essential for B lymphopoiesis in adult bone marrow. Nat Immunol. 2011 doi: 10.1038/ni.2012. 10.1038/ni.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yabas M, et al. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol. 2011 doi: 10.1038/ni.2011. 10.1038/ni.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann T, et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikoff WR, Pendyala G, Siuzdak G, Fox HS. Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. J Clin Invest. 2008;118:2661–2669. doi: 10.1172/JCI34138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlikowska L, et al. A mouse genetic model for familial cholestasis caused by ATP8B1 mutations reveals perturbed bile salt homeostasis but no impairment in bile secretion. Hum Mol Genet. 2004;13:881–892. doi: 10.1093/hmg/ddh100. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 19.Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Groen A, Kunne C, Oude Elferink RP. Increased serum concentrations of secondary bile salts during cholate feeding are due to coprophagy. A study with wild-type and Atp8b1-deficient mice. Mol Pharm. 2006;3:756–761. doi: 10.1021/mp060009t. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci USA. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, et al. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Taura K, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 24.Choi M, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 25.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 26.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049–2066. doi: 10.3748/wjg.15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauad TH, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 28.Paulusma CC, et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 29.Bauman DR, et al. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci USA. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 31.Ray NB, et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16:1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 33.Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.