Abstract
The establishment of the mammalian neocortex is often explained phylogenetically by an evolutionary change in the pallial neuronal progenitors of excitatory projection neurons. It remains unclear, however, whether and how the evolutionary change in inhibitory interneurons, which originate outside the neocortex, has been involved in the establishment of the neocortex. In this study, we transplanted chicken, turtle, mouse, and marmoset medial ganglionic eminence (MGE) cells into the embryonic mouse MGE in utero and compared their migratory behaviors. We found that the MGE cells from all of the species were able to migrate through the mouse neocortical subventricular zone and that both the mouse and marmoset cells subsequently invaded the neocortical cortical plate (CP). However, regardless of their birthdates and interneuron subtypes, most of the chicken and turtle cells ignored the neocortical CP and passed beneath it, although they were able to invade the archicortex and paleocortex, suggesting that the proper responsiveness of MGE cells to guidance cues to enter the neocortical CP is unique to mammals. When chicken MGE cells were transplanted directly into the neocortical CP, they were able to survive and mature, suggesting that the neocortical CP itself is essentially permissive for postmigratory development of chicken MGE cells. These results suggest that an evolutionary change in the migratory ability of inhibitory interneurons, which originate outside the neocortex, was involved in the establishment of the neocortex by supplying inhibitory components to the network.
Keywords: dorsal pallium, GABAergic interneurons, tangential migration, mammalian evolution, interspecies transplantation
During neocortical development, the projection neurons are generated in proliferative zones lying along the ventricle within the neocortex, namely the ventricular zone (VZ) and the subventricular zone (SVZ), and migrate radially to the brain surface (1–3) to form a cortical plate (CP) (4). Newly generated projection neurons migrate past the earlier-born neurons to settle just beneath the marginal zone (MZ), thereby constructing the neocortical CP in a birth date-dependent inside-out fashion (5). Although many (∼65% in humans) interneurons also seem to originate within the neocortical VZ/SVZ in primates (6, 7), most interneurons in most mammals (almost all interneurons in mice) seem to originate in the medial ganglionic eminence (MGE), caudal ganglionic eminence, and preoptic area located in the subpallium and migrate tangentially to the neocortex (8–11). After entering the neocortex, many MGE-derived interneurons, which constitute the majority of the interneurons in mice (9), continue to migrate tangentially through the neocortical SVZ and intermediate zone (IZ), and then, they change their trajectory and migrate to the brain surface so that they enter the CP and the MZ (12, 13).
Phylogenetically, the neocortex is observed only in mammals, and because it evolved from the dorsal pallium of the ancestor of amniotes (mammals and sauropsids), it is homologous to only the sauropsidian dorsal pallium (avian hyperpallium and lizard/turtle dorsal cortex) (14). In contrast to the developing neocortex of mammals, the developing dorsal pallium of sauropsids lacks an SVZ, its CP is thinner and poorly organized, and its CP forms in an outside-in fashion (15, 16). It has been proposed that evolutionary changes in pallial neuronal progenitors and their descendants, projection neurons, in the ancestor of mammals may have made a critical contribution to the structural and functional establishment of the neocortex of mammals (15–19). This proposal is reasonable, but it may not be sufficient to fully explain the functional establishment of the neocortex, because it does not take into account the other essential component of the neocortex, interneurons, which invade from outside the pallium. Although the overall ratio of interneurons to the total number of neurons in the dorsal pallium varies among amniotes (20–23), the tangential migration of interneurons from the MGE to the dorsal pallium is highly conserved across gnathostomes, including amniotes (24–28), and ∼75% of interneurons in the chicken pallium originate from the MGE (24). Interestingly, the mechanism of the tangential migration seems to be conserved across amniotes (25), suggesting that the common mechanism of tangential migration from the MGE to the dorsal pallium was already established in the ancestor of amniotes. Here too, however, it remains unclear whether and how the evolutionary change in MGE-derived interneurons in the ancestor of amniotes was involved in the establishment of the mammalian neocortex.
To answer these questions, we focused our attention on identifying the phylogenetic origins of the migratory competencies of MGE cells within the neocortex. We used a combination of the strategy of interspecies transplantation of MGE cells (25) and a technique of MGE cell transplantation in mice in utero (12, 29) to examine the migratory behavior of chicken, turtle, and marmoset MGE cells within the mouse neocortex in vivo. The results suggested that, although the phylogenetic origin of the MGE cell competencies to enter the neocortical SVZ is the ancestor of amniotes, the competencies of MGE cells to enter the neocortical CP, especially layers 2–4, were acquired in the ancestor of mammals. This evolutionary change in the migratory ability of MGE-derived interneurons in the ancestor of mammals seems to have been involved in the establishment of the neocortex by supplying inhibitory components to the network.
Results
Chicken MGE Cells Migrated Medially Through the Neocortical SVZ/IZ, the Same as Mouse MGE Cells Did, but Failed to Enter the CP/MZ.
We first dissected out MGE cells from embryonic day 6.5 (E6.5) chicken embryos (Fig. S1 A and B), labeled them with a red fluorescent protein (mCherry) by electroporation, and transplanted them into the MGE of E13.5 mouse embryos in utero together with E13.5 mouse MGE cells expressing GFP (Fig. S2). When their distribution in the mouse telencephalon was analyzed at E15.5 (i.e., 2 d after transplantation), many GFP-expressing mouse cells were seen to have dispersed dorsally from the injection site to the dorsal pallium, as reported previously (12), and to have become widely distributed in both the rostral part of the lateral ganglionic eminence and the caudal ganglionic eminence (Fig. S3 A–G). Some GFP-expressing cells were found in the neocortical SVZ/IZ (Fig. S3D). Notably, a similar dorsal dispersion by the mCherry-expressing chicken cells was observed (Fig. S3 A–G). Dorsal dispersion was not observed when cells from the dorsal ventricular ridge (DVR) of E6.5 chicken embryos were transplanted (Fig. S3 H–K), suggesting that the dorsal dispersion of MGE cells was not a nonspecific behavior of the transplanted cells. These results suggest that the proper responsiveness required for dorsal dispersion from the MGE to the neocortex in the mouse telencephalon is largely conserved in chicken MGE cells.
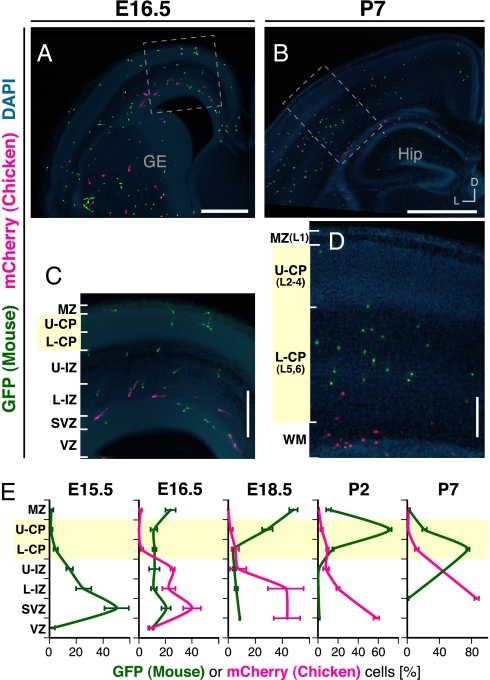
Next, we examined the migratory pathways of the transplanted MGE cells within the mouse neocortex at later stages. Consistent with previous reports (12, 13), most GFP-expressing mouse cells were initially found in the neocortical SVZ/IZ and later in the CP/MZ (Fig. 1 and Fig. S4), suggesting that many transplanted mouse MGE cells first migrated tangentially through the SVZ/IZ, then changed their trajectory, and migrated to the brain surface to enter the CP/MZ. Many mCherry-expressing chicken cells were also initially observed in the SVZ/IZ with their leading process oriented medially (Fig. 1 A, C, and E), suggesting that the proper responsiveness required for tangential migration through the neocortical SVZ/IZ is largely conserved in chicken MGE cells. In contrast to the GFP-expressing mouse cells, however, most mCherry-expressing chicken cells continued to be distributed around the VZ/SVZ/IZ and failed to enter the CP/MZ (Fig. 1 B, D, and E and Fig. S4).
Fig. 1.
Most chicken MGE cells migrated medially through the neocortical SVZ/IZ, the same as mouse MGE cells did, but they failed to enter the CP/MZ. (A and B) Distribution of GFP-expressing mouse MGE cells (green) and mCherry-expressing chicken MGE cells (magenta) in coronal sections through the middle level along the rostrocaudal axis of the host forebrain at E16.5 (A) and P7 (B). Transplantation with chicken MGE cells was performed as illustrated in Fig. S2. (C and D) Enlarged views of the boxed regions in A and B. (E) Quantification of the distribution of GFP-expressing mouse MGE cells and mCherry-expressing chicken MGE cells in embryonic and neonatal neocortical layers. The percentages of GFP and mCherry cells in each layer were analyzed (mean ± SEM) at E15.5 (n = 3 brains, 114 GFP cells), E16.5 (n = 5 brains, 311 GFP and 194 mCherry cells), E18.5 (n = 4 brains, 566 GFP and 102 mCherry cells), P2 (n = 5 brains, 910 GFP and 233 mCherry cells), and P7 (n = 4 brains, 300 GFP and 201 mCherry cells). Because only a few mCherry cells were found in the neocortex at E15.5, they were excluded from the analysis. At E15.5 and E16.5, the neocortical wall was subdivided into seven layers: MZ, upper CP (U-CP), lower CP (L-CP), upper IZ (U-IZ), lower IZ (L-IZ), SVZ, and VZ. Because the border between the IZ and SVZ was indistinct, the SVZ was defined as a zone occupying one-quarter of the thickness of the VZ. The subplate has been omitted for the sake of simplicity. At both E18.5 and P2, the SVZ and VZ were combined into one zone, the SVZ/VZ. At P7, the U-IZ, L-IZ, and SVZ/VZ were combined into one zone as the white matter (WM), and layer 1, layers 2–4, and layers 5 and 6 have been represented as MZ, U-CP, and L-CP, respectively, in the graph to simplify comparisons. GE, ganglionic eminence; Hip, hippocampus; L1-6, layers 1–6; L, lateral; D, dorsal. (Scale bars: A, 500 μm; B, 1 mm; C and D, 200 μm.)
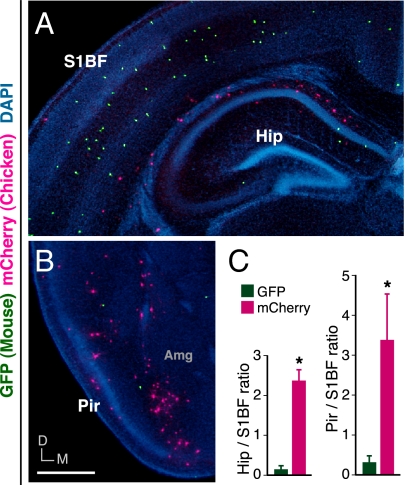
To determine whether the failure of the mCherry-expressing chicken cells to enter the neocortical CP/MZ was attributable to poorer motility than the GFP-expressing mouse cells or inappropriate responsiveness to guidance cues to migrate into the neocortical CP/MZ, we investigated the distribution of labeled cells outside the neocortex, namely in the gray matter of the hippocampus and piriform cortex, which are representative regions of the archicortex and paleocortex, respectively. Comparison with GFP-expressing mouse cells revealed that mCherry-expressing chicken cells were preferentially distributed in the gray matter of the hippocampus and piriform cortex rather than in that of the barrel field of the primary somatosensory area within the neocortex (Fig. 2), suggesting that chicken MGE cells preferentially migrate to the archicortex and paleocortex rather than to the neocortex in contrast to mouse MGE cells. Thus, the failure of chicken MGE cells to enter the neocortical CP/MZ is likely to be attributable to their inappropriate responsiveness to neocortical guidance cues rather than to poorer motility.
Fig. 2.
More chicken MGE cells than mouse MGE cells reached at the gray matter of the hippocampus and piriform cortex but not the gray matter of the neocortex. (A and B) Distribution of GFP-expressing mouse MGE cells (green) and mCherry-expressing chicken MGE cells (magenta) in the barrel field of the primary somatosensory cortex (A), the hippocampus (A), and the piriform cortex (B) at P7. Transplantation with chicken MGE cells was performed as illustrated in Fig. S2. (C Left) The ratios of the number of GFP-expressing mouse MGE cells and mCherry-expressing chicken MGE cells in the gray matter of the hippocampus (n = 6 brains, 35 GFP and 126 mCherry cells) to their numbers in the gray matter of the barrel field of the primary somatosensory cortex (mean ± SEM; n = 6 brains, 207 GFP and 54 mCherry cells) and (Right) the ratios of their numbers in the gray matter of the piriform cortex (n = 6 brains, 26 GFP and 108 mCherry cells) to their numbers in the gray matter of the barrel field of the primary somatosensory cortex (n = 6 brains, 123 GFP and 42 mCherry cells). *P = 0.0022 (Mann–Whitney u test). S1BF, barrel field of the primary somatosensory cortex; Hip, hippocampus; Pir, piriform cortex; Amg, amygdala; M, medial; D, dorsal. (Scale bar: 500 μm.)
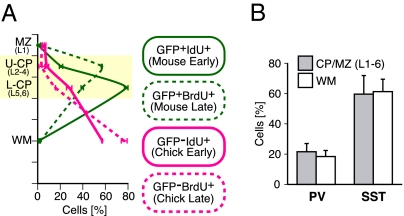
Previous studies have suggested differences between the migratory properties of murine MGE cells born at different developmental stages (29–31). Because chicken E6.5, the time when the donor MGE cells were dissected out, is in the middle of the neurogenic period in the chicken dorsal telencephalon (E5–8) (32), chicken MGE cells born earlier or later than E6.5 may behave differently from E6.5 chicken MGE cells. If that were true, the difference between the neocortical migratory behavior of E13.5 mouse MGE cells and E6.5 chicken MGE cells might be attributable to the difference between their birthdates rather than to the difference in species. To determine which of these two possibilities was correct, we differentially labeled early- and late-born mouse MGE cells and early- and late-born chicken MGE cells (the GFP-expressing mouse MGE cells with IdU at E12.0 and BrdU at E15.0 and the chicken MGE cells with IdU at E5.5 and BrdU at E7.5) and simultaneously transplanted them into a mouse MGE at E13.5 (Fig. S5A). To detect BrdU and IdU, we used an antibody that recognized both BrdU and IdU and an antibody that was specific for BrdU. Cells labeled with the first antibody but not the second were regarded as IdU-positive, and cells labeled with both antibodies were regarded as BrdU-positive (Fig. S5 C–F). Consistent with previous reports (29, 30), we found that, at postnatal day 7 (P7), the GFP/IdU double-positive cells and the GFP/BrdU double-positive cells were preferentially distributed in the upper and lower layers, respectively (Fig. 3A and Fig. S5B), suggesting that both early- and late-born mouse MGE cells entered the CP and preferentially migrated to the upper and lower layers, respectively. Notably, both IdU and BrdU single-positive cells tended to be distributed in the white matter (Fig. 3A and Fig. S5B), suggesting that chicken MGE cells preferentially accumulated in the white matter regardless of their birthdates. Thus, the failure of chicken MGE cells to enter the neocortical CP is likely to represent a species-specific property beyond their birthdates.
Fig. 3.
A majority of chicken MGE cells failed to enter the CP/MZ regardless of their birthdates and interneuron subtypes. (A) Quantification of the distribution of GFP-positive IdU-postive cells (green line), GFP-positive BrdU-postive cells (broken green line), GFP-negative IdU-postive cells (magenta line), and GFP-negative IdU-postive cells (broken magenta line) in the neocortical layers at P7. The percentages of the numbers of the four differentially labeled cells in each layer were analyzed (mean ± SEM; n = 2 brains, 59 slices, 226 GFP-positive IdU-positive cells, 81 GFP-positive BrdU-positive cells, 221 GFP-negative IdU-positive cells, and 349 GFP-negative BrdU-positive cells). Transplantation was performed as illustrated in Fig. S5A. (B) Quantification of the expression of MGE-derived interneuron subtype markers in mCherry-expressing chicken MGE cells in the CP/MZ (layers 1–6) or white matter (mean ± SEM; n = 7 brains for all analyses, 122 mCherry cells in the CP/MZ for PV analysis, 213 cells in the white matter for PV analysis, 85 cells in the CP/MZ for SST analysis, and 187 cells in the white matter for SST analysis). There were no statistically significant differences between the CP/MZ (layers 1–6) and the white matter in the percentages of mCherry cells that were PV-positive (P = 0.805, Mann–Whitney u test) or SST-positive (P = 0.906). Transplantation with chicken MGE cells was performed as illustrated in Fig. S2.
Although a majority of the chicken MGE cells failed to enter the neocortical CP/MZ, a minor fraction (10–40%) of chicken MGE cells entered the CP/MZ (Figs. 1E and 3A). Recent studies have suggested that most murine neocortical GABAegic interneurons derived from the MGE can be classified into two distinct classes according to whether they express parvalbumin (PV) or somatostatin (SST) (9, 33), both of which are also expressed in the avian pallium (34, 35). Thus, the failure to enter the neocortical CP/MZ may be limited to a specific subclass of interneurons derived from the chicken MGE. To investigate this possibility, E6.5 chicken MGE cells labeled with mCherry were transplanted into the MGE of E13.5 mouse embryos together with E13.5 mouse MGE cells expressing GFP (Fig. S2), and both GFP-expressing and mCherry-expressing cells were examined for expression of PV and SST at P23, when the definitive subtype-marker expression in mouse neocortical interneurons should be observed (36). Essentially all of the GFP-expressing mouse cells were distributed in the CP/MZ (layers 1–6; 99.3 ± 0.4%, n = 7 brains, 765 of 769 cells), and ∼54% (53.9 ± 3.3%, n = 7 brains, 453 GFP-expressing cells) and ∼47% (46.5 ± 5.2%, n = 7 brains, 316 GFP-expressing cells) of the GFP-expressing cells expressed PV and SST, respectively, the same as reported previously (12). However, one-third of the mCherry-expressing chicken cells (35.1 ± 1.0%, n = 7 brains, 207 of 607 cells) were distributed in the CP/MZ, and the remaining cells were in the white matter, findings that were roughly consistent with the results of observations of their distribution in embryonic and neonatal stages (Figs. 1E and 3A). We found that ∼20% and ∼60% of the mCherry-expressing cells were positive for PV and SST, respectively (Fig. 3B and Fig. S6), and notably, these percentages were similar in the cells distributed in the CP/MZ and white matter (Fig. 3B). These results suggest that a majority of both PV-positive chicken MGE cells and SST-positive chicken MGE cells failed to enter the neocortical CP/MZ.
We found that the number of chicken MGE cells distributed within the lower CP, corresponding to layers 5 and 6, at P7 was significantly higher than the number distributed within the upper CP corresponding to layers 2–4 (P = 0.029, Mann–Whitney u test; n = 4 brains, 300 GFP-expressing cells, 201 mCherry-expressing cells) (Fig. 1E). In contrast to mouse MGE cells, a similar tendency was observed in regard to both earlier- and later-born chicken MGE cells (Fig. 3A). Thus, most chicken MGE cells failed to enter the neocortical CP, especially layers 2–4.
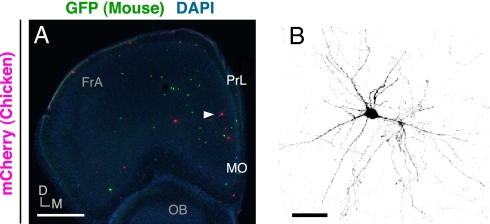
The finding that a minor population of chicken MGE cells that were able to enter the neocortical CP survived, expressed some interneuron subtype markers (Fig. 3B and Fig. S6), and extended many neurites with spine-like structures (Fig. S7) raised the possibility that the neocortical CP was permissive in regard to the postmigratory development of chicken MGE cells. To investigate this possibility, chicken MGE cells labeled with mCherry were transplanted directly into the CP of neonatal medial prefrontal cortex (mPFC) together with GFP-expressing mouse MGE cells, and the survival rate of mCherry-expressing chicken MGE cells within the neocortical gray matter at later stages was compared with that of GFP-expressing mouse MGE cells. We found that the mCherry-expressing chicken cells had morphologically differentiated 4 wk after transplantation (Fig. 4) and that the survival rate of the mCherry-expressing chicken cells was comparable with that of the GFP-expressing mouse cells, at least at 12 wk after transplantation, because the normalized ratio of the number of mCherry-expressing chicken cells to that of GFP-expressing mouse cells at 2 wk after transplantation (100 ± 21.3%, n = 9 hemispheres, 243 mCherry-expressing cells, 535 GFP-expressing cells for comparison with that 12 wk after transplantation; 100 ± 10.3%, n = 7 hemispheres, 720 mCherry-expressing cells, 781 GFP-expressing cells for comparison with that 24 wk after transplantation) was not significantly different from the normalized ratio at 12 wk after transplantation (84 ± 35.1%, n = 7 hemispheres, 79 mCherry-expressing cells, 243 GFP-expressing cells; P = 0.40, Mann–Whitney u test). At 24 wk after transplantation, the normalized ratio of the number of mCherry-expressing chicken cells to that of GFP-expressing mouse cells had decreased significantly (2.6 ± 1.7%, n = 9 hemispheres, 8 mCherry-expressing cells, 372 GFP-expressing cells; P < 0.001, Mann–Whitney u test). Thus, although most chicken MGE cells failed to enter the neocortical CP, for at least a few months after transplantation, the neocortical CP seemed to be essentially permissive in regard to the postmigratory development of chicken MGE cells.
Fig. 4.
The neocortical CP is essentially permissive in regard to the postmigratory development of chicken MGE cells. (A) Distribution of GFP-expressing mouse MGE cells (green) and mCherry-expressing chicken MGE cells (magenta) in a coronal section of host forebrain at 4 wk after transplantation. Transplantation was performed directly into medial prefrontal cortex of P0 neonatal mice. (B) Enlarged view of the cell indicated by the arrowhead in A. FrA, frontal association cortex; PrL, prelimbic cortex; MO, medial orbital cortex; OB, olfactory bulb; D, dorsal, M, medial. (Scale bars: A, 600 μm; B, 25 μm.)
Although Turtle MGE Cells Failed to Enter the Neocortical CP/MZ, the Same as Chicken MGE Cells Did, Marmoset MGE Cells Entered These Regions, the Same as Mouse MGE Cells Did.
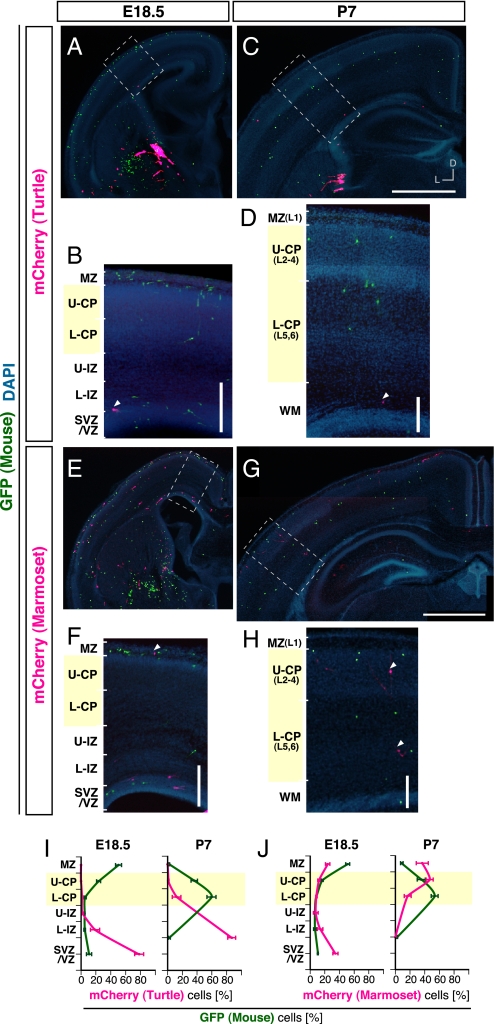
Sauropsids (birds and modern reptiles) diverged from the ancestor of amniotes (mammals and sauropsids) 310 million y ago (MYA) (37, 38), and birds diverged from the ancestor of archosaurs (birds and crocodilians) 240 MYA (37, 38). Thus, there has been a period of 240 million y during which the characters of birds have been able to evolve in a unique direction not shared with other sauropsids. The possibility that they did raises the question of whether the failure of the chicken MGE cells to enter the neocortical CP/MZ was the result of a unique change in a property of the avian MGE cells or represents a more primitive property of sauropsid MGE cells. To answer this question, we examined the migratory behavior of MGE cells derived from turtle embryos. Turtles are classified as sauropsids the same as chickens, but they diverged from the ancestor of archosauromorphs (turtles and archosaurs) 240–260 MYA (37–39). Thus, if turtle MGE cells behaved the same as chicken MGE cells in the mouse neocortex, the behavior would not be specific to bird MGE cells but should represent a primitive property of archosauromorph MGE cells. We, therefore, dissected out MGE cells from E16 turtle embryos (Fig. S1 C and D), labeled them with mCherry, and transplanted them into the MGE of E13.5 mouse embryos in utero together with E13.5 mouse MGE cells expressing GFP (Fig. S2). We found that the mCherry-positive cells had preferentially become distributed in the neocortical VZ/SVZ/IZ at E18.5 (Fig. 5 A, B, and I) and in the white matter at P7 (Fig. 5 C, D, and I), the same as chicken MGE cells had (compare Fig. 5I with Fig. 1E). Thus, the failure of the chicken MGE cells to enter the neocortical CP/MZ, rather than being a specific property of avian MGE cells, seems to represent a primitive property of archosauromorph MGE cells and possibly, sauropsid MGE cells.
Fig. 5.
Most marmoset MGE cells entered the neocortical CP/MZ, but most turtle MGE cells did not. (A and C) Distribution of GFP-expressing mouse MGE cells (green) and mCherry-expressing turtle MGE cells (magenta) in coronal sections through the middle level along the rostrocaudal axis of the host forebrain at E18.5 (A) and P7 (C). Transplantation with turtle MGE cells was performed as illustrated in Fig. S2. (B and D) Enlarged views of the boxed areas in A and C. mCherry cells were often observed outside the CP/MZ (arrowheads). (E and G) Distribution of GFP-expressing mouse MGE cells (green) and mCherry-expressing marmoset MGE cells (magenta) in coronal sections of the host forebrain at E18.5 (E) and P7 (G). Transplantation with marmoset MGE cells was performed as illustrated in Fig. S2. (F and H) Enlarged views of the boxed areas in E and G. mCherry cells were often observed within the CP/MZ (arrowheads). (I and J) Quantification of the distribution of GFP-expressing mouse MGE cells and mCherry-expressing turtle (I) and marmoset (J) MGE cells. (I) The percentages of the numbers of GFP cells and mCherry-expressing turtle MGE cells (n = 6 brains, 1,121 GFP and 54 mCherry cells at E18.5; n = 5 brains, 1,332 GFP and 75 mCherry cells at P7) and (J) marmoset MGE cells (n = 5 brains, 1,060 GFP and 292 mCherry cells at E18.5; n = 4 brains, 338 GFP and 119 mCherry cells at P7) in each layer were analyzed (mean ± SEM). L, lateral; D, dorsal. (Scale bars: A and C, 1 mm; E and G, 1 mm; B, D, F, and H, 200 μm.)
The failure of both chicken and turtle MGE cells to enter the neocortical CP/MZ may also be attributable to a general incompatibility of xenotypic MGE cells rather than to primitive properties of sauropsid MGE cells. Another possibility is that the entrance of mouse MGE cells into the mouse neocortical CP/MZ is attributable to a species-specific compatibility of homotypic MGE cells rather than to a common property of mammalian MGE cells. To investigate these possibilities simultaneously, we examined the behaviors of MGE cells derived from marmosets, which are mammals but clearly different from mice. MGE cells were dissected out from E86 to E93 marmoset embryos (Fig. S1 E and F), labeled with mCherry, and transplanted into the MGE of E13.5 mouse embryos in utero together with E13.5 mouse MGE cells expressing GFP (Fig. S2). The results showed that, at E18.5, the mCherry-expressing marmoset cells were distributed not only in the neocortical VZ/SVZ/IZ but also in the CP/MZ (Fig. 5 E, F, and J), and they were mostly distributed in the CP/MZ at P7 (Fig. 5 G, H, and J), the same as mouse MGE cells. These findings imply that the migratory competency to enter the mouse neocortical CP/MZ is a common property of mammalian MGE cells but not sauropsid MGE cells.
Discussion
The results of this study show that, in contrast to mouse MGE cells, most chicken MGE cells preferentially enter the gray matter of the mouse archicortex and paleocortex but do not enter the gray matter of the neocortex (Fig. S8A). Although both mouse and marmoset MGE cells invaded the mouse neocortical CP/MZ normally during migration, most chicken and turtle MGE cells, regardless of their birthdates and interneuron subtypes, ignored the neocortical CP and passed beneath it (Fig. S8B). Thus, the proper responsiveness of MGE cells to guidance cues to enter the neocortical CP, especially layers 2–4, and MZ is unique to mammals and was established in the ancestor of mammals (Fig. S8C) (additional discussion in SI Discussion). In view of the functional importance of the inhibitory interneurons in the mature neuronal network, we propose that evolutionary changes in the migratory ability of incoming interneurons that originate outside the cortex were important to the establishment of a functional mammalian neocortex by supplying inhibitory components to the network.
Consistent with the previous finding that turtle MGE cells were able to migrate into the mouse neocortex in vitro (25), MGE cells from both chicken and turtle have the competency to migrate tangentially through the neocortical SVZ/IZ, and they have weak competency to enter neocortical layers 5 and 6 (Fig. S8B). In view of the fact that the neocortical SVZ emerged in the ancestor of mammals, the MGE cells in the ancestor of amniotes were preadapted to the mammalian neocortical SVZ in regard to their tangential migration (additional discussion in SI Discussion). The preadaptation of MGE cells in the ancestor of amniotes to migrate tangentially through the neocortical SVZ may provide insight into the phylogenetic cellular mechanism of the tangential expansion of the neocortex during mammalian evolution. One of the morphological hallmarks of the primate neocortex is its folding, which is mainly attributable to its tangential expansion by the addition of radial columns (19). The folding can be observed even in the developing neocortex, especially in the superficial regions such as in the CP/MZ (40). The folding in the superficial regions likely results in the increase in distance that the MGE-derived interneurons need to migrate to reach the medial part of the neocortex if they move through the superficial regions. Thus, the preferential migration of MGE cells within the neocortical SVZ, which is close to the ventricle, may enable them to reach the medial part of the neocortex by a shorter route than by the superficial paths through the CP/MZ. Interestingly, although the dorsal pallium of sauropsids, in which interneurons disperse without forming any migratory streams, is less elaborate than the neocortex of mammals, the lateral pallium and ventral pallium, which are closer to the MGE than the dorsal pallium, are extremely elaborate and form the DVR in sauropsids (14). The DVR is unique to sauropsids and governs cognitive functions, a role that is similar to the role of the neocortex in mammals (41). One intriguing hypothesis, therefore, is that migratory pathways of the interneurons constrained the evolution of the dorsal pallium in amniotes; whereas the establishment of the migratory stream of MGE cells through the SVZ in mammals had enabled the elaboration of the dorsal pallium and establishment of the large neocortex in primates, dispersion of the MGE cells in sauropsids had severely constrained the elaboration of the dorsal pallium, which is far from the MGE, and the DVR, which is closer to the MGE, may have evolved as a result. Direct generation of GABAergic interneurons from the neocortical VZ/SVZ may be another cellular mechanism that evolved to efficiently supply GABAergic interneurons to the tangentially expanded and folded neocortex in primates (19).
The cellular and molecular mechanisms of the evolution of the competency of MGE cells to enter the neocortical CP, especially layers 2–4, and the MZ remain unclear. Several differences in the environment of the migrating MGE-derived cells between mammals and sauropsids may have affected the evolutionary changes in migratory competency. In the developing neocortex of mammals, (i) an SVZ is present, (ii) the main afferents enter the CP from beneath it, (iii) a separate SP layer is present, (iv) the CP forms in an inside-out fashion, and (v) supragranular cells are present. By contrast, in the developing dorsal pallium of sauropsids, (i) there is no SVZ (15, 16), (ii) the main afferents seem to enter the CP from above it (42, 43), (iii) there does not seem to be a separate SP layer (44), (iv) the CP forms in an outside-in fashion (15, 16), and (v) no equivalent of the supragranular cells of mammals seems to exist (45, 46). Interestingly, the migratory defect of chicken MGE cells that prevented them from entering the neocortical CP/MZ (Fig. S8B) is partially phenocopied in mouse MGE cells lacking Lhx6. Lhx6 is a transcription factor that is specifically expressed in MGE cells (13), and the migration of MGE cells from the neocortical SVZ to the CP/MZ seems to be reduced at an early stage in the development of mouse Lhx6 null embryos (47, 48). However, because Lhx6 is expressed in the chicken pallium in a dispersed manner (49) and thus, is likely to be expressed in chicken interneurons derived from the MGE, the downstream cascade of Lhx6 may have changed during mammalian evolution to enable the MGE-derived cells to enter the neocortical CP/MZ.
Although the interpretation of the results of the studies is still being debated, the brains of patients with schizophrenia seem to show abnormalities in the neocortex, especially in layers 2–4; the abnormalities include alterations in both PV-positive interneurons and SST-positive interneurons (50, 51). Interestingly, ∼80% of the chicken MGE cells that failed to invade the neocortical CP, especially layers 2–4, were either PV-positive or SST-positive (Fig. 3B). Thus, the evolutionary change of the migratory ability of MGE-derived PV-positive interneurons and SST-positive interneurons in the ancestor of amniotes may have been essential for establishing the normal function of the neocortex of mammals, including humans.
Materials and Methods
Fertilized chicken and soft-shelled turtle (Pelodiscus sinensis japonicus) eggs were obtained from local hatcheries (chicken; Takeuchi Hatchery and turtle; Tujimura Hatchery). Common marmosets have been maintained in cages measuring 50 × 60 × 70 cm in our laboratory at the Central Institute for Experimental Animals (CIEA) since 1975. Embryonic mouse donor tissue was obtained by crossing ICR WT female mice with homozygous GFP-expressing C57BL/6-Tg(CAG-EGFP)C14-Y01-FM131Osb male mice (52). To obtain chicken, turtle, and marmoset MGE cells, we used E5.5–7.5 chicken embryos (approximately corresponding to Hamburger and Hamilton stages 27–31) (53), E16 turtle embryos (approximately corresponding to Tokita and Kuratani stage 15) (54), and E86–E96 marmoset embryos, respectively. Sauropsid (chicken and turtle) cells and marmoset cells were transfected with pCAG-mCherry (13) using a nucleofector kit (Amaxa Biosystems) with some minor modifications, and they were pelleted by centrifugation. The donor cell suspensions were front-loaded into beveled glass micropipettes (50- to 75-μm caliber at the tip, GD-1; Narishige). Approximately 3 × 104 cells (∼0.03 μL) were injected into the MGE of the left hemisphere of each embryo in utero under a stereomicroscope. The x and y coordinates of the injection sites were estimated from the surface anatomy (Fig. S2), and the z coordinates of the injection sites were ∼0.9 mm below the skin surface. Complete materials and methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. S. Sasaki for his encouragement and technical advice, Dr. M. Okabe for providing GFP-expressing mice, Dr. J. Miyazaki for providing the pCAGGS vector, Drs. Y. Zhu and F. Murakami for providing the pCAG-mCherry plasmid, and Drs. T. Maruyama, N. Sugo, N. Yamamoto, T. Sunabori, H. Okano, S. Tomisato, K. Kohda, M. Yuzaki, and Y. Murakami for technical assistance and advice. This work was supported by the Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, and Science and Technology of Japan, the Keio Gijuku Academic Development Funds, and the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102153108/-/DCSupplemental.
References
- 1.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 2.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 3.Tabata H, Nakajima K. Multipolar migration: The third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 5.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 6.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 7.Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex. 2009;19:249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: Role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- 9.Fogarty M, et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puzzolo E, Mallamaci A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: Generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural Dev. 2010;5:8. doi: 10.1186/1749-8104-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelman DM, et al. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka DH, et al. Random walk behavior of migrating cortical interneurons in the marginal zone: Time-lapse analysis in flat-mount cortex. J Neurosci. 2009;29:1300–1311. doi: 10.1523/JNEUROSCI.5446-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina L, Abellán A. Development and evolution of the pallium. Semin Cell Dev Biol. 2009;20:698–711. doi: 10.1016/j.semcdb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Molnár Z, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung AF, Pollen AA, Tavare A, DeProto J, Molnár Z. Comparative aspects of cortical neurogenesis in vertebrates. J Anat. 2007;211:164–176. doi: 10.1111/j.1469-7580.2007.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura T, Takahashi M, Hara Y, Osumi N. Patterns of neurogenesis and amplitude of Reelin expression are essential for making a mammalian-type cortex. PLoS One. 2008;3:e1454. doi: 10.1371/journal.pone.0001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sessa A, et al. Tbr2-positive intermediate (basal) neuronal progenitors safeguard cerebral cortex expansion by controlling amplification of pallial glutamatergic neurons and attraction of subpallial GABAergic interneurons. Genes Dev. 2010;24:1816–1826. doi: 10.1101/gad.575410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CS, Lu SM, Schmechel DE. Glutamic acid decarboxylase and somatostatin immunoreactivities in rat visual cortex. J Comp Neurol. 1986;244:369–383. doi: 10.1002/cne.902440309. [DOI] [PubMed] [Google Scholar]
- 21.Blanton MG, Shen JM, Kriegstein AR. Evidence for the inhibitory neurotransmitter gamma-aminobutyric acid in aspiny and sparsely spiny nonpyramidal neurons of the turtle dorsal cortex. J Comp Neurol. 1987;259:277–297. doi: 10.1002/cne.902590208. [DOI] [PubMed] [Google Scholar]
- 22.Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veenman CL, Reiner A. The distribution of GABA-containing perikarya, fibers, and terminals in the forebrain and midbrain of pigeons, with particular reference to the basal ganglia and its projection targets. J Comp Neurol. 1994;339:209–250. doi: 10.1002/cne.903390205. [DOI] [PubMed] [Google Scholar]
- 24.Cobos I, Puelles L, Martínez S. The avian telencephalic subpallium originates inhibitory neurons that invade tangentially the pallium (dorsal ventricular ridge and cortical areas) Dev Biol. 2001;239:30–45. doi: 10.1006/dbio.2001.0422. [DOI] [PubMed] [Google Scholar]
- 25.Métin C, et al. Conserved pattern of tangential neuronal migration during forebrain development. Development. 2007;134:2815–2827. doi: 10.1242/dev.02869. [DOI] [PubMed] [Google Scholar]
- 26.Carrera I, Ferreiro-Galve S, Sueiro C, Anadón R, Rodríguez-Moldes I. Tangentially migrating GABAergic cells of subpallial origin invade massively the pallium in developing sharks. Brain Res Bull. 2008;75:405–409. doi: 10.1016/j.brainresbull.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Moreno N, González A, Rétaux S. Evidences for tangential migrations in Xenopus telencephalon: Developmental patterns and cell tracking experiments. Dev Neurobiol. 2008;68:504–520. doi: 10.1002/dneu.20603. [DOI] [PubMed] [Google Scholar]
- 28.Tuorto F, Alifragis P, Failla V, Parnavelas JG, Gulisano M. Tangential migration of cells from the basal to the dorsal telencephalic regions in the chick. Eur J Neurosci. 2003;18:3388–3393. doi: 10.1111/j.1460-9568.2003.03059.x. [DOI] [PubMed] [Google Scholar]
- 29.Pla R, Borrell V, Flames N, Marín O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammond V, et al. Layer positioning of late-born cortical interneurons is dependent on Reelin but not p35 signaling. J Neurosci. 2006;26:1646–1655. doi: 10.1523/JNEUROSCI.3651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo: I. Neuronal birthdates of telencephalic compartments in situ. J Comp Neurol. 1981;198:275–292. doi: 10.1002/cne.901980207. [DOI] [PubMed] [Google Scholar]
- 33.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erichsen JT, Ciocchetti A, Fontanesi G, Bagnoli P. Neuroactive substances in the developing dorsomedial telencephalon of the pigeon (Columba livia): Differential distribution and time course of maturation. J Comp Neurol. 1994;345:537–561. doi: 10.1002/cne.903450406. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhury S, Nag TC, Wadhwa S. Calbindin D-28K and parvalbumin expression in embryonic chick hippocampus is enhanced by prenatal auditory stimulation. Brain Res. 2008;1191:96–106. doi: 10.1016/j.brainres.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E. Transient colocalization of parvalbumin and calbindin D28k in the postnatal cerebral cortex: Evidence for a phenotypic shift in developing nonpyramidal neurons. Eur J Neurosci. 1996;8:1329–1339. doi: 10.1111/j.1460-9568.1996.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 37.Benton MJ. Phylogeny of the major tetrapod groups: Morphological data and divergence dates. J Mol Evol. 1990;30:409–424. doi: 10.1007/BF02101113. [DOI] [PubMed] [Google Scholar]
- 38.Kumazawa Y, Nishida M. Complete mitochondrial DNA sequences of the green turtle and blue-tailed mole skink: Statistical evidence for archosaurian affinity of turtles. Mol Biol Evol. 1999;16:784–792. doi: 10.1093/oxfordjournals.molbev.a026163. [DOI] [PubMed] [Google Scholar]
- 39.Iwabe N, et al. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Mol Biol Evol. 2005;22:810–813. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- 40.Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 41.Jarvis ED, et al. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aboitiz F. Evolution of isocortical organization. A tentative scenario including roles of reelin, p35/cdk5 and the subplate zone. Cereb Cortex. 1999;9:655–661. doi: 10.1093/cercor/9.7.655. [DOI] [PubMed] [Google Scholar]
- 43.Supèr H, Uylings HB. The early differentiation of the neocortex: A hypothesis on neocortical evolution. Cereb Cortex. 2001;11:1101–1109. doi: 10.1093/cercor/11.12.1101. [DOI] [PubMed] [Google Scholar]
- 44.Wang WZ, et al. Comparative aspects of subplate zone studied with gene expression in sauropsids and mammals. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq278. in press. [DOI] [PubMed] [Google Scholar]
- 45.Medina L, Reiner A. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci. 2000;23:1–12. doi: 10.1016/s0166-2236(99)01486-1. [DOI] [PubMed] [Google Scholar]
- 46.Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: Towards an integrated developmental and functional approach. Behav Brain Sci. 2003;26:535–552. doi: 10.1017/s0140525x03000128. [DOI] [PubMed] [Google Scholar]
- 47.Liodis P, et al. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y, et al. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J Comp Neurol. 2008;510:79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abellán A, Medina L. Subdivisions and derivatives of the chicken subpallium based on expression of LIM and other regulatory genes and markers of neuron subpopulations during development. J Comp Neurol. 2009;515:465–501. doi: 10.1002/cne.22083. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 53.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 54.Tokita M, Kuratani S. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae) Zoolog Sci. 2001;18:705–715. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.