Abstract
The discovery of nonmolecular carbon dioxide under high-pressure conditions shows that there are remarkable analogies between this important substance and other group IV oxides. A natural and long-standing question is whether compounds between CO2 and SiO2 are possible. Under ambient conditions, CO2 and SiO2 are thermodynamically stable and do not react with each other. We show that reactions occur at high pressures indicating that silica can behave in a manner similar to ionic metal oxides that form carbonates at room pressure. A silicon carbonate phase was synthesized by reacting silicalite, a microporous SiO2 zeolite, and molecular CO2 that fills the pores, in diamond anvil cells at 18–26 GPa and 600–980 K; the compound was then temperature quenched. The material was characterized by Raman and IR spectroscopy, and synchrotron X-ray diffraction. The experiments reveal unique oxide chemistry at high pressures and the potential for synthesis of a class of previously uncharacterized materials. There are also potential implications for CO2 segregation in planetary interiors and for CO2 storage.
Keywords: high-pressure chemistry, material science, optical spectroscopy
Carbon dioxide and silicon dioxide are two archetypal, group IV oxides of paramount importance for fundamental and applied chemistry and planetary sciences. CO2 is the dominant component of the atmosphere of Earth-like planets, exists in icy forms in outer planets and asteroids, plays an important role in volcanic and seismic processes, is used as a supercritical solvent for chemical reactions, and its anthropogenic production is a major environmental issue. SiO2 is also one of the most abundant components of terrestrial planets, and an important technological material. The chemical relationship between CO2 and SiO2, in particular their reactivity, is thus of interest. Although the two systems are both group IV oxides, they are remarkably different under ambient conditions, because CO2 is molecular and is held together by C═O double bonds, whereas SiO2 forms network structures involving single Si─O bonds. These bonding patterns radically change under pressure. Two nonmolecular CO2 crystalline phases and a glassy form have been discovered above 30 GPa that bear similarities to SiO2 (1–14). The crystalline phases contain carbon in fourfold coordination by oxygen (1–10, 13, 14), and mixtures of CO4 and CO3 units have been found in a glassy form that has been named carbonia (13).
A possible approach to favor the chemical reaction between CO2 and SiO2 is to select a microporous silica polymorph, such as silicalite. At ambient conditions, silicalite is characterized by a framework of four-, five-, six-, and ten-membered rings of SiO4 tetrahedra with 5.5-Å pores (15) (Fig. 1, Left Inset). Recently, it has been found that the pores can be completely filled by simple molecules such as CO2 and Ar under pressure, which prevents pressure-induced amorphization (PIA) and stabilizes the crystalline framework up to at least 25 GPa at room temperature (16). It is well known that pressure can strongly modify the character of materials, and possible SiO2/CO2 alloys have been predicted by density functional theory (DFT) simulations (17). The choice of silicalite is motivated by the large effective surface exposed to the CO2 in the pores (all of the SiO4 tetrahedra are on the surface of the micropores), which is likely to be a crucial factor for enhancing the chemical reaction.
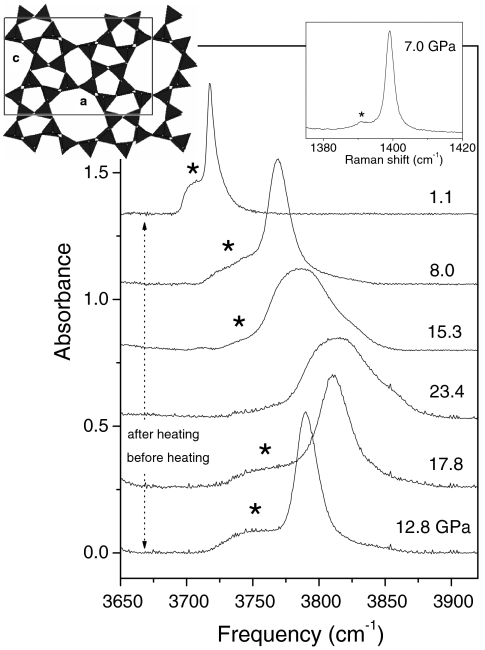
Fig. 1.
IR and Raman (Right Inset) spectra of mixed silicalite and CO2, showing evidence of the chemical reaction. The main figure shows IR spectra in the frequency region of the (ν3 + ν1; ν3 + 2ν2) combination band of CO2. (Right Inset) Raman spectrum in the frequency region of the ν+ line of CO2, measured before the heating. Asterisks indicate peaks of CO2 confined in the micropores of silicalite. The stronger peaks are from bulk, free CO2 (see text). The sample was heated to 600 K for about 1 h. IR spectra before (after) the heating were measured upon increasing (decreasing) pressure. Changes in the line-shape of the band of bulk CO2 are related to phase transitions among phases I, II, III, and IV of molecular CO2 (1, 18). (Left Inset) Schematic representation of the crystal structure of silicalite.
Results and Discussion
We examined chemical reactions between silicalite SiO2 and CO2. We conducted nine experiments at 18–26 GPa and 296–980 K, far from the pressure-temperature (P-T) transformation boundary of molecular to nonmolecular CO2 (18) in the pure system. In Fig. 1, we present the IR (ν3 + ν1; ν3 + 2ν2) and Raman (ν+) spectra of a typical sample. About half of the total sample thickness (20 μm) was taken by silicalite powder with CO2 completely filling the pores, whereas the residual space was occupied by bulk, free CO2. The sample was compressed up to 18 GPa before heating, and the spectra show the peaks of confined and bulk (1, 19) CO2, split with each other by at most 1% in frequency. This indicates that the interaction between CO2 and the silicalite surface is still in the range of van der Waals forces. Despite the weak interaction at room temperature, high-temperature annealing to 600 K was effective in enhancing the chemical reaction. In fact, the IR peak of confined CO2 disappears after the temperature annealing, which indicates evidence of a chemical reaction between silicalite and the confined CO2. Also, the reaction is reversible: The IR peak of confined CO2 appears again upon decreasing pressure below 15 GPa. The Raman spectrum of the transformed material was completely overwhelmed by a large fluorescence background that also indicates a major transformation leading to a compound with a large number of defects.
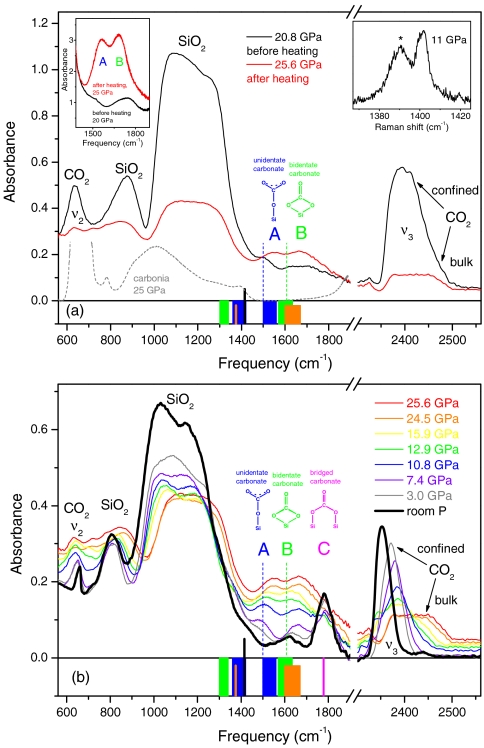
The full picture of the reaction was obtained by a special set of measurements (see Methods) on very thin samples, approximately 1 μm, which avoided saturation of the IR absorption (Fig. 2). A very small amount of bulk CO2 was left in this case. The sample was compressed to 20.8 GPa, then heated to 740 K. In the IR spectrum of the temperature-quenched material (Fig. 2 Upper) the peaks of silicalite are reduced by about 0% of the original intensity, and the peaks of confined CO2 almost completely vanished. In parallel, two strong peaks A and B appeared, which belong neither to molecular CO2 nor to silicalite. Therefore, the most fundamental aspect of a binary chemical reaction is demonstrated: Two reactant substances, silicalite and CO2, react with each other, and a product substance is formed, identified by peaks A and B. Upon lowering the pressure, the peaks shift to lower frequencies and gradually disappear below 15 GPa, and a nonmolecular peak, C, emerges at about 1,780 cm-1. In parallel, we observe the formation of confined CO2 and the intensification of the silicalite peaks again (Fig. 2 Lower). Finally, at room pressure, peak C disappears after a few days along with the peak of residual confined CO2, thereby showing the overall reversibility of the transformation.
Fig. 2.
IR spectra of mixed silicalite and CO2 (thin sample, approximately 1 μm), showing the formation of silicon carbonate. The compound is identified by the A, B, and C peaks, assigned to unidentate, bidentate, and bridged silicon carbonate species, respectively. (Upper) IR spectra before and after the heating to 740 K. Heating lasted for about 1 h. Gray dashed line indicates spectrum of carbonia (13). The peak at 2,400 cm-1 and the high-frequency shoulder at 2,450 cm-1 are assigned to the ν3 mode of confined and bulk CO2, respectively. After heating, the confined CO2 has almost vanished, and the ν3 peak is dominated by the component of bulk CO2, which did not react. (Left Inset) IR spectra of a thick (20 μm) sample, before and after temperature annealing, showing the formation of the strong A and B peaks. (Right Inset) Raman spectrum of the ν+ line of CO2 on the 1-μm-thick sample, before annealing. Peaks of confined (asterisk) and bulk CO2 have an integrated intensity ratio of about 3. (Lower) IR spectra measured upon decreasing pressure, after annealing. The behavior of the ν3 peak shows that confined CO2 forms again (sharp component), starting at about 16 GPa; instead, bulk CO2 (broad component) vanishes, indicating that this material progressively extrudes from the sample chamber. Vertical black sticks (Upper and Lower) indicate frequency of the IR stretching mode of the free CO3 ion (24). Blue, green, and orange bars (Upper and Lower) indicate spectral ranges, at ambient pressure, of the split high- and low-frequency components of the IR stretching mode of the free CO3 ion, as found in unidentate (blue), bidentate (green), and bridging, T-CO3-T (orange) carbonates, obtained from CO2 adsorption on metal oxides and dissolution in silicate melts (see text). The two components correspond to the antisymmetric and the symmetric C─O stretching modes, respectively, involving the two equivalent oxygen atoms. Pink bar (Lower) indicates spectral range, at ambient pressure, for the highest-frequency IR stretching mode of bridging carbonates also obtained from adsorption of CO2 on some metal oxides (see text). Vertical, dashed lines (Upper and Lower) indicate room pressure extrapolated frequencies of the A and B peaks.
The assignment of peaks A, B, and C to C─O stretching modes of silicon carbonates is very straightforward. We note that in covalent, nonmolecular CO2, the stretching modes of C═O double and C─O single bonds exhibit IR absorption around 1,900–2,000 cm-1 and below 1,400 cm-1, respectively (13). These frequency ranges are far away from the region of interest here, proving that covalent double and single bonds are too strong and too weak, respectively, to explain the nature of the present material. Peaks A, B, and C can be assigned to stretching modes of C─O bonds with intermediate strength as those involved in carbonates in ionic compounds. It has been well known for several decades that CO2 can be adsorbed at ambient pressure on the surface of basic metal oxides and a variety of zeolites that contain transition, alkali, or alkaline earth metals (20–27). The adsorption results in the formation of unidentate and bidentate carbonates (see Fig. 2), where the double degenerate IR stretching mode of the free CO3 ion splits in two bands on either side of the unperturbed frequency [1,415 cm-1 (24)]. Remarkably, the high-frequency components of unidentate and bidentate carbonates fit fairly well the frequencies of peaks A and B, respectively, thereby showing that the reaction of confined CO2 with silicalite results indeed in the formation of unidentate and bidentate carbonates, each one involving a single framework silicon atom.
We note that the presence of carbonate groups has been reported from IR studies on CO2-bearing, high silica, aluminosilicate glasses (28–30). A number of different carbonates have been identified in these materials containing various cations, among which T-CO3-T groups (where T is silicon or aluminum in a tetrahedral coordination by oxygen) exhibit large splitting of the IR stretching mode of CO3 (orange bars in Fig. 2). The high-frequency component of these doublets is definitely compatible with peak B. These bridging carbonates would involve two framework silicon atoms in the case of silicalite, which is pure silica, in our study. Bridging CO3 groups were also found in some carbonates obtained by CO2 adsorption on metal oxides (23), whose highest frequency C─O stretching mode fits fairly well to peak C. The high frequency of peak C indicates that these bridging carbonates have more covalent character than the CO3 species discussed above; in a sense, their nature is closer to that of CO2.
A clear picture thus emerges. Confined CO2 and silicalite react with each other at high P-T conditions, above 18 GPa, forming a silicon carbonate compound. Silicalite behaves similarly to ionic metal oxides under these conditions. The material decomposes upon reducing pressure below 15 GPa into silicalite, silicon carbonate with only bridged CO3 groups, and molecular CO2. Finally, the recovered material releases the CO2 and the residual carbonate.
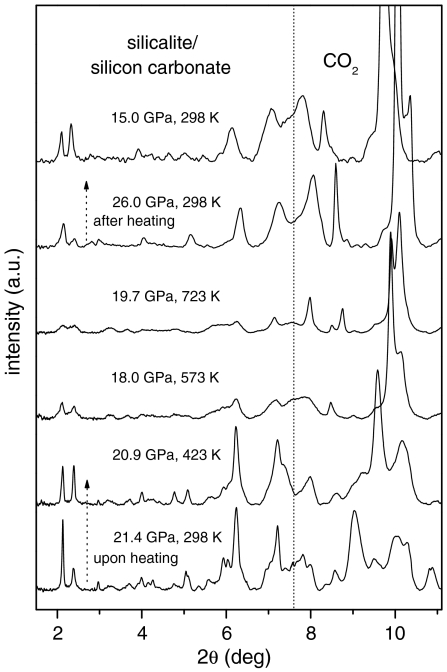
As an additional test of the chemical reaction and of the nature of the compound, we measured the X-ray diffraction (XRD) patterns (Fig. 3) in situ on a sample compressed to 21.4 GPa and then heated to 723 K. The Bragg peaks of silicalite progressively broaden upon increasing temperature. This is a clear indication of the chemical reaction, as diffraction peaks should instead sharpen because of the usual temperature enhancement of the crystal quality and relaxation of any deviatoric stress present. The broad peaks observed upon heating are centered on the sharp reflections observed for orthorhombic silicalite at 298 K before heating, for which the cell parameters were a = 18.000(1) Å, b = 18.000(1) Å, and c = 12.203 Å. Based on the estimated error of the positions of these broad peaks, the cell parameters at 723 K lie within ± 1% of these values. This quantitatively indicates that the CO2 remains in this poorly crystallized material and that the reduction in crystallinity is not due to loss of CO2 followed by the reported collapse of the structure (15) (PIA) to form dense amorphous SiO2, but is a result of the chemical reaction with CO2. Loss of CO2 followed by PIA is also ruled out by the observation that the broadening of Bragg peaks is reversible (see below), whereas PIA is irreversible.
Fig. 3.
High P-T, XRD patterns of silicalite-CO2 mixture, showing the formation of a highly strained, disordered crystal. From the bottom to the top: The sample was heated to 723 K at 18–26 GPa, for about 1 h. The temperature-quenched material was then decompressed to 15 GPa. The sample thickness is about 20 μm, as in Fig. 1. At angles higher than the vertical dotted line the diffraction pattern is dominated by the peaks of bulk, molecular CO2, which undergoes phase transitions between phases I, II, III, and IV. The intensity of the patterns has been normalized to the same acquisition time and X-ray beam intensity.
It is then shown that the chemical reaction takes place progressively, ending up in a product that is a highly strained crystal still exhibiting the structure of the original silicalite. This is not unexpected, because the carbonates form at the pore surface, which in turn does not alter the pore arrangement within the unit cell, but does affect the long-range periodicity of the structure. In fact, the solid is strained, and the coherence length is reduced to 8–10 nm, at the maximum temperature, as deduced from the peak width. This is evidence that the carbonate groups form in a random manner without any long-range order. They can also be expected to induce local geometrical distortions to the framework.
Surprisingly, no peaks of stishovite, the sixfold coordinated cristalline silica, are observed. Stishovite is the thermodynamic stable phase of silica above 9 GPa (31), and it easily forms upon heating cristobalite to 570 K, at similar pressures (32). The fact that we are left with silicon carbonate instead of stishovite and molecular CO2 is evidence of the high chemical stability of this compound. In the temperature-quenched material, several weak peaks of silicalite emerge again from the background below 6° and the two peaks between 1.8° and 2.6° are sharpened. Nevertheless, the crystal is still severely strained. Finally, the sample was decompressed, and the two low-angle peaks sharpened more at 15 GPa, which indicates the reversibility of the reaction, in very good agreement with the IR study.
All of the data presented here consistently show that SiO2 and CO2 undergo high P-T chemical reactions of the type xSiO2 + yCO2↔SixCyO(2x+2y), which results in the formation of one or more silicon carbonate compounds. Although the reaction occurs at the surface of the silicalite micropores, the final product has the nature of a real bulk compound due to the particular structure of zeolitic silicalite. In fact, all of the tetrahedra in silicalite are on the surface of the micropores; thus, a surface reaction can involve, in principle, all the SiO2 as would be the case in a bulk reaction. This is quantitatively shown by the fact that about 50% of the original tetrahedra are indeed transformed (see the discussion on IR spectra). However, the silicalite framework is retained, although highly strained, and the product is thus a nonstoichiometric silicon carbonate. The chemical stability of this compound is surprising. It forms in a large variety of P-T conditions. In several measurements we have found that silicon carbonate forms and is chemically stable up to at least 1,000 K and 60 GPa, which covers the P-T range where stishovite or dense poststishovite phases and nonmolecular CO2 form in pure silica and CO2 samples, respectively (1, 11, 13, 18, 32). Preliminary results show that silicon carbonate is also obtained by a pressure-induced (38 GPa, 296 K) reaction of CO2 with a different precursor material (amorphous silicic acid [SiOx(OH)4-2x]n), which supports the evidence for chemical stability of this compound. Therefore, we found that the reactions between CO2 and silicalite, and CO2 and silica, both yield chemically stable silicon carbonate as compared to dense CO2 and stishovite. However, both of these silicon carbonate materials are disordered and almost certainly metastable with respect to a crystalline, ordered counterpart of these compounds, which could possibly be thermodynamically stable and is likely to exist. This crystal could be rather similar to those predicted by DFT studies: cristobalite-type Si1-xCxO2 mixed oxides, which are expected to be stable with respect to the end members above 6 GPa (17). It is probable that more extreme P-T conditions than those employed here would allow the high energy barriers preventing the crystallization of these solids to be overcome.
In conclusion, the discovery of the pressure-induced chemical reaction between SiO2 and CO2 shows a remarkable affinity between these two important substances, which was completely unexpected from the ambient pressure point of view. We thus show that a unique oxide chemistry exists at high pressures. In fact, the broad class of carbonate compounds has to be extended to include silicon carbonate. More generally, we think that the potential for the synthesis of a whole class of previously uncharacterized materials could be in sight, along with unique opportunities for the storage of CO2. It is also remarkable that our thermodynamic conditions are not very far from those of terrestrial planetary interiors, and future investigations on such planetary interiors should definitely consider possible chemical reactions between silica and CO2.
Methods
IR spectra were measured using Fourier transform infrared spectrometers: Bruker IFS-120 HR and Bruker IFS66V. In the case of Bruker 120 HR, an optical beam condenser based on ellipsoidal mirrors was used. Raman spectra were performed by using the 752.5-, 785-, and 633-nm lines of a Kr+, a diode, and an HeNe laser, respectively, as the excitation sources. Backscattering geometry was used, with 20× and 50× Mitutoyo microobjectives, and the signal was detected by single monochromators: Acton/SpectraPro 2500i, equipped with a CCD detector (Princeton Instruments Spec-10∶100 BR), and Horiba Jobin-Yvon LabRam Aramis, also equipped with a CCD detector. Angle-dispersive XRD patterns have been measured at the ID27 beam line of the European Synchrotron Research Facility, with monochromatic beam (λ = 0.3738 Å) and a Mar CCD detector. The nominal size of the focal spot was 2 μm. The diffraction patterns were analyzed and integrated using the FIT2D program (33).
Hydrophobic silicalite-1-OH, which contains traces of hydroxyl groups, and hydrogen-free silicalite-1-F were obtained from SOMEZ. Identical results were obtained in the reaction of CO2 with both types of silicalite, indicating that hydrogen does not play a relevant role in the reaction.
A resistively heated diamond anvil cell (DAC) was used for the high-pressure experiments. CO2 was loaded in the DAC (IIa diamonds for the IR measurements) cryogenically in the liquid phase, at 25 bar, together with powdered silicalite. A ruby chip was put in the sample chamber for pressure measurements, using the ruby fluorescence method. A special procedure was adopted for preparing the very thin (approximately 1 μm) samples. The 40-μm-thick sample chamber was almost completely filled by a KBr pellet, on the top of which a thin (approximately 1 μm) silicalite pellet was slightly pressed. In the DAC loading procedure, liquid CO2 filled the pores of silicalite, and the amount of bulk, free CO2 was very small, as compared to the thick samples. KBr is transparent in the IR spectral range of this study.
Acknowledgments.
We thank M. Mezouar for discussions and D. Maurin and D. Bourgogne for technical support for Raman and IR measurements at Université Montpellier 2. We acknowledge support from the European Union (European Laboratory for Nonlinear Spectroscopy Contract FP7 G.A. No. 228334 LASERLABEUROPE), the Ente Cassa di Risparmio di Firenze, and the Agence Nationale de la Recherche (Contract ANR-09-BLAN-0018-01). We also thank the ESRF for provision of beam time at ID27. M.S. thanks the Région Languedoc-Roussillon for having supported his research at the Université Montpellier 2, in 2009, and the Université de Nîmes, in 2010, as an Invited Professor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Santoro M, Gorelli FA. High pressure solid state chemistry of carbon dioxide. Chem Soc Rev. 2006;35:918–931. doi: 10.1039/b604306m. [DOI] [PubMed] [Google Scholar]
- 2.Iota V, Yoo CS, Cynn H. Quartzlike carbon dioxide: An optically nonlinear extended solid at high pressures and temperatures. Science. 1999;283:1510–1513. doi: 10.1126/science.283.5407.1510. [DOI] [PubMed] [Google Scholar]
- 3.Yoo CS, et al. Crystal structure of carbon dioxide at high pressure: “Superhard” polymeric carbon dioxide. Phys Rev Lett. 1999;83:5527–5530. [Google Scholar]
- 4.Serra S, Cavazzoni C, Chiarotti GL, Scandolo S, Tosatti E. Pressure-induced solid carbonates from molecular CO2 by computer simulation. Science. 1999;284:788–790. doi: 10.1126/science.284.5415.788. [DOI] [PubMed] [Google Scholar]
- 5.Dong J, Tomfohr JK, Sankey OF. Non-molecular carbon dioxide (CO2) solids. Science. 2000;287:11. [Google Scholar]
- 6.Dong J, Tomfohr JK, Sankey OF. Rigid intertetrahedron angular interaction of nonmolecular carbon dioxide solids. Phys Rev B Condens Matter Mater Phys. 2000;61:5967–5971. [Google Scholar]
- 7.Dong J, et al. Investigation of hardness in tetrahedrally bonded nonmolecular CO2 solids by density-functional theory. Phys Rev B Condens Matter Mater Phys. 2000;62:14685–14689. [Google Scholar]
- 8.Holm B, Ahuja R, Belonoshko A, Johansson B. Theoretical investigation of high pressure phases of carbon dioxide. Phys Rev Lett. 2000;85:1258–1261. doi: 10.1103/PhysRevLett.85.1258. [DOI] [PubMed] [Google Scholar]
- 9.Tschauner O, Mao HK, Hemley RJ. New transformations of CO2 at high pressures and temperatures. Phys Rev Lett. 2001;87:075701. doi: 10.1103/PhysRevLett.87.075701. [DOI] [PubMed] [Google Scholar]
- 10.Santoro M, Lin JF, Mao HK, Hemley RJ. In situ high P-T Raman spectroscopy and laser heating of carbon dioxide. J Chem Phys. 2004;121:2780–2787. doi: 10.1063/1.1758936. [DOI] [PubMed] [Google Scholar]
- 11.Santoro M, et al. Amorphous silica-like carbon dioxide. Nature. 2006;441:857–860. doi: 10.1038/nature04879. [DOI] [PubMed] [Google Scholar]
- 12.Iota V, et al. Six-fold coordinated carbon dioxide VI. Nat Mater. 2007;6:34–38. doi: 10.1038/nmat1800. [DOI] [PubMed] [Google Scholar]
- 13.Montoya JA, Rousseau R, Santoro M, Gorelli F, Scandolo S. Mixed threefold and fourfold carbon coordination in compressed CO2. Phys Rev Lett. 2008;100:163002. doi: 10.1103/PhysRevLett.100.163002. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, et al. High-pressure polymeric phases of carbon dioxide. Proc Natl Acad Sci USA. 2009;106:6077–6081. doi: 10.1073/pnas.0812624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haines J, et al. Topologically ordered amorphous silica obtained from the collapsed siliceous zeolite, silicalite-1-F: A step forward “perfect glasses”. J Am Chem Soc. 2009;131:12333–12338. doi: 10.1021/ja904054v. [DOI] [PubMed] [Google Scholar]
- 16.Haines J, et al. Deactivation of pressure induced amorphization in silicalite SiO2 by insertion of guest species. J Am Chem Soc. 2010;132:8860–8861. doi: 10.1021/ja1034599. [DOI] [PubMed] [Google Scholar]
- 17.Aravindh A, et al. SixC1-xO2 alloys: A possible route to stabilize carbon-based silica-like solids? Solid State Commun. 2007;144:273–276. [Google Scholar]
- 18.Santoro M, Gorelli FA. Constraints on the phase diagram of nonmolecular CO2 imposed by infrared spectroscopy. Phys Rev B Condens Matter Mater Phys. 2009;80:184109. [Google Scholar]
- 19.Gorelli FA, Giordano V, Salvi PR, Bini R. Linear carbon dioxide in the high-pressure high-temperature crystalline phase IV. Phys Rev Lett. 2004;93:205503. doi: 10.1103/PhysRevLett.93.205503. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith JA, Ross SD. Factors affecting the infra-red spectra of planar anions with D3h symmetry-IV The vibrational spectra of some complex carbonates in the region 4000 - 400 cm-1. Spectrochim Acta. 1968;24A:993–998. [Google Scholar]
- 21.Fukada Y, Tanabe K. Infrared study of carbon dioxide adsorbed on magnesium and calcium oxides. Bull Chem Soc Jpn. 1972;46:1616–1619. [Google Scholar]
- 22.Gensse G, Anderson TF, Friplat JJ. Study of oxygen mobility in some synthetic faujasites by isotopic exchange with CO2. J Phys Chem. 1980;84:3562–3567. [Google Scholar]
- 23.Philipp R, Fujimoto K. FTIR study of CO2 Adsorption/desorption on MgO/CaO catalysts. J Phys Chem. 1992;96:9035–9038. [Google Scholar]
- 24.Lavalley JC. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catalysis Today. 1996;27:377–401. [Google Scholar]
- 25.Yagi F, Tsuji H, Hattori H. IR and TPD (temperature-programmed desorption) studies of carbon dioxide on basic site active for 1-butene isomerization on alkali added zeolites X. Microporous Mater. 1997;9:237–245. [Google Scholar]
- 26.Di Cosimo JI, Diez VK, Xu M, Iglesia E, Apesteguia CR. Structure and surface and catalytic properties of Mg-Al basic oxides. J Catalysis. 1998;178:499–510. [Google Scholar]
- 27.Doskocil EJ, Davis RJ. Spectroscopic characterization and catalityc activity of zeolite X containing occluded alkali species. J Catalysis. 1999;188:353–364. [Google Scholar]
- 28.Brooker RA, Kohn SC, Holloway JR, McMillan PF, Carroll MR. Solubility, speciation and dissolution mechanisms for CO2 in melts on the NaAlO2 - SiO2 join. Geochim Cosmochim Acta. 1999;63:3549–3565. [Google Scholar]
- 29.Brooker RA, Kohn SC, Holloway JR, McMillan PF. Structural controls on the solubility of CO2 in silicate melts Part II: IR characteristics of carbonate groups in silicate glasses. Chem Geol. 2001;174:241–254. [Google Scholar]
- 30.Kubicki JD, Stolper EM. Structural roles of CO2 and [CO3]2- in fully polymerized sodium aluminosilicate melts and glasses. Geochim Cosmochim Acta. 1995;59:683–698. [Google Scholar]
- 31.Stishov SM, Popova SV. A new dense modification of silica. Geochemistry (USSR) 1961;10:923–926. [Google Scholar]
- 32.Yamakata M, Yagi T. New polymorph of SiO2 formed under quasi-hydrostatic compression of cristobalite. Rev High Pressure Sci Technol. 1998;7:107–109. [Google Scholar]
- 33.Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Häusermann D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press Res. 1996;14:235–248. [Google Scholar]