Abstract
Vaccines can greatly reduce the spread of and deaths from many infectious diseases. However, many infections have no successful vaccines. Better understanding of the generation of protective CD8 memory T cells by vaccination is essential for the rational design of new vaccines that aim to prime cellular immune responses. Here we demonstrate that the combination of two adjuvants that are currently licensed for use in humans can be used to prime long-lived memory CD8 T cells that protect mice from viral challenge. The universally used adjuvant, aluminum salts, primed long-lived memory CD8 T cells; however, effective cytotoxic T-cell differentiation occurred only in the presence of an additional adjuvant, monophosphoryl lipid A (MPL). MPL-induced IL-6 was required for cytotoxic differentiation. The IL-6 acted by inducing granzyme B production and reducing expression of inhibitory molecule PD1 on the surface of the primed CD8 T cells. CD8 memory T cells generated by antigen delivered with both aluminum salts and MPL provided significant protection from influenza A challenge. These adjuvants could be used in human vaccines to prime protective memory CD8 T cells.
Keywords: cross-presentation, cytokine, Toll-like receptor, MHC tetramer
Most current vaccines act by generating antibodies that either neutralize or otherwise inactivate the pathogen (1). However, many diseases, including malaria and tuberculosis, have no effective vaccine (2, 3). In these cases, a broader immune response encompassing both humoral and cellular immunity could provide increased protection. Memory CD8 T cells that can directly kill infected cells are known to provide protection both in animal models and in humans (4), and thus are an important cell type for a vaccine to generate. Although much is known about the generation of such memory cells after infection with viruses or bacteria (2, 4), there is much less appreciation of how to prime protective CD8 memory T cells by vaccination.
Vaccines fall into two categories: those that contain an attenuated or inactivated microorganism and those made up of parts of the pathogen, subunit vaccines. Whereas vaccines in the former category contain both antigen and innate-stimulating components that provide all signals required to activate the immune response, subunit vaccines must be given with an adjuvant to provide an effective stimulatory environment (5). The only two adjuvants currently licensed for addition to human vaccines in the United States are aluminum salts (alum) and monophosphoryl lipid A (MPL). Alum has been used for many years to enhance antibody responses. The generation of CD8 T-cell responses by vaccines containing alum is less well documented, and alum is often considered a poor CD8 T-cell adjuvant (6, 7), although it has been reported to prime CD8 T cells in vaccinated humans (8, 9). MPL has been added to alum-containing vaccines by GlaxoSmithKline to boost antibody responses (10, 11), but has not been used to prime protective CD8 T cells.
We recently demonstrated that antigen-specific CD8 T cells are activated after immunization of mice with protein and alum (12). In the present study, we examined the properties of memory CD8 T cells activated with antigen delivered with alum in the presence or absence of MPL and determined the signals and cell types required to prime protective memory cells. Memory cells produced in this way are long-lived and protect animals from disease. The addition of MPL enhanced protection by increasing the differentiation of the activated T cells into cytotoxic cells (CTLs), a process that required IL-6 and resulted in the down-regulation of the inhibitory molecule PD1 on the surface of the antigen-specific CD8 T cells.
Results
Protein Delivered with Alum Primes Memory Cells via the Cross-Presentation Pathway.
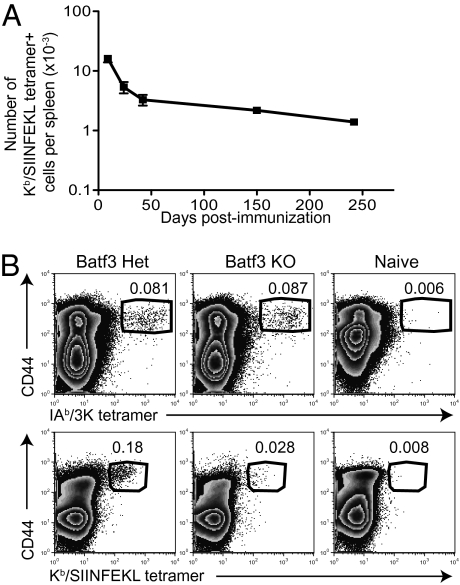
For a CD8 T cell-mediated vaccine to be successful, memory cells must be generated. We examined the number and phenotype of antigen-specific CD8 T cells that were activated after immunization of C57BL/6 (B6) mice with ovalbumin (OVA) protein and alum, with cells detected using Kb/SIINFEKL tetramer (tet) (Fig. S1). The activated cells developed into long-lived memory cells expressing molecules associated with effector memory cells (Fig. 1A and Fig. S2).
Fig. 1.
Protein delivered with alum primes antigen-specific effector memory CD8 T cells by cross-presentation. (A) The numbers of Kb/SIINFEKL tet+ cells in the spleen were examined after immunization with OVA plus alum. Cells were gated on CD8+CD4−B220−F4/80−MHCII− live cells. The data are from two experiments with eight mice per time point. Error bars are SEM. (B) Batf3 heterozygous (Het) and KO mice were primed with 3K-OVA plus alum i.p., and the percentages of CD4 T cells that bound to IAb/3K tet (Upper) and of CD8 T cells (Lower) that bound to the Kb/SIINFEKL tetramer were determined on day 8. Cells were gated on live CD4+CD8−B220−F4/80−MHCII− or CD8+CD4−B220−F4/80−MHCII− lymphocytes. The numbers show the percentage of tet+ CD44hi cells in the gate. Data are representative of three experiments with three mice per group.
Exogenous antigen must enter the cross-presentation pathway to be presented on MHC class I molecules (13). CD8α+ dendritic cells (DCs) are thought to be the main cell type that can carry out this process. These cells are lacking in mice deficient for Batf3 (14), and thus we tested whether alum could prime CD8 T cells in deficient animals. To examine CD4 and CD8 T-cell responses, we covalently conjugated a CD4 epitope, 3K peptide, to OVA protein and used IAb/3K and Kb/SIINFEKL tets to examine the responses. Whereas CD4 T cells responded equivalently in heterozygous and KO mice, the CD8 T-cell response was greatly reduced in KO mice (Fig. 1B). This indicates that the CD8 T-cell response to antigen plus alum occurs via cross-presentation by a specialized subset of DCs.
Alum-Primed CD8 T Cells Produce a Type 1 Rather Than a Type 2 Cytokine Response.
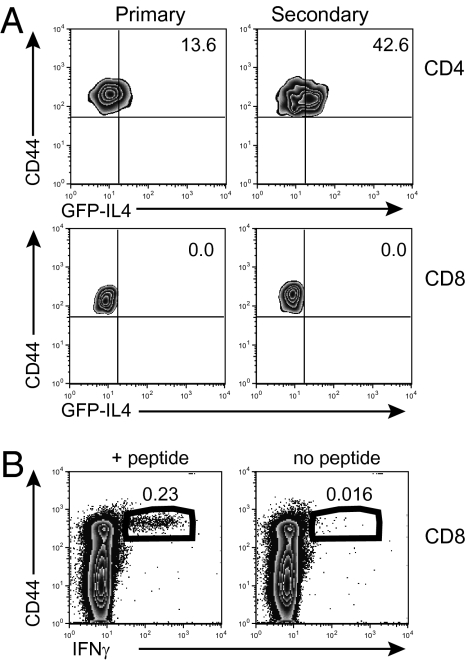
CD4 T cells primed in the presence of alum are known to make a T helper (Th) 2 cell response (15). To examine whether CD8 T cells primed in the presence of alum make a type 2 response, we immunized mice that express GFP in cells that have produced mRNA for IL-4. These animals were immunized with 3K-OVA plus alum to examine CD4 and CD8 T-cell responses. Whereas some IAb/3K tet+ CD4 T cells were GFP+, Kb/SIINFEKL tet+ cells were not (Fig. 2A). In contrast, CD8 T cells from mice primed with OVA plus alum were able to make the type 1 cytokine IFN-γ (Fig. 2B). Therefore, CD8 T cells produce a type 1 cytokine response after activation in the presence of alum, whereas CD4 T cells produce a type 2 response.
Fig. 2.
Alum-primed CD8 T cells produce a type 1 rather than a type 2 cytokine response. (A) 4Get mice on a B6 background were immunized with 3K-OVA plus alum i.p. Either 8 d later (primary) or after 100 d and a second immunization (secondary), IAb/3K or Kb/SIINFEKL tetramers were used to examine responding CD4 and CD8 T cells. The percentage of these cells that were GFP+ was examined in the spleen. Cells are gated as in Fig. 1 and on the tet+ cells. Numbers are the percentage of cells within the indicated quadrant. Data are representative of one of two experiments with four mice per group. (B) Eight days after B6 mice were primed with OVA plus alum, their splenocytes were activated with SIINFEKL peptide for 6 h in the presence of Golgi plug. The cells in the plots are gated on CD8+B220−CD4−MHCII− cells. Numbers indicate the percentage of cells in the gates that were CD44hi IFN-γ+. The data are representative of eight experiments with three or four mice per group.
Alum-Induced PD1 Limits CTL Differentiation.
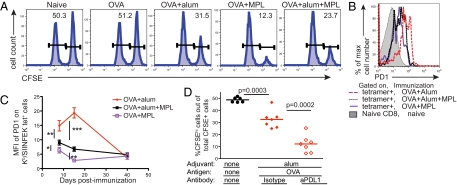
CTLs are associated with protection from infectious diseases both in animal models and in humans (4). To test the cytotoxic response of CD8 T cells primed with antigen and alum, we injected immunized mice with peptide-pulsed and nonpulsed target cells. These could be distinguished because we had labeled them with different intensities of the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE). Mice primed with OVA in the absence of adjuvant were unable to prime a CTL response, whereas the addition of alum resulted in some specific killing (Fig. 3A).
Fig. 3.
Alum-induced PD1 limits CTL differentiation. (A) B6 mice were primed with OVA plus alum, MPL, or both adjuvants i.p. On day 7, these mice and naïve mice were injected with a 1:1 mixture of splenocytes, half of which had been incubated with SIINFEKL peptide and stained with a high level of CFSE and half of which had been stained with a low level of CFSE. Two days later, the percentage of total CFSE+ splenocytes that were CFSEhi was examined. A representative sample from each group is shown. The number indicates the percentage of CFSEhi cells that had been labeled with SIINFEKL out of total CFSE+ cells. The data are representative samples from two experiments with three mice per group. (B) B6 mice were primed with OVA plus alum, MPL, or both adjuvants i.p., and the levels of PD1 expression on the surface of Kb/SIINFEKL tet+ cells were examined on day 8. Cells were gated on Kb/SIINFEKL tet+ cells from the indicated mice (open histograms) or on naive CD8 T cells (filled histogram). (C) The MFI of PD1 on Kb/SIINFEKL tet+ cells was determined after immunization. *P < 0.05; **P < 0.01; ***P < 0.001. The data were combined from between one and three experiments with three or four mice per group for each time point. (D) B6 mice immunized with OVA plus alum were given anti-PDL1 or an isotype control antibody on days 0, 3, and 7 and SIINFEKL pulsed and unpulsed CFSE-labeled cells on day 7. Two days later, the percentage of CFSEhi/ SIINFEKL pulsed cells out of the total CFSE+ cells was examined. Each point represents a mouse, and the line indicates the mean value for the group, combined from two experiments.
Because CTL activity is an important effector CD8 T-cell response, we tested whether this could be improved by providing additional innate stimulation. MPL, which induces inflammatory responses by activating TLR4, has, like alum, been approved for use in human vaccines and enhances antibody responses (11). The MPL used in these studies was a generic form and thus might not have acted in exactly the same way as that produced by GlaxoSmithKline, the form approved for use in vaccines. T cells primed with OVA plus MPL or a combination of both adjuvants plus OVA had equivalent numbers of activated T cells and IFN-γ–producing cells (Fig. S3). CTLs in mice primed with OVA plus MPL killed the peptide-pulsed target cells efficiently, whereas alum inhibited this to some extent (Fig. 3A).
To understand why the addition of MPL enhanced the ability of the CD8 T cells to kill target cells, we examined the expression of various cell surface molecules that can indicate the activation state of T cells. We found that the inhibitory molecule PD1 (16) was expressed at higher levels on T cells primed in the presence of alum, and that MPL reduced its expression (Fig. 3B). In all cases, PD1 expression declined as the activated cells differentiated into memory cells (Fig. 3C).
Blocking a ligand of PD1, PDL1, reignites the functions of exhausted T cells (17). Thus, we tested whether this high PD1 expression inhibited the effector function of the alum-primed cells. Mice immunized with OVA plus alum and treated with anti-PDL1 had similar numbers of antigen-specific cells and IFN-γ–producing cells as immunized mice treated with an isotype control antibody (Fig. S4). However, anti-PDL1 increased the ability of the T cells to kill targets in vivo (Fig. 3D).
MPL-Induced IL-6 Is Required to Enhance the CTL Response and Reduce PD1 Expression.
Our data suggest that MPL may act to increase the cytotoxic response and reduce T-cell expression of PD1 via the same mechanism. The injection of MPL has been reported to increase the production of IL-6 (10), a cytokine associated with CTL function in vitro (18). The number of antigen-specific T cells primed in mice deficient in IL-6 was equivalent to that in WT B6 mice, suggesting that IL-6 is not required for T-cell activation (Fig. S5). IL-6 was required for the MPL-induced cytotoxic response in vivo, as demonstrated by the limited ability to kill of T cells from alum plus MPL–immunized IL-6 KO mice (Fig. 4A).
Fig. 4.
MPL-generated IL-6 enhances the cytotoxic response and reduces PD1 expression. (A) B6 and IL-6 KO mice were immunized with OVA plus alum or alum plus MPL i.p. Seven days later, these mice received SIINFEKL-loaded, CFSE-stained cells as in Fig. 3. The percentage of CFSEhi peptide-loaded cells out of the total CFSE+ cells in the spleen was examined 2 d later. Each symbol represents one mouse, and the line indicates the mean value of the group. Data are representative of two or three experiments with three or four mice per group. n.s., not significant. (B) B6 or IL-6 KO mice were primed with OVA plus alum, MPL, or both adjuvants i.p. on day 0, and some of the mice were treated with an isotype control antibody or anti-PD1 on days 0, 3, and 7. The percentages of Kb/SIINFEKL tet+ cells expressing granzyme B were examined 8 or 9 d later. Each point represents a mouse and the line shows the mean of the group. Data are combined from two experiments with three or four mice per group. (C) B6 (solid lines) or IL-6 KO (dashed lines) mice were immunized with OVA plus alum (Left) or alum plus MPL (Right), and 8 d later the level of PD1 staining on the Kb/SIINFEKL tet+ cells was examined and compared with that on naïve CD8 T cells (filled histogram). Plots are representative of two experiments with three or four mice per group.
We also examined the expression of the cytotoxic molecule granzyme B to determine whether the activated T cells were able to differentiate into effector cells but were blocked from performing this function. Antigen-specific cells from WT mice immunized in the presence of MPL or anti-PDL1 expressed more granzyme B than those immunized with alum and given isotype control antibody (Fig. 4B). This demonstrates that MPL-induced effector cell differentiation was inhibited by PD1. However, antigen-specific CD8 T cells from IL-6 KO mice did not increase their expression of granzyme B in response to MPL. This suggests a link between IL-6 production and the down-regulation of PD1 on the T cells primed in the presence of alum plus MPL. Indeed, MPL was unable to down-regulate the expression of PD1 on antigen-specific cells in IL-6 KO mice immunized with OVA delivered with alum plus MPL (Fig. 4C).
Antigen Delivered with Alum and MPL Protects Mice from Influenza A.
To test whether CD8 T cells primed with antigen and adjuvant(s) could provide protection to an infectious challenge, we primed T cells specific for an influenza A epitope. To do this, we coupled an epitope from the nucleoprotein of influenza A, NP366–74(NP), to a protein (OVA or BSA). We chose this approach so that only CD8 T cells, and not CD4 T cells or B cells, were primed against viral antigens, yet CD4 T-cell help, in the shape of CD4 T cells specific for OVA or BSA, was available. Although it is possible that an antibody response to the peptide could develop, it is unlikely that antibodies to a single epitope within an internal viral protein could provide protection. Thus, the effects of immunization on the course of a subsequent influenza virus infection must be due to the primed CD8 T cells. There are some concerns about the use of OVA, given that soluble OVA can prime a T-cell response after injection of the antigen or of cells incubated with the protein (19, 20). Therefore, we used either OVA or BSA as carriers. Results with the two carriers were indistinguishable, and so the data with OVA and BSA are combined in the discussion of the experiments that follows.
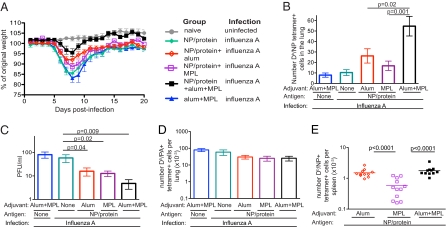
Mice were primed with the NP/protein and alum, MPL or both adjuvants; control mice received protein alone or the two adjuvants in the absence of antigen. Five to 14 wk later, these animals were infected intranasally with influenza A. Control mice lost a significant amount of weight over the first 8 d, then slowly regained some of this weight. Mice primed with NP/protein with either alum or MPL lost less weight (P < 0.05) but did not regain their starting weight. In contrast, mice primed with NP/protein and both adjuvants lost less weight and quickly regained their starting weight (P = 0 .0002) (Fig. 5A).
Fig. 5.
Mice primed with a CD8 epitope from influenza nucleoprotein, alum plus MPL, are protected from influenza A. (A) B6 mice were immunized with NP/OVA or NP/BSA either alone (closed diamonds) or with alum (open diamonds), MPL (open squares), both alum and MPL (closed squares), or the two adjuvants and PBS (closed triangles). Five to 14 wk later, the mice were infected with 150 pfu of influenza A intranasally and weighed each day. The data shown are combined from two experiments with four or five mice per group. (B) The experiments were performed as in Fig. 5A, but the numbers of Db/NP tet+ cells in one lung lobe were examined on day 4. The data shown are combined from three experiments with four or five mice per group. The error bars represent SEM. (C) The experiments were performed as in Fig. 5A, but the viral titers in one lobe of the lung were measured on day 4. The data were combined from three experiments. Error bars represent SEM. (D) The experiments were performed as in Fig. 5A, but the numbers of Db/PA tet+ cells in one lung lobe were examined 8 d after infection. The data are from one of two experiments with four or five mice per group. (E) B6 mice were primed with NP/OVA or NP/BSA with the indicated adjuvant, and the numbers of Db/NP tet+ cells in spleens was examined 6–14 wk later. Each symbol represents a mouse, and the line indicates the group mean. The data are from three experiments with three or four mice per group.
At 4 d after infection, we found NP-specific CD8 T cells in the lungs of mice that had been primed with NP/protein delivered with one or both adjuvants (Fig. 5B and Fig. S6). This early recruitment of memory cells to the lungs correlated with a reduction in viral titers, which was most significant in mice immunized with NP/protein with both adjuvants (Fig. 5C). This indicates that immunization with antigen and both adjuvants was required to provide the most rapid and protective memory response.
We also examined the response to a second immunodominant influenza A epitope, PA224–233. PA-specific CD8 T cells could be clearly identified on day 8 in the lung (Fig. 5D). Therefore, although the mice immunized with NP/protein and both adjuvants had reduced disease, they developed a primary response to new antigens, potentially enhancing future protection.
Protection from influenza A infection requires that the T cells be able to mount a CTL response, as demonstrated by the inability of perforin KO mice primed with NP/protein plus alum plus MPL to control influenza infection (Fig. S7). Therefore, because the addition of MPL to the original vaccination increases CTL differentiation, these memory cells are better equipped to rapidly kill infected cells than are those cells primed with alum alone. To examine why the vaccination was less effective when given with MPL compared with both adjuvants, we examined the number of memory cells present before the challenge. Mice vaccinated with NP/protein with alum and with alum plus MPL had an equivalent number of memory cells, but mice primed with NP/protein plus MPL had significantly fewer memory cells present (Fig. 5E and Fig. S8). Thus, our data indicate that both adjuvants are required to generate protective CD8 memory T cells: alum to generate a long-lived population of memory cells, and MPL to induce CTL differentiation.
Discussion
Here we have demonstrated that a CD8 T-cell vaccine in combination with the safe and universally used adjuvant alum protects mice from some of the weight loss associated with influenza infection. Adding MPL to the formulation did not alter the numbers of memory cells generated with alum alone, but did improve protection by increasing the differentiation of CTLs. Although antigen plus MPL alone activated a good primary response and CTL differentiation, these cells were unable to protect mice from influenza, because of the poor survival of the memory cells. This indicates that alum and MPL complement one another; alum provides signals required for the generation of long-lived memory cells, whereas MPL enhances CTL differentiation.
How alum acts as an adjuvant to generate adaptive immune responses is controversial (15). Although some investigators have reported that inflammasome activation by alum is required for antibody responses (21, 22), we and others have found that T- and B-cell responses are unaffected in alum-primed mice that lack critical components of the inflammasome (12, 23). Alum causes destabilizaton of endocytic vesicles, allowing coinjected antigens to enter the cytosol (24). This should allow all antigen-presenting cells (APCs) that phagocytose antigen delivered with alum to present to CD8 T cells. However, CD8α+ DCs were required to prime CD8 T cells after immunization with alum, suggesting that the presence of antigen in the cytosol is not sufficient for cross-presentation.
Our data show that at least two types of APCs are needed to activate CD4 and CD8 T cells after immunization with alum: CD8α+ DCs and a second APC that primes CD4 T cells even in the absence of the cross-presenting DCs. The identity of this second APC and the signals required for alum to activate it and the CD8α+ DCs remain unclear. Alum is known to form an antigen depot that results in antigen presentation of MHC class I peptides for 12–19 d after immunization (25). Although this presentation may affect the activated T cells, it is unlikely to be required for memory cell generation, because short-term activation of CD8 T cells is sufficient to drive the generation of effective memory cells (26).
The alum-primed CD8 T cells differentiated into IFN-γ–producing cells, but had only limited cytotoxic potential. Clearly, the signals that induce these two CD8 T-cell effector functions are not always linked (27). Coinjection of MPL increased granzyme B expression by the alum-primed cells, which in turn increased the cytotoxic response, a process that required IL-6. Currently, how the IL-6 acts and on which cell types it acts are not clear. MPL is known to enhance the production of IL-6 at the injection site when delivered with alum (10), implying that the IL-6 acts on APCs that migrate from this site to secondary lymphoid organs. However, such cells do not differentiate into the CD8α+ DCs required to prime CD8 T cells. Alternatively, IL-6 also may act either on the priming APCs or directly on the activated CD8 T cells. This possibility is supported by the ability of IL-6 to enhance the differentiation of CTLs in vitro (18).
IL-6 also was required for the down-regulation of PD1 on T cells activated in the presence of alum and MPL. The high expression of PD1 by the alum-primed CD8 T cells led to reduced granzyme B expression, and thus the majority of these cells did not differentiate into cytotoxic cells. Consequently, when the memory cells generated from this response were reactivated by the influenza A infection, they first had to differentiate into CTLs before they could provide protection, possibly explaining why these cells were less protective than those cells primed with both adjuvants.
The influenza nucleoprotein is highly conserved between different strains of influenza A (28), and human CD8 T cells reactive to a number of epitopes within NP respond to a range of viruses (29–31). The cross-reactivity of NP-specific CD8 T cells for different stains of influenza A, although not directly examined here, is well known (32, 33), and such cells have been shown to provide protection from heterosubtypic influenza challenge (34–36). Therefore, immunization with NP protein with alum plus MPL will generate cross-reactive CTLs that could provide protection in the majority of individuals to most, if not all, influenza A subtypes—in effect, a universal vaccine. Importantly, such T cells are thought to protect against the worst forms of disease that occur in individuals with no cross-reactive antibody response (37). Although some previous studies also have found protective influenza-specific CD8 T cells after vaccination, those studies used adjuvants, delivery methods, or viral vectors that have not been used at all or not used as extensively as alum and MPL, which are currently the only adjuvants approved for addition to human vaccines in the United States (34–36, 38). Importantly, alum-delivered vaccines have been shown to prime IFN-γ–producing CD8 T cells in humans immunized with a hepatitis B vaccine (8, 9). Therefore, it is conceivable that an alum-containing influenza vaccine could prime CD8 T cells in humans, and that the addition of MPL would make these cells cytotoxic. A vaccine that includes alum and MPL has clear advantages, because both of these adjuvants have an established safety record. Here we have shown why these adjuvants are needed to prime protective and long-lived memory cells, providing information that could be used for the rational development of CD8 T cell-mediated vaccines.
Methods
Mice, Immunizations, and Infections.
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the National Jewish Health Animal Care and Use Committee. Female B6, perforin KO, and IL-6 KO mice were obtained from Jackson Laboratory. B6 IL-4 reporter (4Get) mice were obtained from Dr. R. Locksley (University of California, San Francisco, CA), and Batf3 KO mice were obtained from Ken Murphy (Washington University, St. Louis, MO). Both of these strains were bred at National Jewish Health in a specific pathogen-free environment. Mice were age-matched within experiments and primed at age 6–10 wk, then immunized with 10 μg of OVA protein (Sigma-Aldrich) or OVA-peptide or BSA-peptide conjugates. OVA or BSA proteins were conjugated to either 3K peptide or NP peptide as described previously (12) using maleimide-activated OVA or BSA (Pierce). Peptides were supplied by JPT. All immunizations were given i.p. Mice were injected with 1 mg of alum (Alydrogel; Brenntag) with or without 10 μg of MPL (InvivoGen). Antigens contained ≤6 EU or 1 ng of LPS per injection, as determined by the limulus amebocyte lysate test (Lonza). Proteins were tumbled with alum with or without MPL for 2 h at room temperature before injection. Infections with PR8 were done on mice anesthetized with isofluorane and 50 μL of PBS containing 150 PFU of virus injected intranasally. The virus was prepared as described previously (39).
Flow Cytometry.
Splenocytes were prepared at the indicated time, and red blood cells were lysed. APC-Kb/SIINFEKL tet, APC-Db/NP, PE-Db/PA, and PE-IAb/3K tet were produced as described previously (40). Single-cell suspensions were stained with MHC tets at 37 °C for 2 h. Antibodies to surface markers were added, and the cells were incubated for a further 20 min at 4 °C, except for anti-CCR7, which was also incubated at 37 °C for 2 h. Additional information on antibodies used is provided in SI Methods. Tet+ cells were defined by gating on live (based on forward side-scatter characteristics), CD8+ or CD4+ cells that were B220−, F4/80−, and MHC class II (MHCII)− and either CD4− or CD8−, respectively.
To examine CD8 T cells in the lung, euthanized mice were perfused to remove blood from the lungs. Then one lobe was cut into small pieces and treated with collagenase and DNase before flow staining, as described above.
For analysis of cytokine production by intracellular staining, splenocytes were activated ex vivo with 1 μg/mL of SIINFEKL and 1 μg/mL of Golgi plug (BD) for 6 h. Cells were stained with surface antibodies and then fixed and permeabilized using the BD Cytofix/Cytoperm Kit according to the manufacturer's instructions. Cells were gated on live CD8+CD4−B220−MHCII− cells. For granzyme B staining, cells were stained with class I tet and surface antibodies, fixed, and permeabilized using the BD Cytofix/Cytoperm Kit, and then stained with anti-human granzyme B (BD) for 40 min.
In all cases, two to five million events were collected on a CyAn ADP (Dakocytomation), and data were analyzed using FlowJo version 8.8 (Treestar).
In Vivo Antibody Treatment.
Mice were given 200 μg i.p. of anti-PDL1 (10F.9G2; BioXCell) on days 0, 3, and 7. Control antibody was anti-DR5 (HB-151, grown and purified at National Jewish Health), delivered as for anti-PDL1.
In Vivo Killing Assay.
A single-cell suspension of B6 splenocytes was prepared, and red cells were lysed. Half of the cells were labeled with 10 μg/mL of SIINFEKL at 37 °C for 2 h, then stained with 1 μM CFSE (Molecular Probes) for 7 min at room temperature. The rest of the cells were labeled with 0.05 μM CFSE. After cells were washed, a 1:1 mixture of these cells, a total of 5 × 106 cells, was injected i.v. Forty-eight hours later, the percentage of CFSEhi cells out of the total CFSE+ cells was measured by flow cytometery.
Plaque Assay.
One lung lobe from mice infected 4 d earlier was homogenized, and supernatants were frozen until use. Then 1 μg/mL of TPCK trypsin was added to the diluted supernatant, which was plated on confluent MDCK cells. The cells were incubated for 1 h at 37 °C, after which the supernatants were removed. Then media containing 1% agar was added, and the plates were again incubated at 37 °C. At 48 h later, agar containing neutral red was added. Plaques were counted after a further 24–36 h of incubation at 37 °C.
Statistics.
To test for a significant drop in weight loss after infection, the percent weight loss was calculated for each mouse on each day after infection. The data were graphed, and the area under the curve was calculated for each mouse. Statistical significance was determined by the Student two-tailed t test using GraphPad Prism version 4.
Supplementary Material
Acknowledgments
We thank Dr. Kevan Hartshorn for supplying the influenza A, Dr. Rebecca Oberley-Deegan for helping with infections, and Dr. Richard Willis for assisting with development of the MHC class I tets. This work was supported by National Institutes of Health Grants AI-18785 and AI-22295 and US Department of Defense Grant USAMRMC W81XWH-07-1-0550.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104588108/-/DCSupplemental.
References
- 1.Plotkin SA. Vaccines: Correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 2.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 3.Good MF, Doolan DL. Malaria vaccine design: Immunological considerations. Immunity. 2010;33:555–566. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 5.McKee AS, MacLeod MK, Kappler JW, Marrack P. Immune mechanisms of protection: Can adjuvants rise to the challenge? BMC Biol. 2010;8:37. doi: 10.1186/1741-7007-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Newman MJ, et al. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992;148:2357–2362. [PubMed] [Google Scholar]
- 8.Höhn H, et al. Longitudinal analysis of the T-cell receptor (TCR)-VA and -VB repertoire in CD8+ T cells from individuals immunized with recombinant hepatitis B surface antigen. Clin Exp Immunol. 2002;129:309–317. doi: 10.1046/j.1365-2249.2002.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman F, et al. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 10.Didierlaurent AM, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 11.Giannini SL, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.McKee AS, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 18.Renauld JC, Vink A, Van Snick J. Accessory signals in murine cytolytic T cell responses: Dual requirement for IL-1 and IL-6. J Immunol. 1989;143:1894–1898. [PubMed] [Google Scholar]
- 19.Carbone FR, Bevan MJ. Class I–restricted processing and presentation of exogenous cell–associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staerz UD, Karasuyama H, Garner AM. Cytotoxic T lymphocytes against a soluble protein. Nature. 1987;329:449–451. doi: 10.1038/329449a0. [DOI] [PubMed] [Google Scholar]
- 21.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munks MW, et al. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood. 2010;116:5191–5199. doi: 10.1182/blood-2010-03-275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peixoto A, et al. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med. 2007;204:1193–1205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiny AT, et al. Evolutionarily conserved protein sequences of influenza a viruses, avian and human, as vaccine targets. PLoS ONE. 2007;2:e1190. doi: 10.1371/journal.pone.0001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreijtz JH, et al. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008;82:5161–5166. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LY, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, et al. CTL epitopes for influenza A including the H5N1 bird flu: Genome-, pathogen-, and HLA-wide screening. Vaccine. 2007;25:2823–2831. doi: 10.1016/j.vaccine.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 32.Townsend AR, McMichael AJ, Carter NP, Huddleston JA, Brownlee GG. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 33.Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen JP, Doherty PC, Branum KC, Riberdy JM. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J Virol. 2000;74:11690–11696. doi: 10.1128/jvi.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuya Y, et al. Cytotoxic T cells are the predominant players providing cross-protective immunity induced by γ-irradiated influenza A viruses. J Virol. 2010;84:4212–4221. doi: 10.1128/JVI.02508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulmer JB, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 37.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999;162:7578–7583. [PubMed] [Google Scholar]
- 38.Poon LL, et al. Vaccinia virus-based multivalent H5N1 avian influenza vaccines adjuvanted with IL-15 confer sterile cross-clade protection in mice. J Immunol. 2009;182:3063–3071. doi: 10.4049/jimmunol.0803467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, et al. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFNλ-1) in response to influenza A infection. J Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.