Abstract
Type I and type III IFNs bind to different cell-surface receptors but induce identical signal transduction pathways, leading to the expression of antiviral host effector molecules. Despite the fact that type III IFN (IFN-λ) has been shown to predominantly act on mucosal organs, in vivo infection studies have failed to attribute a specific, nonredundant function. Instead, a predominant role of type I IFN was observed, which was explained by the ubiquitous expression of the type I IFN receptor. Here we comparatively analyzed the role of functional IFN-λ and type I IFN receptor signaling in the innate immune response to intestinal rotavirus infection in vivo, and determined viral replication and antiviral gene expression on the cellular level. We observed that both suckling and adult mice lacking functional receptors for IFN-λ were impaired in the control of oral rotavirus infection, whereas animals lacking functional receptors for type I IFN were similar to wild-type mice. Using Mx1 protein accumulation as marker for IFN responsiveness of individual cells, we demonstrate that intestinal epithelial cells, which are the prime target cells of rotavirus, strongly responded to IFN-λ but only marginally to type I IFN in vivo. Systemic treatment of suckling mice with IFN-λ repressed rotavirus replication in the gut, whereas treatment with type I IFN was not effective. These results are unique in identifying a critical role of IFN-λ in the epithelial antiviral host defense.
IFNs play a critical role in the antimicrobial host defense. Whereas lymphocyte-derived type II IFN (also called IFN-γ) is associated with resistance against a broad range of intracellular microorganisms, type I and III IFN primarily mediate antiviral protection. IFN-α, IFN-β, and all other type I IFN family members use the same heterodimeric receptor complex (IFNAR) for signaling. Receptor engagement leads to activation of the Jak/STAT signaling pathway and expression of IFN-stimulated genes (ISG), which mediate the antiviral state (1). The type III IFN family consists of three members in humans, IFN-λ1, -λ2, and -λ3 that are also named IL29, IL28A, and IL28B, respectively, whereas mice only express IFN-λ2 and -λ3. Type III IFN are structurally different from type I IFN and bind to a distinct heterodimeric receptor (IL28R), consisting of the IL28Rα, also called IFN-λ receptor 1 (IFN-λR1), and the IL10Rβ chains (2–4). Type I and III IFN are both induced following stimulation of pattern recognition receptors of the innate immune system, such as Toll-like receptors and RIG-like helicases (5–7). IFN-λ–triggered signal transduction events and gene activation profiles are virtually indistinguishable from those of the type I IFN system (2, 3, 8, 9). However, the type I and type III IFN systems differ strikingly with regard to the spectrum of responsive cell types. Whereas receptors for type I IFN seem to be present on most if not all nucleated cells, functional receptors for type III IFN are preferentially expressed on epithelial cells (10).
Recent studies investigating the role of type III IFN in vivo demonstrated a high degree of redundancy of the type I and type III IFN systems (6, 11, 12). Available data suggest that the type III IFN system supports but cannot functionally replace the type I IFN system. For example, influenza and other respiratory viruses replicated more efficiently in the lung of mice lacking both receptor systems than in the lung of mice lacking only functional type I IFN receptors. However, unlike IFNAR-deficient mice, single-knockout mice lacking functional type III IFN receptors showed only slightly enhanced virus susceptibility (12).
Rotavirus represents one of the most common causes of infectious gastroenteritis in humans worldwide with significant morbidity and mortality, particularly in countries with limited access to medical care (13). Rotavirus exhibits a strong epithelial cell tropism with predominant replication in the epithelium of the small intestine. Recent work demonstrated that rotavirus-infected adult STAT1-deficient mice shed substantially more virus than wild-type controls (14). In contrast, IFNAR1-deficient mice exhibited no enhanced rotavirus susceptibility (15). These results prompted us to examine whether IFN-λ might be responsible for rotavirus protection. We found that mice lacking functional IFN-λ receptors are indeed highly susceptible to rotavirus infection. Our work demonstrates that IFN-λ is a functionally nonredundant component of the mucosal antiviral innate immune system.
Results
Enhanced Susceptibility to Rotavirus in Mice Lacking Functional IFN-λ Receptors.
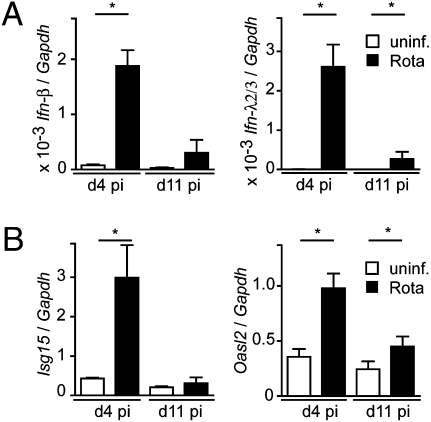
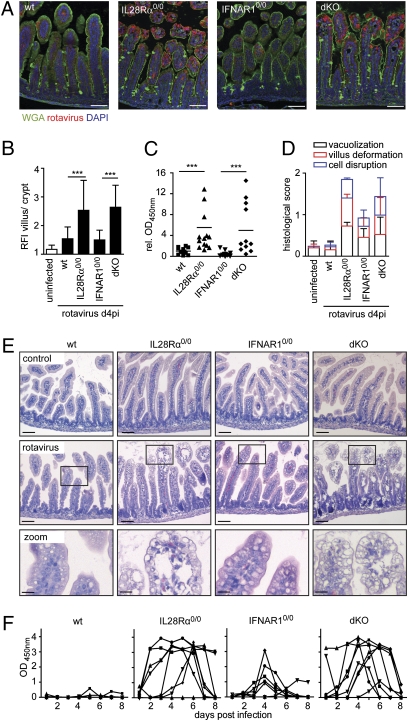
Activation of the enteric IFN system after oral rotavirus infection was determined in freshly isolated intestinal epithelial cells (IECs) from suckling mice. Ifn-β and Ifn-λ mRNA levels were strongly enhanced at day 4 postinfection and still slightly enhanced at day 11 postinfection (Fig. 1A). The infected epithelium readily responded to virus-induced IFN, illustrated by enhanced expression levels of the IFN-stimulated genes Isg15 and Oasl2 (Fig. 1B), suggesting that rotavirus infection represents a suitable model to study the biological importance of type I and type III IFN. Therefore, we compared virus replication in the intestinal tract of mice lacking functional receptors for IFN-λ with animals deficient in type I IFN recognition. Wild-type, IL28Rα0/0, IFNAR10/0, and IFNAR10/0IL28Rα0/0 double-knockout mice were orally infected with rotavirus and the extent of virus replication was determined at day 4 postinfection. Immunohistochemical analysis of thin sections of paraffin-embedded small intestinal tissue revealed abundant viral antigen at the villus epithelium of IL28Rα0/0 and IFNAR10/0IL28Rα0/0 mice, but not wild-type and IFNAR10/0 mice (Fig. 2 A and B). Additionally, average rotavirus antigen levels in colon homogenates of IL28Rα0/0 and IFNAR10/0IL28Rα0/0 mice were significantly higher compared with wild-type mice. In contrast, virus antigen levels in colons of infected IFNAR10/0 mice were comparable to wild-type mice (Fig. 2C). Enhanced rotavirus susceptibility of mice lacking functional receptors for IFN-λ was also associated with more severe pathology. Epithelial vacuolization, villus deformation, and epithelial cell disruption were more pronounced in IL28Rα0/0 single-knockout and IFNAR10/0IL28Rα0/0 double-knockout mice than in wild-type and IFNAR10/0 animals (Fig. 2 D and E).
Fig. 1.
IECs express type I and III IFN genes after rotavirus challenge. Suckling C57BL/6 mice were orally infected with murine rotavirus strain EDIM (5 μL of 1:100 diluted virus stock). IECs were isolated at day 4 (n = 5) and day 11 (n = 5) postinfection (pi, black bars) or from uninfected control mice (open bars, n = 4) and analyzed by quantitative RT-PCR for expression of (A) Ifn-β, Ifn-λ2/3, and (B) Isg15 and Oasl2 genes. Results are representative for at least two independent experiments.
Fig. 2.
Enhanced rotavirus replication and IEC damage in mice lacking functional IFN-λ receptors. (A–E) Suckling wild-type (n = 12), IL28Rα0/0 (n = 14), IFNAR10/0 (n = 14), and IFNAR10/0IL28Rα0/0 (dKO, n = 12) mice were orally infected with murine rotavirus strain EDIM (5 μL of 1:100 diluted stock). Animals were killed 4 d later. (A) Immunostaining for rotavirus antigen (red) in thin sections of paraffin-embedded intestinal tissue. Counterstaining was performed with wheat germ agglutinin (WGA, green) and DAPI (blue). (Scale bars, 50 μm.) (B) Epithelial rotavirus antigen staining intensity was measured to obtain the relative fluorescence intensity (RFI) of villus/crypt epithelium. (C) Viral antigen levels in colon homogenates were determined by ELISA. Combined results of three independent experiments are shown. To facilitate comparison, OD450nm readings were normalized to the mean values obtained from wild-type mice of each experiment. (D) Histological score of virus-induced small intestinal tissue alterations and (E) H&E stainings of rotavirus-infected tissue. [Scale bars, 50 μm (Top and Middle), 10 μm (Bottom).] (F) Virus shedding in feces of adult wild-type, IL28Rα0/0, IFNAR10/0, and IFNAR10/0IL28Rα0/0 (dKO) mice (n = 8 for each group) after oral infection with murine rotavirus strain EDIM. Each line represents the OD450nm values of one individual mouse during the observed time period.
We next asked if rotavirus restriction was also dependent on functional receptors for IFN-λ in adult mice that usually exhibit a higher degree of natural resistance to rotavirus than suckling mice (16). Groups of 4- to 6-wk-old wild-type and mutant mice were orally infected with rotavirus, and kinetics and extent of rotavirus shedding were assessed. As expected, feces of all wild-type mice contained very low or even undetectable levels of rotavirus antigen at all times. In contrast, seven of eight infected IL28Rα0/0 mice shed high concentrations of rotavirus antigen. Virus shedding typically lasted several days, with some variation between individual animals (Fig. 2F). Six of eight infected IFNAR10/0 mice exhibited detectable levels of rotavirus antigen in the feces on at least one occasion, but the extent and duration of virus shedding were substantially reduced compared with IL28Rα0/0 mice. Rotavirus shedding of IFNAR10/0IL28Rα0/0 double-knockout mice was indistinguishable from IL28Rα0/0 single-knockout mice (Fig. 2F).
IFN-λ–Mediated Resistance to Rotavirus Is Attributed to an Epithelial Cell-Specific Response.
In an attempt to understand the basis for the surprising observation that IFN-λ rather than IFN-α/β determines rotavirus resistance, we visualized the type I and type III IFN responses at the cellular level. Mx1 is an IFN-induced protein that rapidly accumulates in the nucleus of cells from mice that carry functional Mx1 alleles (17). We previously demonstrated that staining for Mx1 in IFNAR10/0 or IL28Rα0/0 mice represents a powerful tool to identify cells that specifically respond to IFN-λ or IFN-α/β (12).
In tissue sections of the small intestine from rotavirus-infected suckling wild-type mice, we observed only few virus antigen-positive cells but strong Mx1 staining in the nucleus of epithelial cells (Fig. 3, Top). In infected IL28Rα0/0 mice, however, rotavirus antigen was abundantly present in epithelial cells. Mx1-positive cells were also detected but, interestingly, did not stain for the epithelial cell marker E-cadherin, identifying them as lamina propria cells (Fig. 3, second row). In contrast, rotavirus antigen-positive cells were rare in infected IFNAR10/0 mice, and Mx1 staining was restricted to E-cadherin–positive cells (Fig. 3, third row). As expected, in infected IFNAR10/0IL28Rα0/0 mice, many epithelial cells contained high levels of rotavirus antigen but lacked Mx1 staining (Fig. 3, fourth row). These results suggested that IECs vigorously respond to IL28R stimulation but are only weakly stimulated by type I IFN in vivo, a finding that was confirmed by quantitative RT-PCR for Isg15 and Mx1 expression in primary IECs isolated from infected mice (Fig. S1).
Fig. 3.
IECs of infected mice mainly respond to virus-induced IFN-λ. Intestinal tissue from rotavirus-infected suckling wild-type, IL28Rα0/0, IFNAR10/0, and IFNAR10/0IL28Rα0/0 (dKO) mice was harvested at day 4 postinfection. Paraffin-embedded samples were subjected to simultaneous staining for Mx1 (green), rotavirus antigen (white), and E-cadherin (red). Counterstaining was performed with DAPI (blue). (Right) Zoom column represents larger magnifications of the adjacent boxed areas. (Scale bars, 50 μm in first three columns; 20 μm in the zoom column.)
The results presented above seemed to violate the accepted concept that most if not all nucleated cells express type I IFN receptors (reviewed in ref. 18). To exclude rotavirus-induced inhibitory effects on the epithelial type I IFN response, the in vivo IFN responsiveness of IECs was investigated in the absence of viral infection. Therefore, muscle cells of mice were transfected in vivo with expression plasmids for either mouse IFN-α or mouse IFN-λ, leading to prolonged synthesis and systemic spread of biologically active IFN (10). Epithelial Mx1 expression was low in IL28Rα0/0 mice expressing a plasmid encoding IFN-α, whereas lamina propria cells contained high levels of Mx1 (Fig. 4A). In contrast, IECs stained very strongly for nuclear Mx1 in IFNAR10/0 mice expressing a plasmid encoding IFN-λ (Fig. 4B). Control experiments in which a plasmid encoding IFN-λ was administered to IL28Rα0/0 mice, a plasmid encoding IFN-α was administered to IFNAR10/0 mice, or empty control vectors were administered to either mouse strain, yielded no specific staining for Mx1 (Fig. 4 A and B, and Fig. S2).
Fig. 4.
IFN-λ induces an epithelial cell-specific response. (A and B) The spatial response to in vivo electroporation of plasmids encoding mouse IFN-λ or mouse IFN-α was monitored by immunofluorescence staining for Mx1 in duodenal tissue sections of (A) IFNAR10/0 or IL28Rα0/0 mice expressing IFN-α, and (B) in IL28Rα0/0 and IFNAR10/0 mice expressing IFN-λ. (Scale bar, 50 μm.) (C–E) Stat1 tyrosine phosphorylation was detected by immunoblotting after stimulation with IFN (human IFN-αB/D, 2,000 U/mL; mouse IFN-β, 500 U/mL; or mouse IFN-λ, 20 ng/mL). (C) IECs isolated from adult C57BL/6 wild-type mice were stimulated ex vivo for 1 h with the indicated IFN. Unstimulated IECs served as negative control. The images show triplicates for each condition. (D) Mouse macrophage-like RAW 264.7 cells were stimulated for the indicated time. (E) Rat intestinal epithelial IEC-6 cells were grown to confluency on transwell filters and left untreated or stimulated either apically or basolaterally with the indicated IFN. Actin staining was included to demonstrate equal protein loading. The images are representative of three independent experiments.
Interestingly, freshly isolated IECs responded well to ex vivo treatment with mouse IFN-β and mouse IFN-λ2, as visualized by pronounced tyrosine phosphorylation of the transcription factor STAT1 (Fig. 4C). The response of macrophage-like RAW264.7 cells was restricted to type I IFN (Fig. 4D). The observed difference in type I responsiveness of primary IECs in vivo and ex vivo was explained in transwell chamber experiments with polarized intestinal epithelial IEC-6 cells, which demonstrated that STAT1 phosphorylation occurs only after apical but not basolateral stimulation with type I IFN. In contrast, IFN-λ readily induced STAT1 phosphorylation in IEC-6 cells from both, the apical and basolateral site (Fig. 4E).
IFN-λ Confers Protection from Rotavirus Infection.
Next, we determined if administration of exogenous IFN-λ would ameliorate rotavirus resistance of suckling mice. Groups of 10-d-old wild-type mice received subcutaneous injections of mouse IFN-λ2 or human IFN-αB/D. IFN-αB/D induced enhanced expression of the IFN response genes Isg15 and Oasl2 in spleen and liver tissue but not IECs. In contrast, IFN-λ2 treatment led to enhanced expression of Isg15 and Oasl2 in IECs but not spleen and liver cells (Fig. S3). Mice received mouse IFN-λ2 or human IFN-αB/D 8 h before oral infection with a high or low dose of rotavirus. Additional doses of mouse IFN-λ2 and human IFN-αB/D were administered on days 1 and 2 postinfection and viral antigen load in the colon was determined at day 3 postinfection. Virus antigen levels were uniformly high in mock-treated control animals infected with the high virus dose and uniformly low in IFN-λ-treated animals (Fig. 5A). Of the 10 animals that received human IFN-αB/D, eight failed to control the infection, whereas the other two contained little virus antigen in the colon. If a low dose of rotavirus was used for infection (Fig. 5B), viral antigen levels in the mock-treated group were less uniform but all 10 animals became ELISA-positive. Under low-dose infection conditions, treatment with mouse IFN-λ2 was very effective and viral antigen levels in the colon of all 10 animals of this group remained below the detection limit of the assay. Human IFN-αB/D was also partially effective under these less stringent virus challenge conditions (Fig. 5B).
Fig. 5.
Administration of IFN-λ mediates rotavirus protection. Suckling wild-type mice (10 animals per group) were given subcutaneous injections of mouse IFN-λ2 (1 μg per injection), human hybrid IFN-αB/D (1 μg per injection), or buffer only (mock) 8 h before oral infection with (A) high dose (5 μL of 1:103 diluted virus stock) or (B) low dose (5 μL of 1:104 diluted virus stock) of murine rotavirus strain EDIM. The IFN treatment was repeated on days 1 and 2 postinfection. OD450nm values representing the viral antigen levels in colon homogenates on day 3 postinfection are shown.
Discussion
Our work is unique in providing direct experimental evidence for a distinct and critical role of IFN-λ in the mucosal antiviral host defense that cannot be compensated for by IFN-α/β. Previous infection studies in which respiratory viruses were used to dissect the role of IFN-λ indicated only a minor contribution of IFN-λ to antiviral protection that appeared largely masked by the type I IFN system (6, 11, 12). The apparent discrepancy between the conclusions of the current and previous studies may be a result of the exceptional tissue tropism of rotavirus. Rotavirus replicates mostly, if not exclusively, in epithelial cells of the small intestine (19).
Strikingly, our results suggest that the small intestinal epithelium responds far more strongly to IFN-λ than to type I IFN. The epithelium-specific response to IFN-λ is presumably because of the restricted receptor expression (10, 20, 21). The poor responsiveness to type I IFN after either viral infection or systemic administration, in contrast, may result from the subcellular restriction of the type I IFN receptor at the intestinal epithelium. The finding that the type I IFN response of polarized IECs depends on whether IFN acts from the apical or from the basolateral site, provides an explanation for the apparent contradiction between type I IFN susceptibility of in vitro stimulated primary IECs and the lack of type I IFN induced epithelial stimulation in vivo after rotavirus infection or administration of an IFN-α–expressing vector. Thus, our results are in accordance with previous studies that suggested that all nucleated cell types express the IFN-α/β receptor (18). These results, however, also explain why parenteral administration of type I IFN in our study, as well as work published by Angel et al. (15), did not result in an improved outcome after rotavirus infection in suckling mice. Finally, these results illuminate why the gastrointestinal tissue failed to respond to parenteral IFN-α/β administration in an in vivo reporter system (22).
A previous report, which was difficult to interpret at the time, indicated that rotavirus replicates much better in STAT1-deficient than IFNAR1-deficient mice (14, 15). Our results suggest that IFN-λ represents the elusive additional STAT1-dependent mechanism of antiviral host defense and offer a simple explanation for the seemingly discrepant published findings. Similar to the situation after rotavirus infection, STAT1-deficient mice also exhibited a more susceptible phenotype after oral norovirus challenge compared with mice lacking functional receptors for type I and type II IFN (23). Furthermore, after infection with respiratory syncytial virus, lung tissue from IFN-α/β and IFN-λ receptor double-deficient and STAT1-deficient mice contained similarly high virus titers, which were clearly exceeding the titers found in IFNAR1-deficient animals (12). Thus, STAT1-mediated signaling through IFN-λ appears to contribute to antiviral protection both at the lung and intestinal mucosa.
Although the presence of IFN-λ signaling critically determined the viral load in the course of infection, clearance of the infection in adult animals did not depend on IFN signaling and no mortality was observed in IFN receptor-deficient mice. In accordance, STAT1−/− mice harbored higher virus titers during the acute phase of rotavirus infection but viral clearance was not delayed and correlated with the appearance of the specific IgA response (14). Despite the strong link between type I interferons, the clonal expansion of CD8 T cells (24–26), and the development of humoral immunity (27), rotavirus clearance therefore appears to be independent of the IFN-mediated instruction of the adaptive immune system.
In conclusion, our results are unique in revealing a distinct, nonredundant function of IFN-λ in vivo. Using the model of oral rotavirus infection, we demonstrate a striking cell-type specificity of the IFN-λ–mediated antiviral host response and its significant role in the antiviral immune defense at the intestinal epithelium. IL-28Rα–induced signaling might therefore represent a promising therapeutic target to protect the intestinal epithelium from viral infection.
Materials and Methods
Mice and Reagents.
Standard C57BL/6 mice were obtained from Charles River. B6.A2G-Mx1 wild-type mice carrying intact Mx1 alleles (WT), B6.A2G-Mx1-IFNAR10/0 mice lacking functional type I IFN receptors (IFNAR10/0), B6.A2G-Mx1-IL28Rα0/0 mice lacking functional type III IFN receptors (IL28Rα0/0), and B6.A2G-Mx1-IL28Rα0/0IFNAR10/0 double-knockout mice (IL28Rα0/0IFNAR10/0) lacking functional receptors for both type I and type III IFN and SV129 mice were bred locally (11). Animals were housed in accordance with the guidelines defined by the Federation for Laboratory Animal Science Associations (www.felasa.eu/recommendations) and the national animal welfare body Die Gesellschaft für Versuchstierkunde (www.gv-solas.de/index.html), and experiments were performed in compliance with the German animal protection law (TierSchG) and approved by the local animal welfare committees of the universities of Freiburg, Hanover, and Brussels. Human hybrid IFN-αB/D (28), mouse IFN-β (PBL), and mouse IFN-λ2 (IL-28A; PeproTech) were used at the concentrations indicated in the figure legends. Human IFN-αB/D was previously shown to be highly active on many mouse cell types in vitro and in vivo (29, 30).
Virus Infection and Monitoring.
Murine rotavirus strain EDIM was provided by Lennart Svensson (Molecular Virology, Linköping University, Sweden). Virus stocks for infection experiments were prepared from pooled colon contents collected 4 d postinfection of suckling mice. Suckling mice (4–15 days old) were infected by oral application of 5-μL samples of diluted virus stock (1:100–1:10,000 dilutions as indicated in the figure legends). Age-matched animals were used for individual experiments. Adult mice (4–6 wk old) were orally infected with 20 μL of 20-fold diluted virus stock. A comparative ELISA measurement using a dilution series of a rhesus rotavirus stock with known fluorescence focus units in parallel with the virus stock used in this study indicated a virus titer of ∼3 ×108 IU/mL. This approach, however, appeared to somewhat underestimate the number of infectious viral particles, because in vivo challenge of neonatal mice resulted in ELISA-positive fecal shedding after oral challenge with as little as 5 μL of a 1:108 dilution of the virus stock. To determine the viral antigen concentration in colon homogenates or stool samples, the samples were homogenized in the dilution buffer supplied with the RIDASCREEN Rotavirus ELISA Kit from R-Biopharm and the ELISA was performed according to the manufacturer's instructions. Samples were diluted to allow measurement within the linear range of the assay.
Cell Culture and Isolation of Primary IECs and Gene-Expression Analysis.
The rat intestinal epithelial cell line IEC-6 was kindly provided by E. Cario (Gastroenterology and Hepatology, University Duisburg/Essen, Germany) (31) and cultured in DMEM (Invitrogen) supplemented with 4 mM glutamine (Invitrogen), 10% FCS (Sigma), 0.1 units/mL insulin (Sigma), and 4.5 g/L glucose (Sigma). For stimulation experiments, cells were differentiated 4 to 6 d on transwells (pore size 0.4 μm, Greiner Bio-One). RAW264.7 were cultured in DMEM (Invitrogen) supplemented with 4 mM glutamine (Invitrogen) and 10% FCS (Sigma). For isolation of adult IECs, intestinal tissue was removed, cut into 3-cm long pieces, and inverted with the mucosal surface outwards. Inverted tissues were pulled on a plastic stick, incubated for 10 min in 30 mM EDTA and subjected to centrifugal force using a motor-driven biovortexer purchased from Sigma using eight pulses with ∼1- to 2-s duration. Epithelial cell aggregates were separated from contaminating lymphoid and myeloid cells by threefold sedimentation at 1 × g for 20 min. All steps except the incubation in EDTA were performed at 4 °C (32). For the preparation of neonatal IECs, total small intestinal tissue was cut in small pieces and incubated for 10 min in 30 mM EDTA. After vigorous shaking, epithelial cells were separated from the underlying tissue by filtration through a 100-μm pore size cell strainer (BD Falcon). This method resulted in a somewhat less pure fraction of IECs. RNA was isolated from isolated epithelial cell preparations, spleen, and liver tissue with TRIzol (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed using 1 to 2 μg of total RNA with RevertAid reverse transcriptase (Fermentas). Real-time PCR was quantified using Sybr green (Invitrogen) and analyzed using the Pfaffl method to express relative expression of the target gene to the housekeeping gene Gapdh (33). The following primers were used in this study: Isg15 (gagctagagcctgcagcaat, ttctgggcaatctgcttctt), Mx1 (tctgaggagagccagacgat, actctggtccccaatgacag), Oasl2 (ggatgcctgggagagaatcg, tcgcctgctcttcgaaactg), Ifn-λ 2/3 (agctgcaggccttcaaaaag, tgggagtgaatgtggctcag), Ifn-β (tcagaatgagtggtggttgc, gacctttcaaatgcagtagattca), and Gapdh (tgcaccaccaactgcttagc, ggcatggactgtggtcatgag) (10, 34).
In Vivo Plasmid Electrotransfer.
Plasmid electrotransfer-mediated expression of IFN was performed as described previously (10). Twenty-five micrograms of empty plasmid or plasmid expressing either IFN-α6T or IFN-λ3 was injected in the left and right tibialis anterior muscles and electric pulses were administered using a Cliniporator system. At day 7 after electrotransfer, organs were harvested for histological analysis as described in ref. 10.
Immunohistology.
Antigen retrieval in deparaffinized paraformaldehyde-fixed tissue sections was performed with 0.01 M sodium citrate buffer. Slides were blocked with normal donkey serum (Jackson ImmunoResearch) and stained with the rabbit anti-Mx1 polyclonal antiserum (AP5) (35), a mouse monoclonal anti–E-cadherin antibody (BD Bioscience Pharmingen), or a polyclonal goat anti-rotavirus antiserum (NCDV; Meridian LS) followed by the appropriate AF555-, AF488-, Cy3-, or Cy5-conjugated secondary antibody (Molecular Probes, Jackson ImmunoResearch). Counterstaining was performed with fluorescein-conjugated wheat germ agglutinin (Vector Laboratories) and slides were mounted in DAPI-containing Vectashield (Vector Laboratories). Tissue sections were visualized using an ApoTome-equipped Axioplan 2 microscope connected to an AxioCam Mr digital Camera (Carl Zeiss MicroImaging, Inc.). The rotavirus antigen staining intensity at the level of the villus and crypt epithelium was analyzed separately over a total area of greater than 7 × 104 μm2 per mouse using the Axiovision software (Carl Zeiss). The fluorescence intensity per square micrometer of the villus epithelium was divided by the respective value determined for the crypt epithelium to obtain relative fluorescence intensity. H&E staining was performed according to Mayer's protocol with reagents from Roth. Epithelial vacuolization (grade 0–3), villus deformation (grade 0–1), and breakage of the epithelial barrier (number of sites with lost epithelial integrity) per villus were recorded in a blinded fashion in eight visual fields in two tissue sections per mouse, with three mice per experimental group. The grades recorded were normalized to the maximal value obtained for each individual criterium to obtain a balanced histological score.
Western Blot Analysis.
Cell lysates were prepared as recently described (32), separated by SDS-PAGE, and transferred onto nitrocellulose membranes (Millipore). Membranes were incubated with polyclonal rabbit antibody recognizing phosphorylation at tyrosine 701 of STAT1 (Cell Signaling) and monoclonal mouse actin antibodies (Sigma). Horseradish peroxidase-labeled secondary antibodies were purchased from Jackson Immunoresearch and detected using the chemiluminescence detection system from Pierce.
Statistical Analysis.
Results are presented as means ± SD. The Mann-Whitney U test was used for statistical analysis using the GraphPad Prism Software 4.00. P values are indicated as follows: ***P < 0.001; **P < 0.01, and *P < 0.05.
Supplementary Material
Acknowledgments
We thank Lennart Svensson, Gerry McInerney, Elke Cario, and André Bleich for technical support, and Heinz-Kurt Hochkeppel for providing human IFN-αB/D. This work was supported in part by the individual Grant Ho2236/5-3 and the Collaborative Research Center SFB621 and SFB900 from the German Research Foundation and Grants DLR 01GU0825 and 01KI0752 from the Federal Ministry of Education and Research (to M.W.H.); a grant of the International Research Training Group IRTG 1273 (to C.U.D.); a Federation of European Biochemical Societies postdoctoral fellowship (to S.S.) and an Austrian Programme for Advanced Research and Technology fellowship from the Austrian Academy of Sciences at Hannover Medical School (to S.S.); German Research Foundation Grant SFB 620 (to P.S.); and Grant FRSM 3.4576.08 from Fonds de la Recherche Scientifique Médicale (to T. Michiels).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100552108/-/DCSupplemental.
References
- 1.Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407–422. doi: 10.1038/sj.cr.7290309. [DOI] [PubMed] [Google Scholar]
- 2.Kotenko SV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard P, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 4.Gad HH, et al. Interferon-lambda is functionally an interferon but structurally related to the interleukin-10 family. J Biol Chem. 2009;284:20869–20875. doi: 10.1074/jbc.M109.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onoguchi K, et al. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- 6.Ank N, et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 7.Yang K, et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumoutier L, et al. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: Similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, et al. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mordstein M, et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mordstein M, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Global Deaths Under Age Five Attributable to Rotavirus Infection. World Health Organization, Geneva, Switzerland; 2004. [Google Scholar]
- 14.Vancott JL, McNeal MM, Choi AH, Ward RL. The role of interferons in rotavirus infections and protection. J Interferon Cytokine Res. 2003;23:163–170. doi: 10.1089/107999003321532501. [DOI] [PubMed] [Google Scholar]
- 15.Angel J, Franco MA, Greenberg HB, Bass D. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J Interferon Cytokine Res. 1999;19:655–659. doi: 10.1089/107999099313802. [DOI] [PubMed] [Google Scholar]
- 16.Franco MA, Greenberg HB. Immunity to homologous rotavirus infection in adult mice. Trends Microbiol. 2000;8:50–52. doi: 10.1016/s0966-842x(99)01682-0. [DOI] [PubMed] [Google Scholar]
- 17.Dreiding P, Staeheli P, Haller O. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology. 1985;140:192–196. doi: 10.1016/0042-6822(85)90460-x. [DOI] [PubMed] [Google Scholar]
- 18.Randall RE, Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 19.Boshuizen JA, et al. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J Virol. 2003;77:13005–13016. doi: 10.1128/JVI.77.24.13005-13016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasfar A, et al. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 21.Brand S, et al. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G960–G968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- 22.Pulverer JE, et al. Temporal and spatial resolution of type I and III interferon responses in vivo. J Virol. 2010;84:8626–8638. doi: 10.1128/JVI.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW., 4th STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 24.Aichele P, et al. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 25.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 26.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bon A, et al. Cutting edge: Enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 28.Horisberger MA, de Staritzky K. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J Gen Virol. 1987;68:945–948. doi: 10.1099/0022-1317-68-3-945. [DOI] [PubMed] [Google Scholar]
- 29.Pichlmair A, et al. Thogoto virus lacking interferon-antagonistic protein ML is strongly attenuated in newborn Mx1-positive but not Mx1-negative mice. J Virol. 2004;78:11422–11424. doi: 10.1128/JVI.78.20.11422-11424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumpey TM, et al. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J Virol. 2007;81:10818–10821. doi: 10.1128/JVI.01116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotz M, et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockinger S, et al. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 35.Meier E, et al. A family of interferon-induced Mx-related mRNAs encodes cytoplasmic and nuclear proteins in rat cells. J Virol. 1988;62:2386–2393. doi: 10.1128/jvi.62.7.2386-2393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.