Abstract
Stem cell antigen (Sca)-1/Ly6A, a glycerophosphatidylinositol-linked surface protein, was found to be associated with murine stem cell– and progenitor cell–enriched populations, and also has been linked to the capacity of tumor-initiating cells. Despite these interesting associations, this protein's functional role in these processes remains largely unknown. To identify the mechanism underlying the protein's possible role in mammary tumorigenesis, Sca-1 expression was examined in Sca-1+/EGFP mice during carcinogenesis. Mammary tumor cells derived from these mice readily engrafted in syngeneic mice, and tumor growth was markedly inhibited on down-regulation of Sca-1 expression. The latter effect was associated with significantly elevated expression of the TGF-β ligand growth differentiation factor-10 (GDF10), which was found to selectively activate TGF-β receptor (TβRI/II)-dependent Smad3 phosphorylation. Overexpression of GDF10 attenuated tumor formation; conversely, silencing of GDF10 expression reversed these effects. Sca-1 attenuated GDF10-dependent TGF-β signaling by disrupting the heterodimerization of TβRI and TβRII receptors. These findings suggest a new functional role for Sca-1 in maintaining tumorigenicity, in part by acting as a potent suppressor of TGF-β signaling.
Stem cell antigen (Sca)-1 is a member of the Ly6A superfamily of glycerophosphatidylinositol (GPI)-anchored membrane proteins (1), which is associated with murine stem and progenitor cell populations in several tissues. In the mammary gland, Sca-1–positive cells are able to reconstitute the cleared fat pad (2, 3) and give rise to alveolar and ductal structures (4). In addition to Sca-1’s normal role in stem cell self-renewal, Sca-1 expression is elevated in malignant tissues, such as retinoblastomas (5), prostate tumors (6), mammary tumors (7, 8) and chronic myeloid leukemia (9), which generally reflects a more aggressive phenotype (10). Despite these associations, the role of Sca-1 in these processes remains largely unknown. To address this question, we used Sca-1+/EGFP mice, in which EGFP is under the control of the Sca-1 locus (11), to study mammary tumorigenesis. We found that Sca-1 suppresses TGF-β signaling by inhibiting expression of the TGF-β family ligand GDF10 and binding to the type I TGF-β receptor (TβRI), thereby inhibiting ligand-induced TGF-β receptor complex formation and Smad3 phosphorylation. This study is the first to demonstrate specific functions for Sca-1 and GDF10 in tumorigenesis.
Results
Sca-1 Is Increased Early in Mammary Tumorigenesis.
To explore the role of Sca-1 in tumorigenesis, we induced mammary tumors in heterozygous Sca-1+/EGFP mice (11) using medroxyprogesterone (MPA) and 7,12-dimethylbenz[a]anthracene (DMBA) (8, 12, 13). Cultures of primary mammary epithelial cells exhibited enhanced EGFP fluorescence coincident with increased Sca-1 expression immediately after treatment with MPA and DMBA ((Fig. S1 A and B ), suggesting that the Sca-1 locus is activated at the onset of carcinogenesis. Both Sca-1+/EGFP and WT mice developed tumors within 2 mo of MPA/DMBA treatment, with no significant difference in the incidence of tumor formation (Fig. S1C). Primary cultures derived from mammary tumors showed a fourfold higher level of Sca-1-expressing cells than cultures from normal mammary gland (Fig. S1D). To explore the role of Sca-1, we established a tumor cell population, designated 34T, from an adenocarcinoma induced in Sca-1+/EGFP mice. We used these 34T cells, which were >80% positive for Sca-1 (Fig. S1E), to examine the signaling pathways regulated by Sca-1.
Attenuation of Sca-1 Expression Reduces Cell Growth and Tumorigenicity.
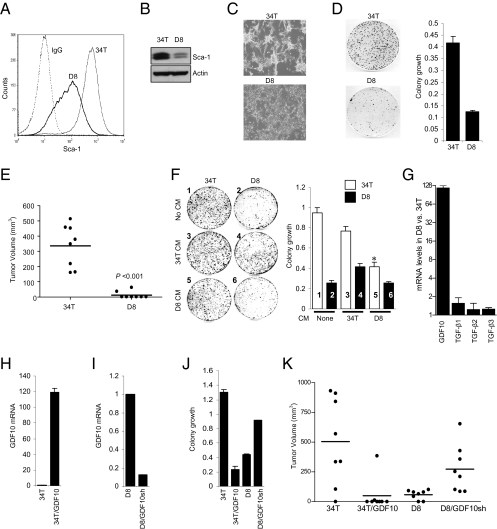
To examine whether Sca-1 plays a tumorigenic role, we transduced 34T cells with a lentivirus expressing either GFP shRNA as a control or Sca-1 shRNA to generate a stable cell population with reduced Sca-1 expression (Fig. S2). 34T cells ex-pressing Sca-1 shRNA D8 (D8 cells) exhibited a 90% reduction of Sca-1 protein expression compared with 34T/GFP shRNA cells (34T cells) (Fig. 1 A and B). 34T cells grew as clusters with a spheroid morphology, whereas D8 cells lost the spheroid morphology (Fig. 1C) and displayed reduced anchorage-independent colony formation (Fig. 1D). To test the tumorigenic potential of Sca-1, we engrafted syngeneic mice with 34T and D8 cells. D8 cells either failed to develop tumors or grew as small tumors, whereas 34T cells formed large tumors after 25 d (Fig. 1E).
Fig. 1.
Silencing Sca-1 expression decreases colony formation and tumorigenic potential, and increases GDF10 expression. 34T cells were transduced with a lentivirus expressing either GFP shRNA as a control or a Sca-1 shRNA (Fig. S2). D8 cells expressing Sca-1 shRNA cells exhibited a 90% reduction in Sca-1 expression compared with control 34T cells, as assessed by FACS analysis (A) or Western blot analysis (B). (C) Down-regulation of Sca-1 expression reduces spheroid cluster morphology. 34T cells grew as spheroid clusters, whereas D8 cells grew as a flattened monolayer with contact-inhibited growth. (D) Silencing Sca-1 expression reduces colony formation. Colony-formation assay in D8 cells vs. 34T cells showed that growth was reduced by 70% in D8 cells vs. 34T cells. (E) Down-regulation of Sca-1 expression markedly reduces tumorigenicity. 34T cells and D8 cells were implanted s.c. at an inoculum of 100,000 cells into opposite flanks of eight syngeneic C57BL/6 mice. Tumor formation was monitored over 25 d, and tumor volume was calculated. Isografts from D8 cells exhibited little growth compared with isografts from 34T cells (P < 0.001, two-tailed Student t test). (F) Conditioned medium (CM) from 34T cells enhanced colony formation of D8 cells, whereas conditioned medium from D8 cells markedly reduced colony formation of 34T cells. The bar graph shows quantification of colony growth; the number in each bar corresponds to the plate number in the left panel. (G) Real-time qPCR analysis of TGF-β ligands. The fold increase in expression of each ligand in D8 cells relative to 34T cells is shown. D8 cells expressed >100-fold higher levels of GDF10 mRNA compared with 34T cells. (H) 34T/GFP shRNA cells stably expressing GDF10 (34T/GDF10) exhibit a >100-fold increase in GDF10 mRNA expression compared with control cells (34T). (I) D8 cells transfected with a GDF10 shRNA (D8/GDF10sh) show 90% reduced GDF10 mRNA expression compared with cells expressing a control shRNA (D8). (J) Modulation of GDF10 expression results in changes in colony formation. Expression of GDF10 in 34T cells (34T/GDF10) reduced colony formation by 80%, whereas silencing GDF10 expression in D8 cells (D8/GDF10sh) increased colony formation. (K) GDF10 modulates tumorigenicity. Cells were implanted into eight syngeneic C57BL/6 mice, and tumor growth was assessed as in E. Tumor volume was reduced in isografts of 34T/GDF10 cells compared with tumors from 34T cells (P < 0.001, two-tailed Student t test). In contrast, D8/GDF10sh cells showed increased tumor volume compared with D8 cells (P < 0.001, two-tailed Student t test).
Sca-1 Controls Cell Behavior and Tumorigenicity Through Inhibition of GDF10 Expression.
To determine whether the Sca-1–associated changes in growth is related to secreted growth inhibitory factors, we assessed colony formation in the presence of conditioned media from 34T and D8 cells. Conditioned medium from control 34T cells partially restored the growth of D8 cells, whereas the medium from D8 cells inhibited the growth of 34T cells (Fig.1F). To further assess the phenotypic changes associated with Sca-1 expression, we carried out gene microarray analyses in both cell populations. Among the 57 expressed genes that exhibited a threefold or greater change in D8 cells, the TGF-β ligand GDF10 exhibited the largest change (Fig. S3 and Table S1). Because several TGF-β ligands are known to inhibit cell proliferation, the expression of additional TGF-β ligands was determined in 34T and D8 cells (Fig. 1G). Only GDF10 expression was significantly increased in D8 cells; no changes in TGF-β1, TGF-β2, and TGF-β3 expression were seen.
We next evaluated the role of GDF10 in tumor cell growth by generating 34T cells that stably express GDF10 (Fig. 1H) and D8 cells that express a GDF10 shRNA and thus have reduced GDF10 expression (Fig. 1I). Increased GDF10 expression in 34T/GDF10 cells resulted in reduced colony formation, whereas reducing GDF10 expression enhanced the colony formation of D8 cells (Fig. 1J and Fig. S4A). In addition, increasing GDF10 expression reduced the spheroid morphology and restored the contact inhibition of 34T cells, whereas down-regulation of GDF10 expression had an opposite effect in D8 cells (Fig. S4B). We then evaluated the tumorigenic properties of these cells in isograft experiments in syngeneic mice (Fig. 1K). 34T cells formed large tumors after 25 d (Fig. 1E), whereas 34T/GDF10 cells formed considerably smaller tumors. Conversely, D8 cells failed to grow as isografts (Fig. 1E), but decreasing GDF10 expression conferred tumorigenicity (Fig. 1K). Thus, Sca-1 regulates tumorigenicity in part by repressing GDF10 expression.
Sca-1 and GDF10 Regulate Signaling Through Smad3.
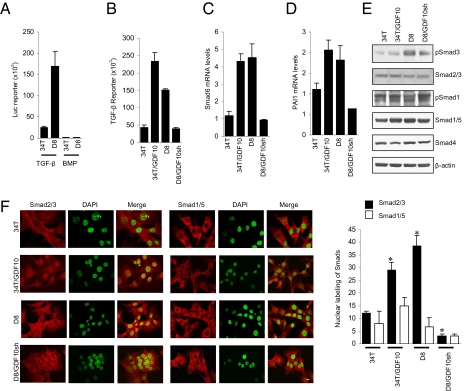
GDF10 was previously identified as a TGF-β ligand related to BMP-3 (14), although there is little information on its mechanism of action. Among several TGF-β family ligands, TGF-βs and activins signal primarily through activation of Smad2 and Smad3, whereas BMPs act through Smad1 and 5 (15, 16). To determine the effect of increased GDF10 expression in D8 cells, we compared the autocrine Smad signaling in 34T and D8 cells using reporter assays specific for Smad3 (TGF-β) and Smad1/5 (BMP) signaling (Fig. 2A). The Smad3 reporter activity was strongly increased in D8 cells, in contrast to the low and unchanged Smad1/5 reporter activity, demonstrating a correlation of decreased Sca-1 expression and increased GDF10 expression with increased Smad3 activity. In addition, GDF-10 overexpression in 34T cells increased TGF-β reporter activity, and silencing GDF10 expression in D8 cells produced the opposite effect (Fig. 2B). This outcome is consistent with increased expression of the TGF-β target genes Smad6 (Fig. 2C) and PAI-1 (Fig. 2D) in 34T/GDF10 cells and decreased expression of these genes when GDF10 shRNA was introduced in D8 cells. These results indicate a correlation of GDF10 expression and signaling with activation of Smad3 signaling. To verify this notion, we examined the Smad3 activation in 34T and D8 cells (Fig. 2E). We found a much higher level of C-terminal Smad3 phosphorylation in the D8 cells than in the 34T cells, consistent with the significant increase in GDF10 production. Furthermore, the phosphoSmad3 level in 34T cells was increased by GDF10 overexpression, and the high level of phosphoSmad3 in D8 cells was reduced on down-regulation of GDF10 expression. These results are correlated with the extent of nuclear localization of Smad2/3 and Smad1/5 in 34T, 34T/GDF10, D8 and D8/GDF10sh cells (Fig. 2F).
Fig. 2.
Silencing Sca-1 expression activates GDF10-dependent signaling through Smad3. (A) D8 cells exhibit increased Smad3 (TGF-β), but not Smad1/5 (BMP) reporter activity. D8 cells expressed a >20-fold increase in Smad3 activity compared with 34T cells. (B) GDF10 expression increases Smad3 activity. Reporter activity was increased in GDF10-overexpressing 34T cells (34T/GDF10), whereas silencing GDF10 expression in D8 cells (D8/GDF10sh) reduced the activity. (C and D) Expression of the TGF-β target genes Smad6 (C) and PAI1 (D) is regulated by GDF10 expression. Both Smad6 and PAI1 expression were increased by overexpression of GDF10 in 34T cells and decreased by down-regulation of GDF10 expression in D8 cells. (E) GDF10 expression regulates Smad3 activation, as assessed by immunoblotting for phosphoSmad3 (pSmad3). The levels of Smads and pSmads were determined by Western blot analysis. pSmad3 was increased in 34T/GDF10 cell, compared with 34T cells and was reduced in D8/GDF10sh cells compared with D8 cells. No difference in pSmad1/5 was observed. (F) GDF10 expression correlates with Smad2/3 nuclear localization. Fluorescence was measured by confocal microscopy using a Smad2/3 or Smad1/5 antibody and an Alexa Fluor 594-conjugated secondary antibody; nuclei were stained with DAPI and pseudo-colored in green. The merged image shows nuclear localization of Smad2/3 in 34T/GDF10 and D8 cells, but not in 34T and D8/GDF10sh cells. The bar graph quantifies Smad2/3 and Smad1/5 nuclear localization. *P < 0.01, Student two-tailed t test. (Scale bar: 10 μm.)
GDF10 Activates Smad3 Signaling via TβRI and TβRII.
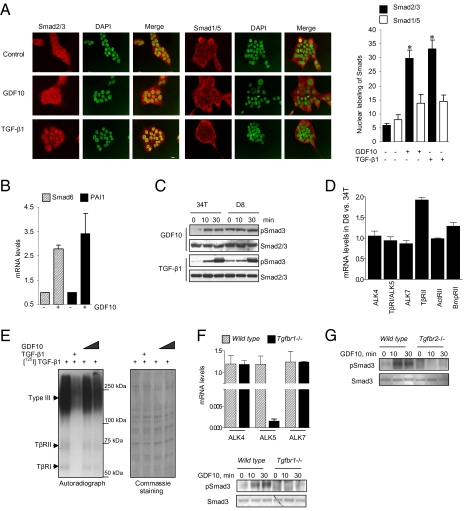
To determine whether GDF10 directly activates Smad3 similar to TGF-β, we treated NMuMG cells with recombinant GDF10 or TGF-β1 (Fig. 3A). Both GDF10 and TGF-β1 induced the nuclear localization of Smad2/3 but not of Smad1/5, suggesting that they act through a common receptor–Smad pathway. Accordingly, GDF10 induced the expression of the TGF-β target genes Smad6 and PAI-1 (Fig. 3B), consistent with our results using 34T or D8 cells with increased or decreased GDF10 expression (Fig. 2 C and D). In addition, GDF10 induced Smad3 phosphorylation in 34T cells as rapidly as TGF-β1, although at 30 min, TGF-β1 induced a higher level of Smad3 activation than GDF10 (Fig. 3C). Adding GDF10 to D8 cells, which express high levels of GDF10, enhanced the constitutive activation of Smad3 only minimally; however, this was further enhanced in response to TGF-β1 (Fig. 3C). Thus, although both GDF10 and TGF-β activate Smad3, TGF-β1 is more potent in activating Smad3 compared with GDF10.
Fig. 3.
GDF10 activates Smad2/3 signaling through the TGF-β pathway. (A) GDF10 increases Smad3 nuclear localization. Treatment of NMuMG cells for 30 min with 50 ng/mL of GDF10 increased nuclear localization of Smad2/3. Treatment with 5 ng/mL of TGF-β1 served as a positive control. The bar graph quantitates nuclear Smad2/3 and Smad1/5. (Scale bar: 10 μm.) (B) GDF10 induces Smad6 and PAI1 mRNA expression. NMuMG cells treated overnight with GDF10 and TGF-β as in A expressed increased levels of mRNAs for the TGF-β target genes Smad6 and PAI1, as determined by real-time qPCR. (C) GDF10 treatment activates Smad3. 34T and D8 cells were treated with either GDF10 or TGF-β1, and pSmad3 levels were determined by Western blot analysis. pSmad3 was increased at 10 min in 34T cells to a greater extent than in D8 cells due to the higher basal level in the latter cells; pSmad3 at 30 min was equivalent in both cell lines. TGF-β1 increased pSmad3 to a greater extent than GDF10 in both 34T and D8 cells. (D) TGF-β receptor expression in 34T and D8 cells. Receptor mRNA was quantified by real-time qPCR. Only TβRII mRNA was significantly different in D8 and 34T cells (P < 0.05, two-tailed Student t test). (E) GDF10 competes with TGF-β1 for receptor binding. Mv1Lu cells were surface-labeled with 5 ng/mL of [125I]TGF-β1 and competed with either 100 ng/mL of unlabeled TGF-β1 or 100–200 ng/mL of unlabeled GDF10. Subsequent chemical cross-linking of [125I]TGF-β1 to its receptors, SDS/PAGE, and autoradiography indicate that GDF10 competes with TGF-β1 for binding to TβRI (55 kDa), TβRII (70 kDa), and betaglycan (type III receptor). Competition with 100 ng/mL of cold TGF-β served as a positive control. (Right) An identical gel was stained with Coomassie blue to determine protein loading. (F) GDF10 activation of Smad3 is TβRI-dependent. Expression of type I TGF-β receptor mRNAs in WT and Tgfbr1−/− MEFs was quantified by real-time qPCR and confirmed the expression of ALK4 and ALK7 and loss of TβRI/ALK5 in Tgfbr1−/− MEFs. (Right) Treatment with 50 ng/mL GDF10 increased pSmad3 levels in WT, but not Tgfbr1−/−, MEFs. (G) GDF10 activation of Smad3 is TβRII-dependent. pSmad3 was quantified by Western blot analysis in WT and Tgfbr2−/− MEFs. Treatment with 50 ng/mL of GDF10 increased pSmad3 in WT, but not Tgfbr2−/−, MEFs.
As assessed by real-time qPCR, 34T and D8 cells expressed the ALK4, TβRI/ALK5, and ALK7 type I receptors, which all are able to activate Smad2 and Smad3, as well as the TβRII, ActRII, and BMPRII type II receptors (Fig. 3D). These receptors were expressed at similar levels with the exception of TβRII, which was increased twofold in D8 cells. To determine whether GDF10 used the same type I and type II receptors as TGF-β1 (i.e., TβRII and TβRI), we carried out [125I]TGF-β receptor binding assays of intact cells (17–19) using unlabeled GDF10 as a competitor (Fig. 3E). [125I]TGF-β cross-linking visualized the abundant betaglycan (type III TGF-β receptor), as well as the 70-kDa TβRII and 53-kDa TβRI. Unlabeled TGF-β1 and GDF10 were effective at competing with [125I]TGF-β binding to betaglycan, TβRII, and TβRI, although TGF-β1 was more effective in competing than GDF10. We next compared the effects of GDF10 and TGF-β1 in WT versus Tgfbr1−/− mouse embryo fibroblasts (MEFs). Both had equal expression of ALK4 and ALK7, whereas the latter specifically lacked the expression of TβRI (Fig. 3F, Upper). Treatment of WT, but not Tgfbr1−/− cells, with GDF10 resulted in a time-dependent increase in pSmad3 (Fig. 3F, Lower), indicating that GDF10 signals through TβRI. In addition, GDF10 was unable to induce Smad3 activation in MEFs with inactivated expression of TβRII (Fig. 3G), indicating the requirement for TβRII. Taken together, our results indicate that GDF10 acts through the same TβRII/TβRI receptor complex as TGF-β to activate Smad3, and that increased GDF10 expression as a result of down-regulation of Sca-1 expression results in autocrine activation of TβRI/II signaling.
Sca-1 Associates with TβRI to Inhibit Smad3 Signaling.
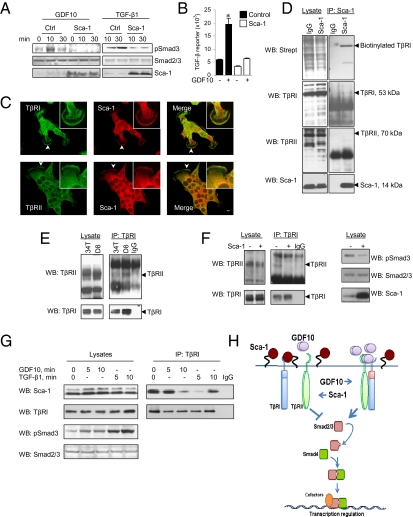
To evaluate the direct effect of Sca-1 on ligand-induced Smad3 activation, we treated WT and Sca-1–overexpressing NMuMG cells with GDF10 or TGF-β1. Both ligands induced Smad3 activation in control cells, and Sca-1 abrogated this effect (Fig. 4A). This inhibition also was reflected in the activation of Smad3-mediated transcription in response to GDF10 (Fig. 4B). Because Sca-1 is a GPI-anchored cell surface protein, we evaluated its colocalization with TβRI and TβRII in 34T cells by confocal microscopy (Fig. 4C). Sca-1 colocalized with TβRI and TβRII at the plasma membrane (Fig. 4C, Insets). We next examined whether Sca-1 interacted with TβRI and TβRII at the cell surface. 34T cells were labeled with membrane-impermeable biotin and cross-linked, and Sca-1 was immunoprecipitated. Immunoblotting revealed that Sca-1 was associated with biotinylated TβRI, but not with TβRII (Fig. 4D).
Fig. 4.
Sca-1 interacts with cell surface TGF-β receptors to inhibit Smad3 activation. (A and B) NMuMG cells were transfected with either a Sca-1 or control plasmid. Treatment of cells with either 50 ng/mL of GDF10 or 5 ng/mL of TGF-β1 resulted in increased pSmad3 (A) and transcription from a Smad3-responsive (TGF-β) reporter (B) in control cells, but not in Sca-1-expressing cells. (C) Sca-1 colocalizes with TβRI and TβRII in 34T cells. Fluorescence was visualized by confocal microscopy using either a TβRI or a TβRII mAb and an Alexa Fluor 488-conjugated secondary antibody or an Sca-1 mAb and an Alexa Fluor 594-conjugated secondary antibody. The merged images indicate that Sca-1 colocalizes with TβRI (Upper) and TβRII (Lower). Arrowheads indicate the cell membrane regions magnified in the insets. (Scale bar: 10 μm.) (D) Cell surface TβRI, but not TβRII, associates with Sca-1. Cell surface proteins in 34T cells were labeled with a membrane-impermeable biotin conjugate, and Sca-1–interacting proteins were isolated by immunoprecipitation with an Sca-1 antibody or IgG isotype control. Immunoprecipitated proteins were analyzed by Western blot using either a streptavidin-conjugated antibody for biotinylated proteins or TβRI, TβRII, and Sca-1 antibodies. Sca-1 associated with a biotinylated band of the same mobility as TβRI, but not with TβRII. (E) Silencing of Sca-1 expression facilitates TβRI and TβRII receptor complex formation. TβRI was immunoprecipitated from lysates of 34T and D8 cells, and associated TβRII was detected by Western blot analysis. D8 cells exhibited greater association of TβRI with TβRII compared with 34T cells. (F) Sca-1 reduces TβRI and TβRII receptor complex formation and Smad3 activation. D8 cells were transfected with a Sca-1 or control plasmid and cell lysates were immunoprecipitated with a TβRI antibody, and TβRI and TβRII were detected by Western blot analysis. (Left) Overexpression of Sca-1 inhibited the association of TβRI with TβRII. (Right) It also reduced pSmad3 levels. (G) Association kinetics of Sca-1 with TβRI. 34T cells were treated with either 50 ng/mL of GDF10 or 5 ng/mL of TGF-β1, and TβRI was immunoprecipitated. Sca-1, TβRI, pSmad3, and Smad2/3 were detected by Western blot analysis. Both GDF10 and TGFβ1 increased pSmad3, although phosphorylation was greater in the presence of TGFβ1. Sca-1 was found to be associated with TβRI in the absence of ligand, which was reduced after treatment with GDF10 or TGF-β1, whereas dissociation occurred more rapidly in the presence of TGF-β1. (H) Schematic representation of the regulation of TGF-β signaling by Sca-1. Sca-1 is depicted as interacting with TβRI to interfere with TβRI–TβRII complex formation and the subsequent activation of Smad3. When Sca-1 expression is reduced, GDF10 expression is increased, leading to stabilization of the receptor complex and Smad3 activation.
The association of Sca-1 with TβRI at the cell surface raised the possibility that Sca-1 could prevent ligand-induced TβRI and TβRII complex formation, which would explain the inhibition of ligand-induced Smad3 activation (Fig. 4 A and B). This implies that D8 cells, with their down-regulated Sca-1 expression and high GDF10 expression, would have a higher level of receptor complex than 34T cells, and indeed this was the case (Fig. 4E). In addition, ectopic overexpression of Sca-1 in D8 cells disrupted the TβRI–TβRII receptor complex in parallel with reduced Smad3 activation (Fig. 4F). Finally, adding recombinant GDF10 or TGF-β1 impaired the association between Sca-1 and TβRI (Fig. 4G), suggesting that this is a dynamic interaction regulated by ligand. This result suggests that the level of activation of TGF-β signals may depend on the relative abundance of GDF10 and Sca-1, as well as on a possible competition between GDF10 and Sca1 for receptor binding.
Discussion
Sca-1 is generally considered to be a stem cell marker of normal tissues, where Sca-1–positive cells have been shown to have a regenerative capacity (3, 20–26), despite the fact that its function is poorly described. In this study, we have defined a role for Sca-1 in tumor progression based on three key findings. First, Sca-1 was necessary for maintaining the tumorigenicity of mammary tumor cells. Its tumor-promoting role is associated with inhibited expression of GDF10, a minimally characterized TGF-β family ligand that acts as a tumor suppressor in our tumor isograft assays. Second, GDF10 acted through TβRI and TβRII and conferred Smad3 activation similarly to, but weaker than, TGF-β1. GDF10 was previously linked to the BMP-like action of osteoblast differentiation (27, 28), but the specific receptor complex associated with GDF10 had not been identified. The ability of GDF10 to act as a tumor suppressor thus resembles the well-documented role of TGF-β signaling as a tumor-suppressor pathway through TβRI and TβRII and Smads early in tumor development (29–31). Third, Sca-1 interacted with TβRI at the cell surface to attenuate ligand binding to the TβRI/TβRII receptor complex and ligand-activated Smad3 signaling, thus providing a complementary mechanism to inhibit tumor suppression by TGF-β or GDF10 signaling.
These findings support a model in which Sca-1 counters the inhibitory role of TGF-β signaling in mammary gland and tumor cells (Fig. 4H). Tumor growth suppression by Sca-1 occurs through an autocrine mechanism, as is apparent from the marked suppression of colony formation by conditioned medium from tumor cells with reduced Sca-1 expression. Furthermore, the expression of Sca-1 early in the tumorigenic process suggests a primary role in tumor-initiating cells, as demonstrated by the marked reduction of tumor growth in Sca-1 knockdown cells. The role of Sca-1 in tumorigenesis may be especially relevant to our understanding of the link between Sca-1 expression and tumor formation (7, 9, 32) and metastatic behavior (6, 10, 33), as well as to the radiation resistance of Sca-1–positive mammary epithelial progenitor cells (34, 35). In relation to our findings, expression of dominant-negative TβRII was found to increase Sca-1 expression in mammary epithelial cells (36), although no mechanism for this effect was presented. Although, Sca-1 is murine-specific, the Ly6 gene family has been associated with the 8q24.3 amplicon in breast cancer (37–39), and expression of the homologs Ly6D and Ly6K has been linked to basal cell and metastatic breast cancer (37–39), as well as to head and neck cancer (40).
The finding that Sca-1 promotes tumor growth through suppression of GDF10-dependent TGF-β signaling may be particularly relevant to breast cancer. TGF-β signaling through TβRI and TβRII, and then through Smad2 and Smad3 as intracellular effectors (16, 41, 42), is known to exert an inhibitory effect on mammary gland development and tumorigenesis. Increased expression of activated TGF-β1 in mammary epithelial cells of MMTV-TGF-β1 transgenic mice results in impaired ductal elongation and involution, and disappearance of stem cells in the terminal buds (43). These mice are resistant to carcinogenesis and exhibit markedly suppressed tumor formation when crossed with MMTV-TGFα mice (44). Conversely, conditional loss of TGF-β1 expression in MMTV-PymT mice accelerates tumor formation and metastasis (45). However, it should be noted that after inactivation of TGF-β–induced growth inhibitory signaling, increased TGF-β production and autocrine signaling help promote cancer progression through their effects on the microenvironment and their ability to promote epithelial plasticity, which results in invasion and metastasis (29–31). In summary, we conclude that Sca-1 maintains the growth and invasive characteristics of tumor cells in part by suppressing the expression of GDF10 and in part by attenuating the accessibility of the TGF-β receptor complex for ligand binding, both of which result in inhibition of TGF-β signaling through GDF10.
Materials and Methods
More detailed information is presented in SI Materials and Methods.
Reagents and Plasmids.
GDF10 was obtained from R&D Systems, TGF-β1 was obtained from Sigma-Aldrich, and [125I]TGF-β1 was obtained from Perkin-Elmer. The Smad3-responsive TGF-β (46) and Smad1/5-responsive BMP (47) reporter plasmids were provided by Dr. Peter ten Dijke, Leiden University Medical Center, Leiden, The Netherlands.
shRNAs and Cell Lines.
Five Sca-1 shRNAs cloned into pLKO.1 were obtained from Open Biosystems and screened for activity in Sca-1–expressing 293T cells; GFP shRNA was used as a control. shRNA D8 containing the sense sequence GAA-CAA-TCT-TTG-CTT-ACC-CAT produced the greatest reduction in Sca-1 protein level (Fig. S2) and was used for further studies. Lentivirus was produced in 293T cells by cotransfection of the pMD2.g and VSVG vectors together with the GFP shRNA or Sca-1 shRNA construct. At 24 h after transfection, the medium was replaced and virus was collected. 34T cells were infected with lentivirus for 24 h in the presence of 4 mg/mL of polybrene, the medium was changed after 24 h, and selection carried out with 2 μg/mL of puromycin. Cell lines were maintained thereafter in media containing 2 μg/mL of puromycin. 34T cells were transfected with pcDNA3.1-GDF10 and selected in 1 mg/mL of G418. D8 cells were transfected with a GDF10 shRNA plasmid or a control shRNA plasmid, obtained from SA Biosciences, and cells were selected with 1 mg/mL of G418, as well as 2 μg/mL of puromycin.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 CA111482 and Contract N01 CN43309 (to R.I.G.) and Grant RO1 CA136690 (to R.D.). This investigation was conducted using the Animal Research, Flow Cytometry, Macromolecular Analysis, and Microscopy and Imaging Shared Resources supported by National Center for Research Facilities Research Facilities Improvement Grant C06 RR14567, National Cancer Institute Cancer Center Support Grant 1P30-CA-51008, and National Institutes of Health Shared Instrumentation Grant 1 S10 RR019291-01A2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103441108/-/DCSupplemental.
References
- 1.Holmes C, Stanford WL. Concise review. Stem cell antigen-1: Expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 2.Welm B, Behbod F, Goodell MA, Rosen JM. Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 2003;36(Suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welm BE, et al. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 4.Deugnier MA, et al. Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dβ cell line. Dev Biol. 2006;293:414–425. doi: 10.1016/j.ydbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–832. [PMC free article] [PubMed] [Google Scholar]
- 6.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Y, Yuan H, Zeng X, Kopelovich L, Glazer RI. Inhibition of peroxisome proliferator–activated receptor gamma increases estrogen receptor–dependent tumor specification. Cancer Res. 2009;69:687–694. doi: 10.1158/0008-5472.CAN-08-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Caro M, et al. Cancer induction by restriction of oncogene expression to the stem cell compartment. EMBO J. 2009;28:8–20. doi: 10.1038/emboj.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treister A, et al. Expression of Ly-6, a marker for highly malignant murine tumor cells, is regulated by growth conditions and stress. Int J Cancer. 1998;77:306–313. doi: 10.1002/(sici)1097-0215(19980717)77:2<306::aid-ijc22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Hanson P, Mathews V, Marrus SH, Graubert TA. Enhanced green fluorescent protein targeted to the Sca-1 (Ly-6A) locus in transgenic mice results in efficient marking of hematopoietic stem cells in vivo. Exp Hematol. 2003;31:159–167. doi: 10.1016/s0301-472x(02)01021-4. [DOI] [PubMed] [Google Scholar]
- 12.Yin Y, et al. Characterization of medroxyprogesterone and DMBA-induced multilineage mammary tumors by gene expression profiling. Mol Carcinog. 2005;44:42–50. doi: 10.1002/mc.20119. [DOI] [PubMed] [Google Scholar]
- 13.Yin Y, et al. Peroxisome proliferator–activated receptor δ and γ agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005;65:3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham NS, et al. Growth/differentiation factor-10: A new member of the transforming growth factor-β superfamily related to bone morphogenetic protein-3. Growth Factors. 1995;12:99–109. doi: 10.3109/08977199509028956. [DOI] [PubMed] [Google Scholar]
- 15.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 2009;19:385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: Molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 17.Ebner R, Chen RH, Lawler S, Zioncheck T, Derynck R. Determination of type I receptor specificity by the type II receptors for TGF-β or activin. Science. 1993;262:900–902. doi: 10.1126/science.8235612. [DOI] [PubMed] [Google Scholar]
- 18.Ebner R, et al. Cloning of a type I TGF-β receptor and its effect on TGF-β binding to the type II receptor. Science. 1993;260:1344–1348. doi: 10.1126/science.8388127. [DOI] [PubMed] [Google Scholar]
- 19.Massagué J, Like B. Cellular receptors for type β transforming growth factor: Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 1985;260:2636–2645. [PubMed] [Google Scholar]
- 20.Linke A, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell PO, et al. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol. 2005;283:240–252. doi: 10.1016/j.ydbio.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Chen HC, Frissora F, Durbin JE, Muthusamy N. Activation induced differential regulation of stem cell antigen-1 (Ly-6A/E) expression in murine B cells. Cell Immunol. 2003;225:42–52. doi: 10.1016/j.cellimm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Rosen JM. Stem/progenitor cells in mouse mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2005;10:17–24. doi: 10.1007/s10911-005-2537-2. [DOI] [PubMed] [Google Scholar]
- 24.Luna G, Paez J, Cardier JE. Expression of the hematopoietic stem cell antigen Sca-1 (LY-6A/E) in liver sinusoidal endothelial cells: Possible function of Sca-1 in endothelial cells. Stem Cells Dev. 2004;13:528–535. doi: 10.1089/scd.2004.13.528. [DOI] [PubMed] [Google Scholar]
- 25.Burger PE, et al. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA. 2005;102:7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tateishi K, et al. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J Cell Sci. 2007;120:1791–1800. doi: 10.1242/jcs.006122. [DOI] [PubMed] [Google Scholar]
- 27.Hino J, Matsuo H, Kangawa K. Bone morphogenetic protein-3b (BMP-3b) gene expression is correlated with differentiation in rat calvarial osteoblasts. Biochem Biophys Res Commun. 1999;256:419–424. doi: 10.1006/bbrc.1999.0341. [DOI] [PubMed] [Google Scholar]
- 28.Kaihara S, et al. Overexpression of bone morphogenetic protein-3b (BMP-3b) using an adenoviral vector promote the osteoblastic differentiation in C2C12 cells and augment the bone formation induced by bone morphogenetic protein-2 (BMP-2) in rats. Life Sci. 2003;72:1683–1693. doi: 10.1016/s0024-3205(02)02477-3. [DOI] [PubMed] [Google Scholar]
- 29.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 30.Ikushima H, Miyazono K. TGFβ signaling: A complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 31.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grange C, Lanzardo S, Cavallo F, Camussi G, Bussolati B. Sca-1 identifies the tumor-initiating cells in mammary tumors of BALB-neuT transgenic mice. Neoplasia. 2008;10:1433–1443. doi: 10.1593/neo.08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohn MA, Kramerov D, Hulgaard EF, Lukanidin EM. The differentiation antigen Ly-6E.1 is expressed in mouse metastatic tumor cell lines. FEBS Lett. 1997;403:181–185. doi: 10.1016/s0014-5793(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 34.Chen MS, et al. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- 35.Woodward WA, et al. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-β or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res. 2009 doi: 10.1186/bcr2244. 10.1186/bcr2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naylor TL, et al. High-resolution genomic analysis of sporadic breast cancer using array-based comparative genomic hybridization. Breast Cancer Res. 2005;7:R1186–R1198. doi: 10.1186/bcr1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herschkowitz JI, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007 doi: 10.1186/gb-2007-8-5-r76. 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi SH, Kong HK, Park SY, Park JH. Metastatic effect of LY-6K gene in breast cancer cells. Int J Oncol. 2009;35:601–607. doi: 10.3892/ijo_00000371. [DOI] [PubMed] [Google Scholar]
- 40.Nieuwenhuis EJ, et al. Quantitative molecular detection of minimal residual head and neck cancer in lymph node aspirates. Clin Cancer Res. 2003;9:755–761. [PubMed] [Google Scholar]
- 41.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor-β isoforms in mouse mammary gland development. Development. 1991;113:867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- 44.Pierce DF, Jr, et al. Mammary tumor suppression by transforming growth factor β-1 transgene expression. Proc Natl Acad Sci USA. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bierie B, et al. Transforming growth factor-β regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–1819. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 46.Dennler S, et al. Direct binding of Smad3 and Smad4 to critical TGF β–inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein–specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.