Abstract
During peripheral nerve development, Schwann cells ensheathe axons and form myelin to enable rapid and efficient action potential propagation. Although myelination requires profound changes in Schwann cell shape, how neuron–glia interactions converge on the Schwann cell cytoskeleton to induce these changes is unknown. Here, we demonstrate that the submembranous cytoskeletal proteins αII and βII spectrin are polarized in Schwann cells and colocalize with signaling molecules known to modulate myelination in vitro. Silencing expression of these spectrins inhibited myelination in vitro, and remyelination in vivo. Furthermore, myelination was disrupted in motor nerves of zebrafish lacking αII spectrin. Finally, we demonstrate that loss of spectrin significantly reduces both F-actin in the Schwann cell cytoskeleton and the Nectin-like protein, Necl4, at the contact site between Schwann cells and axons. Therefore, we propose αII and βII spectrin in Schwann cells integrate the neuron–glia interactions mediated by membrane proteins into the actin-dependent cytoskeletal rearrangements necessary for myelination.
Rapid and efficient action potential propagation in vertebrates depends on axon ensheathement by a multilammelar membrane sheath called myelin. Myelin is made by Schwann cells in the peripheral nervous system (PNS). During development, neuron–glia interactions induce reciprocal differentiation such that axons regulate Schwann cell differentiation, migration, and myelination, and Schwann cells regulate the organization of axonal membrane domains (1–3). The mechanisms regulating PNS myelination still remain poorly understood. In particular, myelination requires dramatic and dynamic changes in the Schwann cell cytoskeleton, leading to the profound changes in cell shape that accompany axonal ensheathement and wrapping. However, how axon-Schwann cell interactions converge on the Schwann cell cytoskeleton to induce these changes is unknown.
A recent study suggests that submembranous cytoskeletal proteins, called spectrins, may contribute to myelination: a dominant-negative human mutation in αII spectrin causes severe cerebral hypomyelination (4). Spectrins are a family of extended, flexible cytoskeletal molecules consisting of α and β subunits (5). β-Spectrins interact with both the actin cytoskeleton and various membrane proteins via scaffolding proteins, such as ankyrins or 4.1 proteins. Spectrins are thought to (i) stabilize membrane protein complexes, (ii) provide mechanical support for cell membrane integrity, and (iii) serve as a multifunctional regulatory platform for cell signaling (6). Spectrins are abundantly expressed in the nervous system, but have traditionally been assumed to be mostly neuronal. For example, spectrins contribute to stabilization of axonal membrane domains including the node of Ranvier (7). Although spectrins were previously reported in myelinating Schwann cells, their specific isoforms and functions are not known (8).
Here, we demonstrate that the submembranous cytoskeletal proteins in Schwann cells consist of αII and βII spectrin. In Schwann cells, these spectrins acquire a polarized distribution along axons, and during development they colocalize together with other signaling molecules known to modulate myelination in vitro. Silencing expression of spectrins by short-hairpin RNA (shRNA) inhibited myelination in vitro and remyelination in vivo. Lack of αII spectrin caused myelin defects in zebrafish motor nerves. The amount of both F-actin and the Nectin-like (Necl) protein 4 on the surface of Schwann cells were reduced by loss of spectrin. Together, our results suggest that αII and βII spectrin in Schwann cells function to integrate the neuron–glia interactions mediated by membrane proteins into the actin-dependent cytoskeletal rearrangements necessary for PNS myelination.
Results
αII and βII Spectrin Form a Special Cytoskeleton in Myelinating Schwann Cells.
To determine the composition of the submembranous cytoskeleton in mature myelinating Schwann cells, we immunostained teased fibers from adult rat sciatic nerves with antibodies against spectrins (αII, βII, βIII, and βIV), ankyrins (ankB, ankG, and ankR), and 4.1 proteins (4.1B, 4.1G, and 4.1N). Among these different proteins, αII and βII spectrin and protein 4.1G were highly enriched in Schwann cell cytoplasm channels, called bands of Cajal, along the outer surface of myelin (Fig. 1 A and B and Fig. S1A) (8–10). Neither αII spectrin nor βII spectrin staining colocalized with dystrophin-related protein 2 (DRP2) immunoreactivity, which instead is located at the abaxonal surface of the myelin sheath where the cytoplasm is absent (Fig. 1A). During development, βII spectrin was incorporated into bands of Cajal concomitant with myelin maturation (Fig. S1 B–E) (11). Immunoblot analysis of sciatic nerves and cultured Schwann cells further confirmed the presence of αII and βII spectrin and protein 4.1G (Fig. S1 F and G). As in other cell types (5), coimmunoprecipitation from Schwann cell extracts demonstrate that αII and βII spectrin form a complex (Fig. S1H). Thus, the submembranous cytoskeleton in myelinating Schwann cells consists mainly of αII spectrin, βII spectrin, and protein 4.1G.
Fig. 1.
Polarized localization of spectrins in Schwann cells. (A and B) Teased sciatic nerve fibers from adult rats immunostained for βII, αII spectrin, or DRP2, as indicated. Arrowheads indicate nodes of Ranvier. (C and D) Teased sciatic nerve fibers from E18 rat immunostained for βII, αII spectrin, or Par-3, as indicated, showing asymmetrical localization (arrowheads) of these proteins. (E) DRG neuron and Schwann cell cocultures 2 d after inducing myelination immunostained for βII spectrin (green) and Par-3 (red). [Scale bars, 20 μm (A and B) and 10 μm (C–E).]
The Spectrin Cytoskeleton Is Polarized in Premyelinating Schwann Cells.
Because spectrins coordinate interactions between membrane proteins and actin in most polarized cells, and because in mature myelinating Schwann cells spectrins are highly polarized to bands of Cajal (Fig. 1), we considered whether spectrins also contribute to polarity in Schwann cells. Previous studies demonstrated that myelination is regulated by the polarity protein Par-3 in premyelinating Schwann cells (12). We found that αII spectrin, βII spectrin, and protein 4.1G showed an asymmetric staining pattern that colocalized with Par-3 in premyelinating Schwann cells in embryonic day (E) 18 rat sciatic nerves (Fig. 1 C and D and Fig. S2 A and B), and in coculture with dorsal root ganglion (DRG) neurons (Fig. 1E and Fig. S2 C and D). Thus, spectrins are highly polarized at axon-glia contact sites in premyelinating Schwann cells.
Silencing Spectrin Expression in Schwann Cells Inhibits Myelin Formation in Vitro.
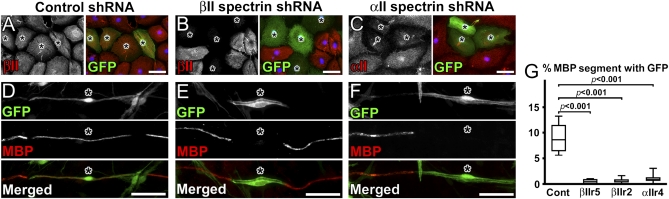
Based on the asymmetric localization of spectrins and their colocalization with Par-3 in premyelinating Schwann cells (Fig. 1 C–E), we hypothesized that αII and βII spectrin might have important functions during PNS myelination. To test this possibility, we silenced spectrin expression by RNA interference in Schwann cells, and then examined the consequence during myelination. ShRNAs for βII spectrin (βIIr5, βIIr2) and αII spectrin (αIIr4) were incorporated into adenoviral vectors together with a membrane-bound GFP. As a control, a highly specific and efficient ankG shRNA in the same adenoviral vector (13) was used, because we did not detect ankG in mature myelin. Whereas control shRNA did not alter spectrin expression, shRNAs for αII or βII spectrin markedly reduced each targeted spectrin (Fig. 2 A–C and Fig. S3). Immediately after viral transduction, Schwann cells were harvested, added to cultured DRG neurons, and 1 wk later myelination was induced. Some of the transduced cells were plated in wells without DRG neurons, and the adenovirus infection efficiency was determined to be 81.0, 84.3, 73.8, and 90.7% for control, βIIr5, βIIr2, and αIIr4 shRNAs, respectively (average of three independent experiments). The βII spectrin staining at the axon-glial contact sites was reduced by βII spectrin shRNA (Fig. S3 J and K). Two weeks after inducing myelination, Schwann cells transduced with control shRNA produced myelin sheaths labeled with myelin basic protein (MBP) (Fig. 2D) or DRP2 (Fig. S4A). In contrast, Schwann cells with spectrin silencing failed to show MBP or DRP2 staining, although those cells aligned properly with axons (Fig. 2 E and F and Fig. S4B). The frequency of adenovirus-infected cells producing myelin segments was quite significantly reduced by all three spectrin shRNAs compared with the control shRNA (Fig. 2G). Importantly, noninfected Schwann cells aligning along the same axon formed normal myelin segments (Fig. 2 E and F and Fig. S4B), suggesting that the lack of myelin was not because of altered signaling from axons. Taken together, these results suggest that the spectrin-based submembranous cytoskeleton modulates PNS myelination.
Fig. 2.
Spectrin silencing in Schwann cells inhibits myelination in vitro. (A–C) Cultured rat Schwann cells 7 d after infection with adenovirus containing control (A), βII spectrin (B), or αII spectrin (C) shRNAs. Infected cells are identified by GFP (asterisks). (D–F) Myelinating coculture of rat DRG neurons and Schwann cells with shRNA immunostained for MBP in red. Asterisks indicate infected cells. (G) Frequency of the GFP signal in myelin segments shown in box-and-whisker plots. Data were obtained from six independent experiments (∼300 MBP+ segments in each experiment). GFP+ myelin segments are significantly less frequent in cells transduced with spectrin shRNAs compared with control Schwann cells. There were no significant differences among the three spectrin shRNAs. (Scale bars, 50 μm.)
βII Spectrin Silencing in Vivo Prevents Remyelination.
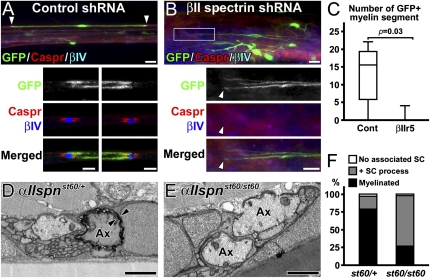
To confirm the in vitro results (Fig. 2), we next examined the consequence of RNA interference in vivo using a sciatic nerve crush injury model characterized by Wallerian degeneration followed by rapid axon regeneration and remyelination (14). We used a remyelination model for two reasons: (i) it is difficult to examine the role of spectrins during developmental myelination because PNS myelination begins immediately after birth, but there is a lag time between adenovirus infection and considerable spectrin reduction, and (ii) the spectrin localization during remyelination after crush injury mimics developmental processes (Fig. S5). We injected the adenovirus with shRNA into the sciatic nerve trunk distal to the injury site 1 d after crush, and the distal segment was allowed to regenerate. Infection of demyelinating Schwann cells, degeneration and regeneration of nerve fibers, and the frequency of Schwann cells per adenovirus-infected cells during regeneration were comparable between control and βIIr5 adenovirus-injected nerves (Fig. S6). At 21 d postcrush (dpc), all peroneal branches of sciatic nerves were dissected, teased, and immunostained. Despite the considerable number of GFP+ cells in nerves, we observed only a few axons with GFP signal, indicating the axonal spectrins are intact. To identify remyelinating Schwann cells, we used the paranodal axonal molecule Caspr, which is only associated with myelin segments. In the nerves injected with control shRNA, we found remyelinating Schwann cells with GFP immunoreactivity (Fig. 3 A and C). In contrast, very few were found in βII spectrin shRNA-injected nerves, despite a considerable number of GFP+ cells (Fig. 3 B and C). To exclude the possibility that the shRNA alters the demyelination process rather than remyelination per se, we also injected the adenovirus at 5 dpc. Similarly, at 21 dpc, 12 GFP+ remyelinating Schwann cells were found in control nerves, whereas none could be found with the βII spectrin shRNA (from one animal each). Taken together, these results strongly suggest that spectrins play important roles in PNS myelination in vivo.
Fig. 3.
Disruption of spectrins in Schwann cells inhibits myelination in vivo. (A–C) βII spectrin silencing during sciatic nerve regeneration. The adenoviruses with shRNAs were injected 1 dpc. (A) A myelin segment produced by a Schwann cell with control shRNA at 21 dpc. The nodes (βIV spectrin in blue) and paranodes (Caspr in red) on both sides (arrowheads) are enlarged in lower panels. (B) Considerable GFP expression in cells not producing myelin in sciatic nerves injected with βIIr5 at 21 dpc. The boxed area in the upper panel is enlarged in the lower panels. The arrowhead indicates the tip of GFP+ cells with no overlapping nodes or paranodes. (C) The number of remyelinating Schwann cells with GFP signal in peroneal nerves shown in box-and-whisker plots. Remyelinating Schwann cells are significantly fewer in βIIr5 injection compared with control. A total of 76 remyelinating segments with GFP was found in five of six control animals, whereas only four were found in one of six animals with βIIr5 injection. (D–F) Control and αII spectrin mutant zebrafish. Transmission electron microscopy of transverse sections of 7-dpf larval motor nerves. Ax indicates axon, and arrowheads indicate multiple layers of myelin. One of the large primary motor axons in the heterozygous sibling has a myelin sheath (D), whereas the large axons from the mutant do not (E). (F) Quantitation of large motor nerve axons with no associated Schwann cells (SC), associated with SC process, or myelin. Data were obtained from 33 large axons from 21 nerves (four larvae) in heterozygous control or 53 large axons from 35 nerves (three larvae) in the mutant at 7 dpf. [Scale bars, 20 μm (A and B, Upper), 5 μm (A, Lower), 10 μm (B, Lower), and 1 μm (D and E).]
Loss of αII Spectrin Impairs Myelination in Zebrafish Motor Nerves.
To further confirm that spectrins modulate PNS myelination in vivo, we analyzed PNS myelin in a zebrafish mutant lacking αII spectrin (αIIspnst60/st60) (15). In heterozygous siblings (αIIspnst60/+), all motor nerves examined (30 of 30 nerves from four embryos) had one to two myelinated axons at 7 d postfertilization (dpf) (Fig. 3D). In contrast, 58.3% (21of 36 nerves from four embryos) of homozygous mutant (αIIspnst60/st60) motor nerves lacked myelinated axons (Fig. 3E), whereas most mutant large axons were associated with the Schwann cell processes (Fig. 3F). The axons that were myelinated in αIIspnst60/st60 mutants appeared normal. Similarly, at 9 dpf all heterozygous motor nerves (36 of 36 nerves from three embryos) contained at least one myelinated axon, whereas 42.3% (11 of 26 nerves from four embryos) of αIIspnst60/st60 motor nerves still had no myelinated axons. Surprisingly, myelination in posterior lateral line sensory nerves was comparable between heterozygous siblings and αIIspnst60/st60 mutant fish (Fig. S7 and ref. 15). This difference may reflect neuron–glia interactions disrupted in αIIspnst60/st60 fish motor nerves that remain intact, or can be compensated for in sensory nerves. Furthermore, normal myelination in the sensory nerves suggests that the myelin reduction in motor nerves is not simply a developmental delay. Taken together, these results further support the important role of Schwann cell spectrins in PNS myelination.
βII Spectrin Knockdown Reduces the Spectrin–Actin Complex.
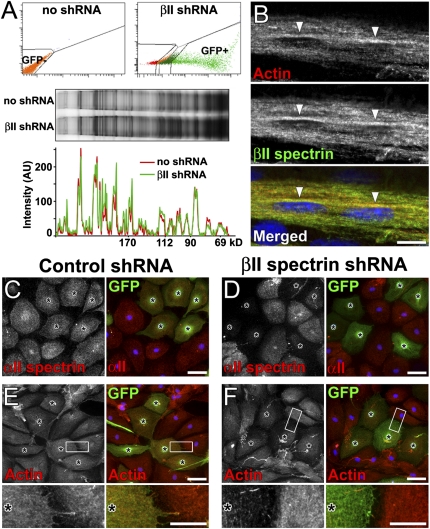
How do Schwann cell spectrins contribute to PNS myelination? Because spectrins function both as structural elements of the cytoskeleton and as scaffolds to link various proteins to the actin cytoskeleton, we expected that spectrins might participate in many different cell biological pathways that converge on Schwann cell differentiation and myelination. Thus, loss of spectrin might lead to profound changes in the expression levels or stability of many different proteins. To test this possibility, we isolated Schwann cells transduced with βII spectrin shRNA by FACS, size-fractionated total proteins by SDS/PAGE, then compared protein amounts with noninfected cells by silver staining (Fig. 4A). Surprisingly, despite robust βII spectrin knockdown (Fig. 2B and Fig. S3), we did not see any dramatic change in protein expression levels, suggesting that the loss of Schwann cell spectrin disrupts specific signaling pathways in subtle ways rather than causing widespread cellular dysfunction. As an alternative method, we evaluated changes in the expression levels and localization of specific proteins previously reported to interact with spectrins or to play important roles in PNS myelination. For example, αII and βII spectrin form an obligatory complex with each other and link membrane proteins to the actin cytoskeleton (5). Interestingly, actin in Schwann cells is reported to play two distinct and necessary roles during differentiation and myelination: one in mediating cell shape changes and another regulating myelin gene expression (16). In E18 rat sciatic nerves, F-actin accumulated asymmetrically in premyelinating Schwann cells, colocalizing with βII spectrin (Fig. 4B). Furthermore, after βII spectrin silencing, both αII spectrin and F-actin were remarkably reduced in cultured Schwann cells (Fig. 4 C–F), while β-tubulin was unaffected (Fig. S8A). Surprisingly, we observed no apparent reduction in protein 4.1G in Schwann cells with βII spectrin silencing (Fig. S8B). These findings suggest the loss of myelination observed here may be related to disruption of the proper actin filament organization necessary for myelination.
Fig. 4.
βII spectrin silencing reduces related cytoskeletal proteins. (A) Comprehensive analysis of Schwann cell proteins. Noninfected (GFP−) or GFP+ βIIr5-transduced cells were collected by FACS. Silver stain of proteins is shown in the middle panel; note that samples were matched for the same cell number. Line scans of staining intensity for each lane in the gel is shown below. (B) Polarized localization of F-actin (red) in premyelinating Schwann cells in E18 rat sciatic nerves, colocalizing with βII spectrin (green, arrowheads). (C–F) Changes in cytoplasmic proteins in cultured Schwann cells with control shRNA (C and E) or βII spectrin shRNA (D and F) (asterisks). The expression of αII spectrin (D) and F-actin (F) is reduced by βII spectrin shRNA, but unchanged by control shRNA (C and E). Higher magnification images in boxed areas are shown in lower panels (E and F). [Scale bars, 10 μm (B), 50 μm (C, D, and E, and F, Upper), and 20 μm (E and F, Lower).]
Spectrin Silencing Alters Axon–Schwann Cell Interactions Mediated by Necl Proteins.
Because spectrins contribute to the stabilization of membrane proteins, we considered whether loss of spectrin affects the membrane proteins required for axon–glia interactions or myelination. Necl proteins are members of the IgCAM superfamily and Necl4 expressed in Schwann cells interacts with Necl1 on axons, thereby mediating axon–glia contact during PNS myelination (17, 18). Importantly, Necl4 was polarized and colocalizes with βII spectrin in premyelinating Schwann cells in vivo and in vitro (Fig. 5 A and B). The amount of Necl4 together with the binding of Necl1-Fc was reduced on the surface of Schwann cells with silenced expression of βII spectrin compared with uninfected Schwann cells or cells expressing the control shRNA (Fig. 5 C and D). Furthermore, the polarized localization of Necl4 in premyelinating Schwann cells was also disrupted by all three spectrin shRNA constructs (Fig. 5 E–G). In contrast, spectrin knockdown in cultured Schwann cells did not cause any apparent changes in other molecules tested, including the neuregulin-1 receptor ErbB2 (3), Par-3, N-cadherin, or β-catenin (Fig. S8), although we cannot exclude the possibility that spectrins function downstream of these molecules during myelination. Indeed, N-cadherin and β-catenin are involved in establishing Schwann cell polarity and the timely initiation of myelination (19), and α-catenin, a binding partner of β-catenin, directly interacts with the αII and βII spectrin complex (20). Taken together, these findings strongly support the conclusion that loss of the spectrin-based cytoskeleton in Schwann cells impairs multiple mechanisms that contribute to proper PNS myelination.
Fig. 5.
Spectrin silencing alters axon-Schwann cell interactions mediated by Necl proteins. (A) E18 rat embryo sciatic nerve showing asymmetric, polarized colocalization of Necl4 (red) and βII spectrin (green) (arrows). (B) DRG neuron coculture 2 d after inducing myelination showing colocalization of Necl4 (red) and βII spectrin (green) at axon-Schwann cell contact sites (arrowheads). (C and D) Cultured Schwann cells expressing control (C) or βIIr5 (D) shRNA (asterisks) incubated with soluble Necl1-Fc. Higher magnification images in boxed areas show bound Necl1-Fc (C and D). The lines in the middle indicate the boundary between the two cells. (E) Polarized Necl4 localization in Schwann cells is altered by spectrin shRNA in DRG neuron coculture 2 d after inducing myelination. Data were collected from two independent experiments. (F and G) DRG neurons cocultured with Schwann cells with control (F) or βIIr5 shRNA (G) 2 d after inducing myelination. Line scans (white lines) of staining intensity are shown below. The GFP value was normalized to the maximum value in the scan. The area where the axon is located can be identified by a decrease in GFP signal. Necl4 staining is reduced at the axon-Schwann cell interface (arrowheads) in the Schwann cell with βII spectrin shRNA (G). [Scale bars, 10 μm (A, B, F, G, and enlarged boxed areas in C and D) and 20 μm (C and D).]
Discussion
In this study, we showed that αII and βII spectrin in Schwann cells modulate PNS myelination. These conclusions are based on several observations: (i) αII and βII spectrin occupy a polarized location in premyelinating Schwann cells at axonal contact sites; (ii) αII and βII spectrin silencing by shRNA in cultured Schwann cells inhibits myelination in coculture with DRG neurons; (iii) βII spectrin knockdown in Schwann cells inhibits remyelination following sciatic nerve crush injury; (iv) myelinated fibers in motor nerves are reduced in αII spectrin mutant zebrafish; and (v) Necl4 and actin, important regulators of myelination, are reduced after spectrin knockdown.
We used an RNA interference approach to elucidate the function and importance of spectrins, because mice lacking βII spectrin have gastrointestinal, liver, neural, and heart defects, and die in utero (21), and αII spectrin-null mice have not been reported. Although the possibility of “off-target” effects always exists in knockdown experiments using shRNA, we consider this unlikely because all three different constructs—one for αII spectrin and two for βII spectrin—had identical biological effects in Schwann cells: they inhibited myelination in DRG neuron cocultures and induced a significant reduction in polarized Necl4 in DRG-Schwann cell cocultures. Furthermore, our complementary results using αII spectrin mutant zebrafish strongly support the conclusion that Schwann cell spectrins play important roles in PNS myelination. At present, this mutant fish is the only animal model available to investigate myelin formation following loss of spectrins. However, we cannot exclude the possibility that the loss of axonal spectrins affects myelination in zebrafish motor nerves. It will be important to elucidate the role of axonal spectrins on myelination, especially to understand the mechanism of West syndrome caused by αII spectrin mutations (4).
How do Schwann cell spectrins contribute to PNS myelination? Our results suggest that spectrins regulate both the actin cytoskeleton and axon–glia interactions mediated by Necl proteins. Loss of Schwann cell spectrin decreased F-actin, which regulates cell shape and myelin gene expression during Schwann cell differentiation and myelination (16). Thus, spectrins may organize the Schwann cell actin cytoskeleton and contribute to its reorganization during myelination. Furthermore, loss of αII and βII spectrin caused a significant reduction in the amount of Necl4 found in the Schwann cells at axonal contact sites. Similar to our results (Fig. 2), knockdown of Necl4 by shRNA (18) or expression of a dominant-negative Necl4 (17) in Schwann cells inhibited myelination in vitro. Thus, similar to the role of spectrins to stabilize membrane proteins in specific axonal domains (7), Schwann cell spectrins may function to stabilize Necl4 at the membrane and promote its association with axonal Necl1. How spectrins interact with Necl4 remains unclear. One possibility is that the protein 4.1G links spectrins and Necl4, because 4.1 proteins can associate with both β spectrin (5) and Necl proteins (22). Consistent with this idea, protein 4.1G colocalizes with αII and βII spectrin and Necl4 in premyelinating Schwann cells. However, 4.1G expression in cultured Schwann cells was not altered by βII spectrin knockdown. The protein 4.1G may be stabilized by interacting with other molecules in cells with spectrin silencing. Alternatively, other unknown proteins may facilitate interactions between spectrins and Necl4.
It remains unclear if Necl proteins have critical roles on myelination in vivo. Injection of soluble Necl4 inhibited remyelination after lysolecithin-induced demyelination in rat sciatic nerves, presumably by competing with endogeneous Necl4 (17). However, Necl1 mutant mice have normal Schwann cell maturation and myelination of sciatic nerves (23). Chronic loss of Necl proteins in vivo may be compensated for by related Necl molecules or other mechanisms. In contrast, our results in βII spectrin knockdown during nerve repair and zebrafish motor nerves lacking αII spectrin, together with a recent report of hypomyelination caused by an αII spectrin mutation in human disease (4), strongly suggest that spectrins are important molecules in myelination in vivo. Because spectrins are capable of participating in multiple interactions with diverse proteins, it may be difficult for Schwann cells to compensate for their loss. Based on our results, we propose that spectrins function to couple extracellular neuron–glia interactions to actin-dependent cytoskeletal rearragements necessary for myelination.
In addition to a clearer description of the spectrin-dependent protein–protein interactions that contribute to developmental PNS myelination, our observations raise important questions regarding the cytoskeleton in myelinating glia in health and disease. For example, what roles do spectrins have in mature PNS myelin? In the premyelinating Schwann cells, spectrins are enriched at the contact sites with axons. During the process of myelination, spectrins become concentrated around periaxonal regions and the structures associated with cytoplasm including bands of Cajal, but excluded from compact myelin (8). Spectrins enriched in abaxonal bands of Cajal in mature myelin may have roles distinct from those during development. Loss of β spectrin from axons in the model organism Caenorhabditis elegans results in fragile axons that easily break (24). Similarly, spectrins may contribute to maintenance of mature myelin by mechanical support of the myelin membrane. Finally, there is accumulating evidence that spectrins in myelinating glia play key roles in the pathogenesis of various human nervous system diseases and injuries, such as αII spectrin mutations recently described in patients with West syndrome (4), or spectrin breakdown by calpain, a calcium-dependent cysteine protease, in the CNS demyelinating disease multiple sclerosis (25). It is possible that dysregulation and disruption of the spectrin-based submembranous cytoskeleton in both Schwann cells and oligodendrocytes contributes to demyelination and failure to remyelinate in neurological diseases. Future studies will be needed to determine the role of spectrins in CNS myelination and remyelination.
Taken together, our results support a model where αII and βII spectrin function as key regulators of PNS myelination to link signals between axons and Schwann cells to actin cytoskeleton rearrangement.
Materials and Methods
Animals.
Adult Sprague-Dawley rats were obtained from Harlan Sprague–Dawley Inc. All animal procedures were approved by the Institutional Animal Care Committee at the University of Connecticut Health Center or Baylor College of Medicine, and conform to the United States Public Health Service Policy on Human Care and Use of Laboratory Animals.
Antibodies.
The list of primary and secondary antibodies used in this study is provided in SI Materials and Methods.
Constructs for shRNA and Adenovirus.
ShRNAs and adenovirus were prepared as described elsewhere (13). A 19-nucleotide sequence corresponding to the targeted gene was selected: βIIr5, 5′-GGAUGAAAUGAAGGUGCUA-3′; βIIr2, 5′-GCAUGUCACGAUGUUACAA-3′; and αIIr4, 5′-AGCAUGAUGUUCAAACACU-3′. The H1 promoter driving shRNA expression and the shRNA sequence were inserted to pENTR11 (Invitrogen) via the EcoRI and HindIII sites. The CAG promoter driving expression of membrane-bound gap-EGFP was inserted using the HindIII and XhoI sites. All shRNAs and EGFP sequence were incorporated into an adenovirus. The pENTR11 plasmids were recombined with pAd/PL-DEST using ViraPower Adenoviral Promoterless Gateway Expression kit (Invitrogen). Adenovirus was produced using HEK 293 cells. Production of Necl1-Fc is described elsewhere (17).
Cell Cultures.
Primary culture of Schwann cells were obtained from sciatic nerves from P2 Sprague-Dawley rats. Binding assays of Necl1-Fc were performed as described previously (17). Purified DRG neuron cultures were obtained from E18 Sprague-Dawley rat embryos. The purified Schwann cells were infected with adenovirus containing shRNAs, then mixed with DRG neuron culture for myelinating coculture. The detailed methods are described in SI Materials and Methods.
Nerve Crush and Intraneural Adenovirus Injection.
Adult rats were anesthetized by ketamine (80 mg/kg body weight) and xylazine (16 mg/kg body weight). For peripheral nerve-crush injury, the left sciatic nerve was exposed and crushed for 10-s periods at the level of the sciatic notch using forceps (No. 5). The next day or 5 dpc, intraneural injection of adenovirus was performed as described previously (17, 26), with modifications. The peroneal and tibial branches of sciatic nerve were each injected with 2 μL of adenovirus (1 × 105-6 pfu) in sterile Locke's solution (pH 7.4) by using a glass micropipette.
Immunofluorescence Studies.
Immunostaining of sciatic nerves and cultured cells was performed as described previously (27, 28). For teased fiber preparation, sciatic nerves were teased apart gently and spread on gelatin-coated coverslips after fixation, and air-dried.
Immunoblotting and Immunoprecipitation.
Adult rat brains, sciatic nerves, and neonatal rat sciatic nerves (collected from 10 P1 pups), or cultured rat Schwann cells were homogenized, then immunoblotting was performed as described (28). Coimmunoprecipitation was done as described previously (28) using monoclonal IgG1 antibodies to αII or βII spectrin, and control monoclonal IgG1 that does not react with any antigen in animal cells (Abcam).
FACS.
Noninfected (GFP−) or infected with adenovirus with βIIr5 (GFP+) cultured Schwann cells (85,000 each) were collected in the Baylor College of Medicine Cytometry and Cell Sorting Facility.
Transmission Electron Microscopy.
Identification of the αII spectrin mutant has been described (15). For transmission electron microscopy, αIIspnst60/st60 mutants and heterozygous siblings were identified by PCR-based genotype assay before embedding. Larvae were fixed and processed as described (29). Motor nerves were imaged at the level of the middle of the notochord where two primary motor axons and a larger number of smaller secondary motor axons are present. Images were collected with a JEOL1230.
Statistical Analysis.
For knockdown experiments in DRG neuron-rat Schwann cell coculture, differences were compared using Kruskal-Wallis test followed by Scheffé’s post hoc test. For in vivo knockdown experiments, the Mann-Whitney test was used. Differences are considered significant at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants NS044916 (to M.N.R.), NS050223 (to W.S.T.), and NS050220 (to E.P.); the Dr. Miriam and Sheldon Adelson Medical Research Foundation; and the United States–Israel Binational Science Foundation. M.N.R. is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society. A.R.R. is supported by a Stanford Graduate Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019600108/-/DCSupplemental.
References
- 1.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 2.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 3.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Saitsu H, et al. Dominant-negative mutations in α-II spectrin cause West syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. Am J Hum Genet. 2010;86:881–891. doi: 10.1016/j.ajhg.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 6.Hund TJ, et al. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susuki K, Rasband MN. Spectrin and ankyrin-based cytoskeletons at polarized domains in myelinated axons. Exp Biol Med (Maywood) 2008;233:394–400. doi: 10.3181/0709-MR-243. [DOI] [PubMed] [Google Scholar]
- 8.Trapp BD, Andrews SB, Wong A, O'Connell M, Griffin JW. Co-localization of the myelin-associated glycoprotein and the microfilament components, F-actin and spectrin, in Schwann cells of myelinated nerve fibres. J Neurocytol. 1989;18:47–60. doi: 10.1007/BF01188423. [DOI] [PubMed] [Google Scholar]
- 9.Ohno N, et al. Expression of protein 4.1G in Schwann cells of the peripheral nervous system. J Neurosci Res. 2006;84:568–577. doi: 10.1002/jnr.20949. [DOI] [PubMed] [Google Scholar]
- 10.Court FA, et al. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431:191–195. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- 11.Court FA, et al. A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. J Neurosci. 2009;29:3908–3919. doi: 10.1523/JNEUROSCI.5672-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JR, et al. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- 13.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolthers M, Moldovan M, Binderup T, Schmalbruch H, Krarup C. Comparative electrophysiological, functional, and histological studies of nerve lesions in rats. Microsurgery. 2005;25:508–519. doi: 10.1002/micr.20156. [DOI] [PubMed] [Google Scholar]
- 15.Voas MG, et al. αII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol. 2007;17:562–568. doi: 10.1016/j.cub.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene-expression associated with Schwann cell myelination. J Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel I, et al. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci. 2007;10:861–869. doi: 10.1038/nn1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurel P, et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–874. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewallen KA, et al. Assessing the role of the cadherin/catenin complex at the schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31:3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pradhan D, Lombardo CR, Roe S, Rimm DL, Morrow JS. α-Catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem. 2001;276:4175–4181. doi: 10.1074/jbc.M009259200. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, et al. Disruption of transforming growth factor-β signaling in ELF β-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, et al. Nectin-like molecule 1 is a protein 4.1N associated protein and recruits protein 4.1N from cytoplasm to the plasma membrane. Biochim Biophys Acta. 2005;1669:142–154. doi: 10.1016/j.bbamem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Park J, et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–12819. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking β-spectrin. J Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijkhuizen PA, et al. Adenoviral vector-mediated gene delivery to injured rat peripheral nerve. J Neurotrauma. 1998;15:387–397. doi: 10.1089/neu.1998.15.387. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa Y, et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa Y, et al. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons DA, Naylor SG, Scholze A, Talbot WS. Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat Genet. 2009;41:854–858. doi: 10.1038/ng.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.