Abstract
The mosquito's body temperature increases dramatically when it takes a blood meal from a warm-blooded, vertebrate host. By using the yellow fever mosquito, Aedes aegypti, we demonstrate that this boost in temperature following a blood meal prompts the synthesis of heat shock protein 70 (Hsp70). This response, elicited by the temperature of the blood meal, is most robust in the mosquito's midgut. When RNA interference is used to suppress expression of hsp70, protein digestion of the blood meal is impaired, leading to production of fewer eggs. We propose that Hsp70 protects the mosquito midgut from the temperature stress incurred by drinking a hot blood meal. Similar increases in hsp70 were documented immediately after blood feeding in two other mosquitoes (Culex pipiens and Anopheles gambiae) and the bed bug, Cimex lectularius, suggesting that this is a common protective response in blood-feeding arthropods.
The evolution of blood feeding in arthropods is well recognized as an adaptation fraught with challenges in finding hosts, avoiding host detection, mechanically penetrating the vertebrate skin, and then avoiding the host immune response to use the highly specialized, albeit protein-rich, food source (1). Imbibing a huge blood meal also quickly generates osmotic stress that requires an efficient excretory system for rapid removal of excess water (2–5). However, one overlooked challenge of feeding on mammalian blood is the high temperature of the blood that rushes into the gut. Mosquitoes and other blood-feeding ectotherms are vulnerable to stress caused by swings in environmental temperature, thus raising the possibility that ingestion of a hot blood meal may cause stress that must be ameliorated to accommodate this lifestyle.
Heat shock elicits diverse behavioral, biochemical, and physiological responses in insects (6, 7). The most conspicuous molecular response is a rapid increase in abundance of heat shock proteins (Hsps), proteins that primarily function as molecular chaperones, preserving the function of enzymes and other critical proteins (6). At least 12 Hsps increase in abundance following exposure to high temperature, and of these, Hsp70 is the most widely studied as a responder to heat and numerous other stresses (6, 8, 9). As with nearly all organisms, mosquito Hsp70s have been documented to increase during environmental stress (8, 10), and recent microarray studies have identified several hsp genes among the multitude of genes showing higher expression following a blood meal (11–13). This suggests that the heat shock response may be triggered by blood feeding. We test that idea in this study by documenting a huge and rapid elevation in body temperature of the yellow fever mosquito in response to blood feeding and demonstrate that a successful Hsp response is essential for processing the blood meal and for subsequent egg production.
Results and Discussion
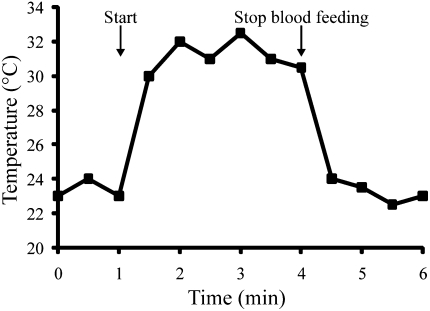
Upon imbibing blood, body temperature of the female mosquito quickly spiked (Fig. 1). Body temperature on the surface of the mosquitoes increased from 22 to 32 °C within 1 min, a rate increase of 0.167 °C/s. We observed similar temperature changes in response to blood feeding in two other mosquitoes, Culex pipiens and Anopheles gambiae, and in the bed bug, Cimex lectularius (Fig. S1). Increases of this magnitude are among the most rapid rate increases documented for any ectotherm (14). At the cessation of feeding, body temperature again decreased to ambient levels within the course of a few minutes.
Fig. 1.
Rapid elevation of body temperature in an adult female mosquito, A. aegypti, in response to blood feeding. This profile is representative of temperature changes recorded in 10 individuals.
Temperature changes of similar magnitude (10–15 °C) occur on a daily basis, especially during the transition from night to day, but these diurnal changes occur much more slowly and enable insects to gradually adjust their physiological state to accommodate the change (15). Rapid temperature changes such as we documented in response to blood feeding rarely are observed in nature. Perhaps the rate increases most similar to what we have observed in response to blood feeding are the increases noted when a dark-colored insect is immediately thrust into direct sunlight (16). Exposure to direct sunlight can yield temperature increases of 0.04 to 0.23 °C/s, a rate similar to what we observed in this study of blood-feeding insects (0.07–0.167 °C/s).
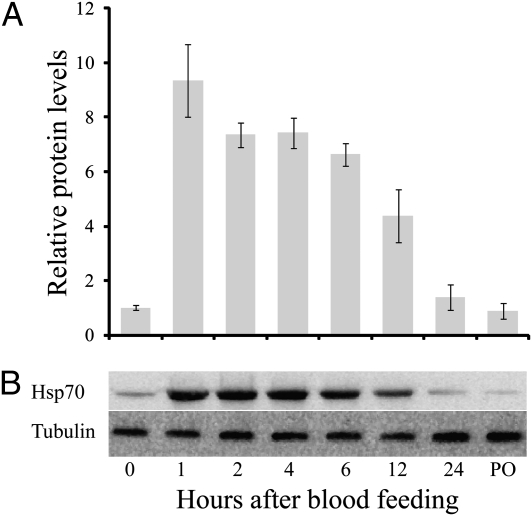
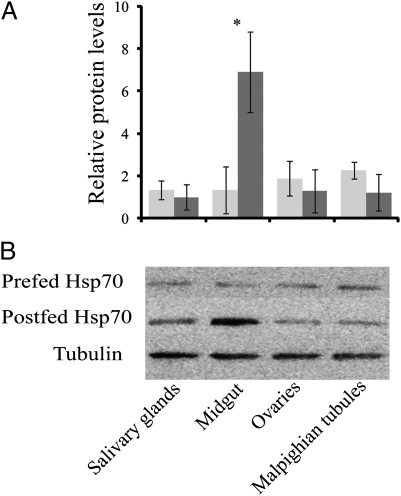
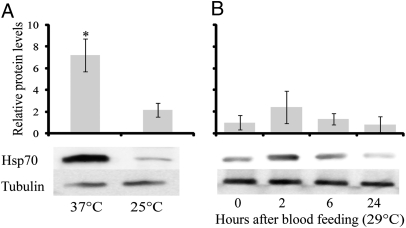
In response to blood feeding, Hsp70 levels increased nearly eightfold within 1 h and remained at least twofold higher than the prefeeding levels for 12 h (Fig. 2). A similar elevation of the hsp70 transcript was noted in Aedes aegypti (Fig. S2), as well as in the mosquitoes A. gambiae and C. pipiens, and the bedbug C. lectularius, following a blood meal (Fig. S2). The Hsp70 increase was most pronounced in the midgut (Fig. 3). Injection of females with 1 μL host-temperature (37 °C) saline solution resulted in an increase in Hsp70, whereas an injection of room-temperature (25 °C) saline solution did not, suggesting that it is elevation in temperature, rather than increase in body volume, that is the likely cause of the increase in Hsp70 (Fig. 4A). To eliminate the possibility that some component of the blood, rather than blood temperature, was eliciting the effect, we enticed mosquitoes to feed on somewhat cooler blood (29 °C), and as shown in Fig. 4B, the Hsp70 level in A. aegypti was not significantly elevated by a “cool” blood meal. Thus, we conclude it is the high temperature of the blood that is responsible for the increase of Hsp70. That the heat shock response is evoked by blood feeding in diverse species of insect disease vectors indicates that this is a common response to blood feeding, an observation congruent with microarray and transcriptome studies documenting an increase in Hsp transcripts following blood feeding in the biting midge, Culicoides sonorensis (17), the American dog tick, Dermacentor variabilis (18), A. aegypti (11), and A. gambiae (12, 19, 20).
Fig. 2.
Western blot analysis of Hsp70 abundance following blood feeding in A. aegypti, as noted from (A) densitometry readings based on (B) protein gels. Hsp70 levels were normalized to tubulin. Values presented as mean ± SE, n = 3.
Fig. 3.
Hsp70 levels in different female tissues of A. aegypti before (light gray) and 2 h after blood feeding (dark gray), showing the most robust response in the midgut, as noted from (A) densitometry readings based on (B) protein gels. Tubulin served as a loading control. Values presented as mean ± SE, n = 3. *Significant difference after blood feeding at P < 0.01.
Fig. 4.
Hsp70 protein levels (A) 2 h following injection of 25 °C or 37 °C (host temperature) PBS solution (mean ± SE, n = 5) and (B) after feeding on 29 °C blood (mean ± SE, n = 3). *Significant at P < 0.01.
Although our temperature records are based on whole body recordings, the tissue in direct contact with the blood, the midgut, would likely experience the most profound effect, and our experiments comparing Hsp70 abundance in several different tissues support the idea that the heat shock response is most robust in the midgut (Fig. 3). Previous studies in Drosophila also suggest that the midgut is especially responsive to heat as demonstrated by pronounced hsp70 expression in the fly midgut (6, 21).
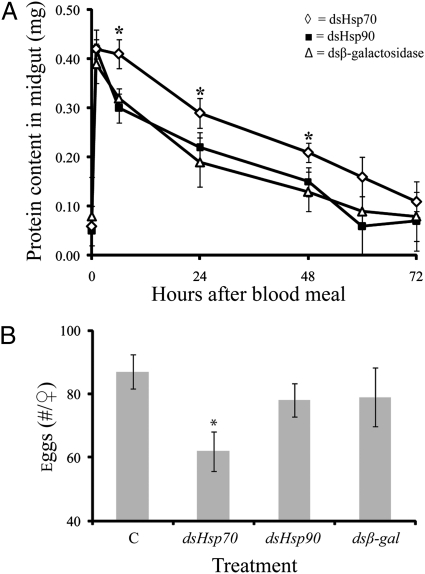
RNA interference suppressed abundance of Hsp70 and its transcript by at least 75% (Fig. S3). In such mosquitoes, the amount of blood ingested was not reduced (Fig. S4), but blood protein levels within the midgut remained elevated for a longer time in flies after Hsp70 suppression, indicating that protein digestion was impaired (Fig. 5A). Egg production was also reduced 25% following knockdown of Hsp70 (Fig. 5B), whereas injection of controls (dshsp90 and dsβ-gal) had no effect. This provides evidence that the Hsp response is essential for successful processing of the blood meal. When Hsps are suppressed, mechanisms for tolerance to heat (22), cold (23, 24), and dehydration (10) are compromised. In similar fashion, suppression of Hsp70 during blood feeding indicates that Hsp70 is critical for successful processing of a hot blood meal. Most likely, cells of the midgut are protected or the function of proteolytic enzymes is preserved from potential temperature damage from the blood meal by the expression of Hsp70. We cannot currently say if the impaired digestion and egg production are a result of reduced digestion efficiency or a slower rate of nutrient uptake from the midgut. It is interesting to note that the ability to up-regulate hsp70 declines with age of the female (20), perhaps resulting in impairment of cellular repair mechanisms (6). Coincidently, mosquito fecundity decreases with female age (25), suggesting a possible interplay between the capacity for Hsp70 production, aging, and egg production. We suspect that the Hsp response we have observed is most prominent in arthropods that feed on warm-blooded vertebrate hosts. The need for such a response would be less apparent in other blood-feeding arthropods that feed on blood from ectothermic vertebrates, such as frogs and lizards (26–28).
Fig. 5.
Responses of female A. aegypti to Hsp70 knockdown by RNA interference. (A) Reduced protein use from the midgut following a blood meal. Noninjected controls were not different from dshsp90- and dsβ-gal–injected controls (data not shown). Each point represents mean ± SE of 20 individuals. (B) Reduction in egg production. Each point represents the mean ± SE of 30 individuals. *Significant difference after RNAi directed against Hsp70.
In summary, these results indicate that a hot blood meal evokes a heat shock response that protects mosquitoes and other blood-feeding insects against the high temperature stress of feeding on a warm-blooded host. The importance of this response suggests yet another pathway that could be targeted for disruption in disease vectors.
Methods
Insects, Samples, and Experimental Conditions.
A. gambiae, A. aegypti, and C. pipiens were reared as described, using chickens as hosts for the blood meal (10, 29). Body temperature of chickens used in the study was 39 °C to 42 °C. Intrathoracic injection of PBS solution was accomplished by immobilizing mosquitoes with CO2 and injecting control mosquitoes with room temperature (25 °C) PBS solution and experimental mosquitoes with host temperature (37 °C) PBS solution. In addition to mosquitoes that were host fed on chickens, a subset of mosquitoes were provided chicken blood through a membrane that was held at 29 °C by using a circulating water bath. TRIzol extraction was used to collect RNA and protein. Each sample was replicated at least three times.
Temperature of Blood Feeding.
To determine temperature changes in the mosquito during blood feeding, a thermocouple (Fisher) was attached to the mosquito using petrolatum, and mosquitoes were placed onto the surface of the host. Temperatures of 10 females of A. aegypti were monitored during feeding with a HHM290 thermometer (Omega). Similar experiments were conducted using females of two other mosquito species, A. gambiae and C. pipiens, and the bed bug, C. lectularius.
Northern and Western Blot Analyses.
RNA and protein were extracted from groups of 10 female mosquitoes using TRIzol reagent (Invitrogen). Northern blots were conducted according to Benoit et al. (10). Hsp70 and tubulin antibodies were acquired from Santa Cruz Biotechnology and used at a concentration of 1/4,000. Equal volumes of protein from three females were combined for each time point, and Western blots were conducted according to standard protocols. Densitometry measurements were conducted using ImageJ (version 1.38x; National Institutes of Health).
dsRNA Preparation and Application.
Hsp70, Hsp90, and β-gal dsRNA were prepared using a MEGAscript T7 transcription kit (Ambion) and applied to the mosquitoes according to Sim and Denlinger (30) and Benoit et al. (10). Mosquitoes were injected with approximately 1 μg dsRNA dissolved in 1 μL of PBS solution and held at 90% RH with access to 10% sucrose during recovery. After 4 d, dsRNA-treated mosquitoes were offered a blood meal. RNA and protein levels were determined 2 h after blood feeding. Each experiment was replicated five times by using 10 mosquitoes each from different mosquito pools.
Efficiency of Digestion.
Midgut protein levels were determined by the Bradford assay (Bio-Rad) to assess the rate of digestion. Midguts were dissected from female mosquitoes at various intervals after blood feeding and stored at −70 °C until analysis, and protein was extracted as before. Five midguts were analyzed for each time point.
Blood Meal Mass.
Thirty mosquitoes were chilled and weighed individually with an Electrobalance (Cahn 35; Ventron) before and after taking a blood meal. Mass of blood ingested was calculated as the difference in mass before and after feeding.
Egg Production.
To conform to standard methods, egg production was assessed according to Harrington et al. (31). Briefly, females were forced to retain their eggs by not being provided an oviposition substrate. Five days after blood feeding, ovaries were dissected in Coast solution containing (in mM) 100 NaCl, 8.6 KCl, 4.0 NaHCO3, 4.0 NaH2PO4•H2O 1.5, CaCl2•2H2O 8.5, MgCl2•6H2O, 24 glucose, 25 Hepes, and 56 sucrose. Developing stages (III/IV) and mature ovarioles were classified according to Christophers (32) and counted.
Supplementary Material
Acknowledgments
We appreciate helpful comments on the manuscript provided by Richard E. Lee (Miami University), Geoffrey M. Attardo (Yale University), Michael J. Lehane (Liverpool School of Tropical Medicine), Edward D. Walker (Michigan State University), and Frank H. Collins (Notre Dame University). This work was supported in part by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant R01 AI058279 and National Science Foundation Grant IOS-0840772.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105195108/-/DCSupplemental.
References
- 1.Lehane MJ. Biology of Blood-Sucking Insects. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 2.Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow-rate and composition of urine during the post-bloodmeal diuresis in Aedes aegypti (L) J Comp Physiol. 1983;153:257–265. [Google Scholar]
- 3.Wheelock GD, Petzel DH, Gillett JD, Beyenbach KW, Hagedorn HH. Evidence for hormonal control of diuresis after a blood meal in the mosquito Aedes aegypti. Arch. Insect Biochem. 1988;7:75–89. [Google Scholar]
- 4.Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol. 2003;206:3845–3856. doi: 10.1242/jeb.00639. [DOI] [PubMed] [Google Scholar]
- 5.Benoit JB, Denlinger DL. Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods. J Insect Physiol. 2010;56:1366–1376. doi: 10.1016/j.jinsphys.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 7.Sørensen JG, Nielsen MM, Kruhoffer M, Justesen J, Loeschcke V. Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell Stress Chaperones. 2005;10:312–328. doi: 10.1379/CSC-128R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross TL, Myles KM, Adelman ZN. Identification and characterization of heat shock 70 genes in Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2009;46:496–504. doi: 10.1603/033.046.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benoit JB, Lopez-Martinez G. In: Hemolymph Proteins and Functional Peptides: Recent Advances in Insects and Other Arthropods. Tufail M, Takeda M, editors. Oak Park, IL: Bentham; 2011. [Google Scholar]
- 10.Benoit JB, Lopez-Martinez G, Phillips ZP, Patrick KR, Denlinger DL. Heat shock proteins contribute to mosquito dehydration tolerance. J Insect Physiol. 2010;56:151–156. doi: 10.1016/j.jinsphys.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- 12.Dana AN, et al. Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genomics. 2005;6:5. doi: 10.1186/1471-2164-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinotti O, Nguyen QK, Calvo E, James AA, Ribeiro JMC. Microarray analysis of genes showing variable expression following a blood meal in Anopheles gambiae. Insect Mol Biol. 2005;14:365–373. doi: 10.1111/j.1365-2583.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich B. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation. Cambridge, MA: Harvard Univ Press; 1993. [Google Scholar]
- 15.Denlinger DL, Lee RE. Low Temperature Biology of Insects. Cambridge, UK: Cambridge Univ Press; 2010. [Google Scholar]
- 16.Willmer PG, Unwin DM. Field analyses of insect heat budgets - reflectance, size and heating rates. Oecologia. 1981;50:250–255. doi: 10.1007/BF00348047. [DOI] [PubMed] [Google Scholar]
- 17.Campbell CL, Vandyke KA, Letchworth GJ, Drolet BS, Hanekamp T, Wilson WC. Midgut and salivary transcriptomes of the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae) Insect Mol Biol. 2005;14:121–136. doi: 10.1111/j.1365-2583.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JM, Sonenshine DE, Valenzuela JG. Exploring the mialome of ticks: an annotated catalogue of midgut transcripts from the hard tick, Dermacentor variabilis (Acari: Ixodidae) BMC Genomics. 2008;9:552. doi: 10.1186/1471-2164-9-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro JMC. A catalogue of Anopheles gambiae transcripts significantly more or less expressed following a blood meal. Insect Biochem Mol Biol. 2003;33:865–882. doi: 10.1016/s0965-1748(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang M-H, et al. Genome-wide patterns of gene expression during aging in the African malaria vector Anopheles gambiae. PLoS ONE. 2010;5:e13359. doi: 10.1371/journal.pone.0013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krebs RA, Feder ME. Tissue-specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. J Exp Biol. 1997;200:2007–2015. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sørensen JG, Dahlgaard J, Loeschcke V. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct Ecol. 2001;15:289–296. [Google Scholar]
- 23.Rinehart JP, et al. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tollarová-Borovanská M, Lalouette L, Kostál V. Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: Role of 70 kDa heat shock protein expression. Cryo Lett. 2009;30:312–319. [PubMed] [Google Scholar]
- 25.Woke PA, Ally MS, Rosenberger CR. The numbers of eggs developed related to the quantities of human blood ingested in Aedes aegypti (L.) Ann Entomol Soc Am. 1956;49:435–441. [Google Scholar]
- 26.Bailey JK. Aedes aegypti as a possible new invertebrate host for frog trypanosomes. Exp Parasitol. 1962;12:155–163. doi: 10.1016/0014-4894(62)90052-8. [DOI] [PubMed] [Google Scholar]
- 27.McIver SB. Host preferences and discrimination by the mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae) J. Med. Ent. 1968;5:422–428. doi: 10.1093/jmedent/5.4.422. [DOI] [PubMed] [Google Scholar]
- 28.Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc Natl Acad Sci USA. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 32.Christophers SR. Aedes aegypti (L.), The Yellow Fever Mosquito. Its Life History, Bionomics and Structure. Cambridge, UK: Cambridge Univ Press; 1960. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.