Abstract
Mutations in polycystin-1 (PC1) lead to autosomal-dominant polycystic kidney disease (ADPKD), a leading cause of renal failure for which no treatment is available. PC1 is an integral membrane protein, which has been implicated in the regulation of multiple signaling pathways including the JAK/STAT pathway. Here we show that membrane-anchored PC1 activates STAT3 in a JAK2-dependent manner, leading to tyrosine phosphorylation and transcriptional activity. The C-terminal cytoplasmic tail of PC1 can undergo proteolytic cleavage and nuclear translocation. Tail-cleavage abolishes the ability of PC1 to directly activate STAT3 but the cleaved PC1 tail now coactivates STAT3 in a mechanism requiring STAT phosphorylation by cytokines or growth factors. This leads to an exaggerated cytokine response. Hence, PC1 can regulate STAT activity by a dual mechanism. In ADPKD kidneys PC1 tail fragments are overexpressed, including a unique ∼15-kDa fragment (P15). STAT3 is strongly activated in cyst-lining epithelial cells in human ADPKD, and orthologous and nonorthologous polycystic mouse models. STAT3 is also activated in developing, postnatal kidneys but inactivated in adult kidneys. These results indicate that STAT3 signaling is regulated by PC1 and is a driving factor for renal epithelial proliferation during normal renal development and during cyst growth.
Autosomal-dominant polycystic kidney disease (ADPKD) is a common life-threatening genetic disease and leading cause of renal failure (1–4). Epithelial-lined cysts develop due to excessive proliferation leading to renal enlargement and destruction of functional renal tissue. No treatment is available to slow disease progression. PKD1 gene mutations cause the majority of cases but the exact function of its gene product, polycystin-1 (PC1), has remained poorly understood. Confusing the issue is the fact that both loss of PC1 as well as PC1 overexpression lead to renal cyst growth in mouse models. Furthermore, in human ADPKD, each clonal cyst within the same patient is thought to exhibit a unique combination of germline and acquired mutations that results in a cyst-by-cyst mosaic of genotypes and resulting variation of expression levels of PC1 harboring various mutations. PC1 has also been implicated in a puzzling variety of intracellular signaling events including JAK-STAT signaling (5, 6) and it has been difficult to elucidate which of these functions may be most important for renal cyst growth in ADPKD.
Disruption of primary cilia in kidney epithelial cells leads to proliferation and cyst growth (7). PC1 has been shown to function in ciliary mechanotransduction, and a potential molecular mechanism emerged from the discovery that PC1 undergoes proteolytic cleavage, releasing the C-terminal cytoplasmic tail (∼30 kDa) from the membrane, followed by nuclear translocation (2, 5). PC1 cleavage is triggered upon the cessation of luminal fluid flow (2). The cleaved PC1 tail interacts with the transcription factors STAT6 and P100, enhances STAT6 activity, and STAT6 translocates between cilia and the nucleus, depending on luminal fluid flow stimulation (5).
In addition to the regulation of STAT6 by PC1, previously it was reported that overexpression of full-length PC1 leads to activation of STAT1 and STAT3 (6). Because this was reported before the discovery of PC1 tail cleavage, it was not investigated whether STAT1/3 signaling involves the PC1 tail and whether it requires the membrane-anchored or soluble form. Furthermore, we reported that the cleaved, soluble PC1 tail does not cause “activation” of STAT6 by tyrosine phosphorylation but rather leads to “coactivation” of STAT6 that was activated by cytokine signaling (5). To distinguish these mechanisms we use the term activation to describe an event that leads to STAT tyrosine phosphorylation. In contrast, coactivation leads to enhancement of the transcriptional activity of an already activated STAT.
We report here that PC1 can both activate and coactivate multiple STATs. STAT activation requires the membrane-anchored PC1 tail, whereas STAT coactivation requires the cleaved, soluble PC1 tail. We show that membrane-anchored PC1 specifically activates STAT3, whereas the soluble PC1 tail can coactivate STAT1, -3, and -6. STAT3 activation requires JAK2, and JAK2 physically interacts with the PC1 tail. In contrast, the soluble PC1 tail sensitizes cells to cytokine and growth factor signaling, leading to increased proliferation or death of renal epithelial cells. ADPKD kidneys accumulate both a large (∼30 kDa) and small (∼15 kDa) PC1 tail fragment, which differ in their ability to coactivate STATs. STAT3 activity is down-regulated after differentiation of renal epithelial cells in vitro and in vivo, but remains highly up-regulated in ADPKD and polycystic kidney mouse models, suggesting that STAT3 promotes renal cyst growth and could be a promising drug target. Overall, these results suggest that PC1 can differentially regulate STAT1, -3, and -6 signaling, depending on the state of apical fluid flow, PC1 cleavage, and the cytokine and growth factor environment. In doing so, PC1 may integrate mechanical and chemical signals and direct the appropriate cellular response.
Results
STAT Activation by Membrane-Anchored PC1.
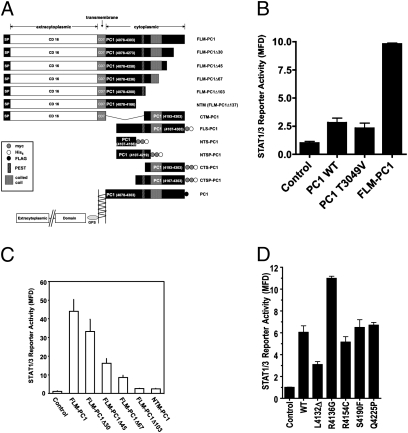
To investigate the mechanisms of STAT activation vs. coactivation by PC1, we used a luciferase reporter responsive to both STAT1 and -3. Expression of full-length PC1 results in a threefold increase of the luciferase signal (Fig. 1B). Next, we investigated which region of PC1 is responsible for STAT1/3 activation. In addition to cleavage of the cytoplasmic tail, the N-terminal ectodomain of PC1 can undergo cleavage at a G protein-coupled receptor proteolytic site (GPS) (8). The noncleavable mutant PC1 T3049V (9) is still capable of STAT1/3 activation (Fig. 1B), indicating that GPS cleavage is not required. To test whether the PC1 tail is involved in STAT1/3 activation, we used membrane-anchored constructs containing the full-length tail (full-length, membrane-anchored PC1 tail construct, FLM-PC1) or smaller deletion constructs. FLM-PC1 causes strong activation of the STAT1/3 reporter, indicating that the membrane-anchored PC1 tail is sufficient. Progressive deletions of the PC1 C terminus decrease its ability to activate STAT1/3, and removal of the coiled-coil domain eliminates activation (Fig. 1C). Neither the N-terminal half (NTM-PC1) nor the C-terminal half (CTM-PC1) alone are capable of STAT1/3 activation (Fig. S1), indicating that the coiled-coil domain is necessary but not sufficient for STAT activation.
Fig. 1.
The membrane-anchored PC1 tail activates STAT3. (A) PC1 expression constructs. Membrane-anchored constructs contain the extracytoplasmic domain and signal peptide (SP) of CD16 and transmembrane domain of CD7. (B–D) Luciferase assays using HEK293T cells transfected with the STAT1/3 luciferase reporter and either control GFP or (B) wild-type PC1, noncleavable GPS-domain mutant PC1, or membrane-anchored PC1 tail or (C) FLM-PC1 truncation constructs or (D) pathogenic mutants in the FLM-PC1 context.
Several PKD1 pathogenic mutations map to the PC1 tail. Five known mutations were introduced in the FLM-PC1 construct. The L4132Δ mutation decreases STAT activation by ∼50% and the R4136G mutation increases STAT activation by ∼100% (Fig. 1D), whereas three other mutations had no effect.
Altogether, these results indicate that the membrane-anchored PC1 tail is sufficient for STAT1/3 activation, that it requires the coiled-coil domain, that the degree of STAT activation is comparable to that mediated by the activated IFNγ receptor, and that some PKD1 germline mutations may influence renal cyst growth due to altered STAT regulation.
STAT3 Activation by PC1 Specifically Activates STAT3 in a JAK2-Dependent Manner.
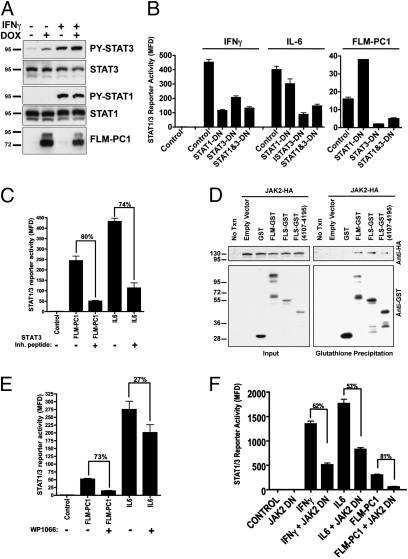
To determine whether PC1 acts via STAT1 and/or -3, we investigated STAT tyrosine phosphorylation. Doxycycline (DOX)-induced expression of FLM-PC1 in stably transfected Madin Darby canine kidney cells (MDCK) cells results in tyrosine phosphorylation of STAT3 but not STAT1 (Fig. 2A), suggesting that the membrane-anchored PC1 tail only activates STAT3 in this renal epithelial cell line. To confirm the specificity for STAT3 activation, we used dominant-negative (DN) STAT mutants (10, 11) (Fig. 2B). STAT-reporter activation by FLM-PC1 was almost eliminated in the presence of STAT3-DN but not by STAT1-DN. Furthermore, a STAT3 inhibitory peptide inhibited reporter activation by FLM-PC1 to the same extent as activation by IL6, a STAT3-specific cytokine (Fig. 2C). These results suggest that FLM-PC1 signaling occurs primarily through activation of STAT3.
Fig. 2.
PC1 specifically activates STAT3 by JAK2 phosphorylation. (A) FLM-PC1 expression was induced with DOX for 16 h in stably transfected MDCK cells followed by a 30-min treatment with IFNγ where indicated. (B, C, E, and F) Luciferase assays using HEK293T cells transfected with the STAT1/3 luciferase reporter and indicated genes. (B) Dominant-negative (DN) STAT1 or STAT3 constructs were expressed with either cytokine treatment or FLM-PC1 expression. (C and E) Treatment with the STAT3-inhibitory peptide (C) or the JAK2 inhibitor WP1066 (E) immediately following transfection, followed by IL6 addition 2 h later. (D) Coprecipitation of JAK2-HA with PC1-GST fusion constructs expressed in HEK293T cells. (F) DN JAK2 was expressed with either cytokine treatment or FLM-PC1 expression.
STAT activation normally requires receptor tyrosine kinases, receptor-bound JAK family kinases, or nonreceptor-associated tyrosine kinases (12, 13). Coprecipitation experiments revealed that JAK2 interacts with the membrane-proximal region (residues 4,107–4,195) of the PC1 tail (Fig. 2D). The pan-JAK inhibitor, pyridone 6, which inhibits all four JAK family kinases (14, 15) (Fig. S2), or the JAK2-specific inhibitor, WP1066 (Fig. 2E), prevents STAT3 activation by FLM-PC1. Overexpression of dominant-negative JAK2 resulted in strong suppression of FLM-PC1–induced STAT3 activity (Fig. 2F). Altogether, these results indicate that JAK2 is the main kinase involved in STAT3 activation by PC1.
Receptor-associated kinases of the JAK family often act by phosphorylating tyrosine residues in the receptor tails. As shown in Fig. S3, mutation of all four tyrosine residues in the PC1 tail (FLM-4Y/F-PC1) does not affect its ability to activate STAT3, indicating that tyrosine phosphorylation of PC1 is not required for its activation of STAT3.
Cleavage and Accumulation of the PC1 Tail in ADPKD Kidneys.
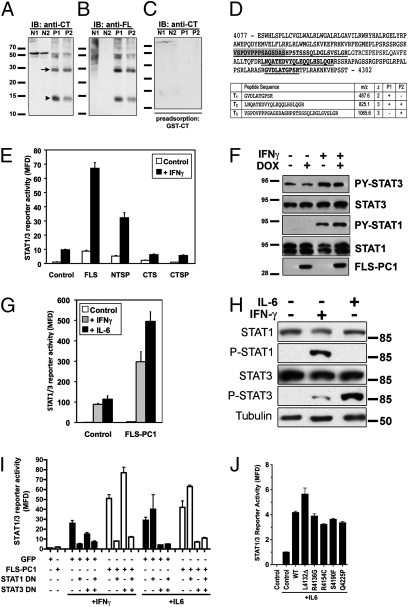
We previously reported that cyst-lining cells in ADPKD kidneys exhibit significant nuclear immunoreactivity to the PC1 tail (5). Immunoblotting with two PC1-tail antibodies labeled prominent bands at ~30 and ~15 kDa in ADPKD kidneys but not normal controls (Fig. 3 A and B). To verify these results independent of antibodies, the ∼15-kDa region of ADPKD samples was analyzed by tandem-mass spectrometry. Three peptides corresponding to the C-terminal half of the PC1 tail were identified (Fig. 3D). These results indicate that the PC1 tail undergoes cleavage in ADPKD kidneys, resulting in at least two defined fragments. On the basis of molecular weight, antibody reactivity, and mass spectrometry, the large fragment (polycystin-1 tail fragment of ∼30 kDa, P30) corresponds to the entire cytoplasmic tail, whereas the small fragment (polycystin-1 tail fragment of ∼15 kDa, P15) corresponds to the C-terminal half of the tail.
Fig. 3.
The cleaved cytoplasmic tail of PC1: Expression in ADPKD and STAT coactivation. (A and B) Immunoblots of total lysates from normal (N1 and N2) and ADPKD (P1 and P2) renal tissues probed with anti-CT antibody (against the C-terminal half of the human PC1 tail) or anti-FL antibody (against the entire human PC1 tail) as indicated. (C) Specificity control: anti-CT antibody preadsorbed with antigen. (D) Mass spectrometric analysis of the ∼15-kDa band from ADPKD patients. The identified peptides detected are underlined in bold in the human PC1 tail sequence. For orientation, the PEST domain is highlighted in gray. (E) STAT1/3 luciferase assay in HEK293T cells transfected with control GFP or soluble PC1 constructs and treated with IFNγ (20 ng/mL) as indicated. (F) FLS-PC1 expression was induced with DOX for 16 h in stably transfected MDCK cells followed by a 30-min treatment with IFNγ where indicated. (G) STAT1/3 luciferase assay with expression of FLS-PC1 and treatment with IFNγ or IL6 as indicated. (H) HEK293T cells were treated with IFNγ or IL6 as indicated for 30 min before lysis and probed with the indicated antibodies. (I) STAT1/3 luciferase assay in the presence of DN-STAT1 and DN-STAT3 and FLS-PC1 as indicated. IFNγ or IL6 treatment for activation of STAT1 and STAT3, respectively. (J) Pathogenic mutants in the FLS-PC1 context do not affect STAT3 coactivation after IL6 stimulation in STAT1/3 luciferase assay.
Soluble PC1 Tail Coactivates STAT3 and STAT1.
We tested whether the cleaved, soluble PC1 tail can affect STAT1 or -3 activities. Full-length, soluble PC1 tail construct (FLS-PC1) (to mimic P30) increased STAT1/3 reporter activity in unstimulated cells ∼10-fold (Fig. 3E). Strikingly, FLS-PC1 in combination with IFNγ led to very strong enhancement of reporter activity over IFNγ alone. C-terminal half, soluble CTS- and C-terminal half, soluble PC1 tail construct containing PEST motif (CTSP-PC1) (to mimic P15) failed to affect STAT1/3 (Fig. 3E). FLS-PC1 did not increase pY-STAT1 or -3, either in the absence or presence of IFNγ (Fig. 3F), suggesting that FLS-PC1 regulates STAT activity by coactivation rather than activation.
We next asked whether FLS-PC1 can coactivate both STAT1 and 3. IFNγ predominantly activates STAT1, whereas IL6 activates only STAT3 (Fig. 3H). FLS-PC1 leads to coactivation of the STAT1/3 reporter in response to either IL6 or IFNγ, indicating that it can coactivate STAT3 and possibly also STAT1 (Fig. 3G). Expression of STAT1/3-DN constructs suppresses the coactivation by FLS-PC1 after activation with the appropriate cytokine (Fig. 3I), indicating that FLS-PC1 can coactivate both STAT1 and STAT3. Coactivation is abolished by the pan-JAK inhibitor (Fig. S4). These results indicate that the cleaved PC1 tail can coactivate both STAT1 and STAT3 by a mechanism that depends on prior cytokine activation and JAK activity.
The same pathogenic mutations as in Fig. 1D were introduced in the soluble PC1 tail context but none of them significantly altered STAT coactivation (Fig. 3J), indicating that sequence requirements for STAT activation and coactivation are distinct.
Coactivation of STAT1 by FLS-PC1 appears to involve a stable complex because both proteins can be coimmunoprecipitated (Fig. S5). We failed to demonstrate a stable complex between STAT3 and FLS-PC1, which may suggest differences in biochemical stability.
It has been suggested that effects of the cleaved PC1 tail may be due to a dominant-negative effect on endogenous, full-length PC1 (16). To test this possibility, we used Pkd1-null mouse embryonic fibroblasts (MEFs). FLS-PC1 still leads to coactivation of the STAT1/3 reporter in these cells (Fig. S6) even in the absence of endogenous PC1, indicating that a dominant-negative effect is unlikely. The lower magnitude of coactivation in MEFs is likely due to the low transfection efficiency in these cells.
To investigate the possibility that FLS-PC1 could act as a stabilizer of activated STATs, MDCK cells were subjected to short cytokine pulses and the decay of the pY-STAT signals was monitored. FLS-PC1 does not appreciably alter the decay of the pY-STAT signals (Fig. S7). Altogether, these results suggest that the enhancement of STAT transcriptional activity by FLS-PC1 is due to nuclear coactivation, possibly by aiding in the assembly of transcriptional complexes.
Soluble PC1 Tail Regulates Proliferation and Cell Death.
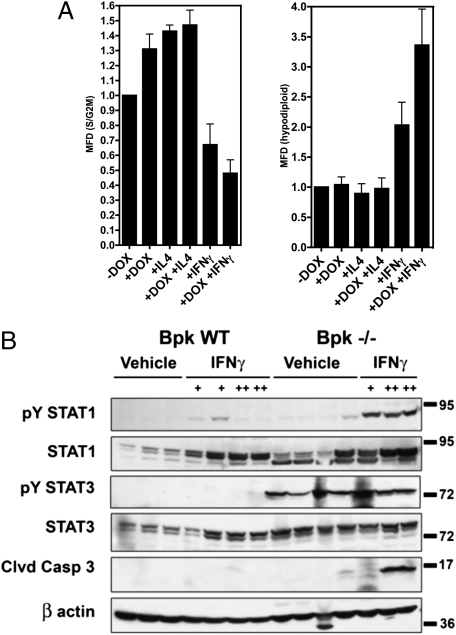
The ability of FLS-PC1 to coactivate STAT1, -3, and -6, and the finding that the cleaved PC1 tail can accumulate in the nuclei of ADPKD renal cysts (5) (Fig. 3), suggest that the cleaved PC1 tail may play a role in regulating cyst growth in ADPKD. We asked how FLS-PC1 may affect proliferation and/or cell death in MDCK cells. Under basal conditions, proliferating MDCK cells express pY-STAT3 (Figs. 2A and 3F) but not pY-STAT1 (Figs. 2A and 3F and Fig. S7) or pY-STAT6 (5). FLS-PC1 overexpression under basal conditions (only pY-STAT3) stimulates proliferation by ∼30% (Fig. 4A). STAT6 activation by IL4 treatment also stimulates proliferation. In contrast, IFNγ (predominantly STAT1 activation) inhibits proliferation and induces cell death (Fig. 4A, Right), and FLS-PC1 further amplifies this effect. No further increase in proliferation is seen with the combination of IL4 and FLS-PC1, suggesting that cell cycling may already be maximal under these conditions. Altogether, these results suggest that FLS-PC1 sensitizes renal epithelial cells to the effects of the activation of STATs and can amplify different biological effects, depending on the activation of diverse signaling pathways and the cytokine environment.
Fig. 4.
The soluble PC1 tail causes hypersensitivity to cytokine signaling. (A) Cell cycle analysis by flow cytometry. Stably-transfetcted FLS-PC1 MDCK cells were induced for 48 h with DOX in conjunction with the indicated cytokines. (Left) Actively cycling cells (S/G2M phase). (Right) Hypodiploid cells. Data are normalized to control (untreated) cells and represented as mean-fold difference ± SE from three independent experiments. (B) Western blots of kidney lysates. Twenty-one-day-old wild-type or bpk cystic animals were treated for 2 (low dose) or 5 (high dose) d with either vehicle, low dose (+, 1.2 μg), or high dose (++, 3 μg) IFNγ as indicated. PY-STAT3 is elevated in cystic animals, independent of treatment. IFNγ-treated cystic animals exhibit increased caspase-3 activation, indicative of apoptosis.
Polycystic Kidneys Are Hypersensitive to IFNγ/STAT1 Signaling.
The cleaved PC1 tail is overexpressed in cyst-lining cells in nonorthologous PKD mouse models (17) and human ADPKD (ref. 5 and Fig. 3). We asked whether the cleaved PC1 tail may hypersensitize cystic epithelial cells to STAT signaling in a nonorthologous PKD mouse model. Balb/c polycystic kidney (bpk) mice were challenged with IFNγ, which resulted in strong renal STAT1 activation (Fig. 4B). Strikingly, STAT3 is already highly activated in kidneys of untreated bpk mice (see below) but no further STAT3 activation is apparent after IFNγ challenge. Neither STAT1 nor STAT3 are activated in kidneys of control mice. Analysis of cleaved caspase 3 reveals a significant apoptotic response in bpk mice challenged with a high dose of IFNγ, whereas WT mice are unaffected (Fig. 4B). These results suggest that polycystic kidneys indeed have an enhanced ability to respond to IFNγ stimulation.
STAT3 Is Activated in PKD.
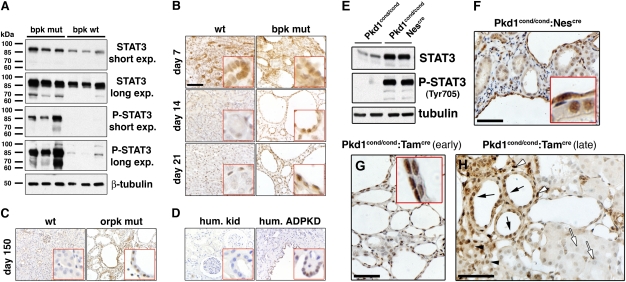
Given our observed PC1-mediated regulation of STAT3 activity, we investigated whether STAT3 is aberrantly regulated in renal cystic disease in mouse models and human ADPKD. As shown in Fig. 5A, kidneys of the early-onset bpk mouse model (18, 19) exhibit extremely high levels of pY-STAT3 compared with WT animals in which pY-STAT3 is barely detectable. Total STAT3 is moderately but consistently increased in bpk kidneys.
Fig. 5.
STAT3 is activated in PKD. Immunoblots of kidney tissue lysates from 21-d-old wild-type and bpk mutant mice (A) or 49-d-old Pkd1cond/cond and Pkd1cond/cond:Nescre mice (E). All other panels: Kidney sections derived from (B) bpk mutant mice, (C) orpk;Tg737Rsq mice, (D) human ADPKD kidney, (F) 49-d-old Pkd1cond/cond:Nescre mice, (G) tamoxifen-treated (P12) 21-d-old Pkd1cond/cond:Tamcre mice, and (H) tamoxifen-treated (P14) 6-mo-old Pkd1cond/cond:Tamcre mice were subjected to immunostaining for pY-STAT3. Representative fields and age-matched controls are shown. (H) Note that pY-STAT3 is highly expressed in cyst-lining cells (black arrows) and normal-appearing tubules (black arrowheads) and interstitial cells (white arrowheads) in close proximity to cysts, whereas normal tubules at a distance from cysts are negative (white arrows). (B–D) (Scale bars, 100 μm.) (F–H) (Scale bars, 50 μm.)
To examine the developmental regulation of STAT3 activity, we analyzed pY-STAT3 in renal sections derived from 7-, 14-, and 21-d-old bpk and WT animals (Fig. 5B). At day 7, renal epithelial cells in WT and bpk mice both exhibit strong pY-STAT3 staining. The predominantly nuclear localization of pY-STAT3 suggests that it is transcriptionally active. There was little detectable pY-STAT3 staining in 14- and 21-d-old WT mice, indicating that STAT3 normally becomes inactive before postnatal day 14. In contrast, nuclear pY-STAT3 signals are strong in cystic epithelial cells of 14- and 21-d-old bpk mutant mice.
Because renal cyst growth in bpk mice temporally coincides with renal development, we also examined the adult-onset Oak Ridge polycystic kidney (orpk)-rescue PKD mouse model (20). Cyst-lining epithelial cells in 150-d-old mutant mice also exhibit strong nuclear pY-STAT3 signals, whereas age-matched WT control animals show no detectable pY-STAT3 (Fig. 5C). Similarly, nuclei of cyst-lining cells in human APDKD kidneys were highly positive for pY-STAT3, whereas normal human kidney showed no detectable pY-STAT3 (Fig. 5D).
Next, we investigated two mouse models in which the Pkd1 gene is conditionally inactivated. The Pkd1cond/cond:Nescre model, characterized by mosaic gene inactivation and a robust renal cystic phenotype (21) exhibits strong STAT3 activation (Fig. 5E) in renal cyst-lining and interstitial cells (Fig. 5F). Pkd1 gene deletion can be temporally controlled by tamoxifen induction in the Pkd1cond/cond:Tamcre model (22). Early tamoxifen induction (day P12) leads to massive renal cystic disease by day 21, and all cysts exhibit strong nuclear pY-STAT3 signals (Fig. 5G). In contrast, later tamoxifen induction (day P14) leads to cyst formation only after a delay of 6 mo, despite the fact that ∼50% of renal epithelial cells have lost PC1 expression in both early and late models (22). Interestingly, in these animals pY-STAT3 is highly expressed in cyst-lining cells (Fig. 5H) and normal appearing tubules and interstitial cells in close proximity to cysts. In contrast, normal tubules at a distance from cysts are negative for pY-STAT3. These results suggest that loss of PC1 expression per se does not lead to STAT3 activation but that diffusible factors—such as cytokines and growth factors—are likely involved in focal STAT3 activation following PC1 disruption.
Altogether, these results demonstrate that STAT3 is normally active in developing kidneys but becomes inactive in adult kidneys. In contrast, STAT3 remains activated in cyst-lining cells in mouse models and human ADPKD, suggesting that it may promote cyst growth.
Discussion
We report here that PC1 is capable of affecting the activity of several STAT transcription factors by two distinct mechanisms. First, membrane-anchored PC1 can activate STAT3 by JAK2-dependent phosphorylation. Second, the proteolytically cleaved, soluble PC1 tail undergoes nuclear translocation and can coactivate STAT1, -3, and -6, which have been previously activated by tyrosine phosphorylation, e.g., by cytokine signaling. Hence, PC1 is a membrane protein that can both activate a STAT at the membrane and then coactivate the STAT in the nucleus after its own cleavage. This dual mechanism on multiple STATs suggests that PC1 can integrate diverse signals and orchestrate different biological responses to these inputs.
The membrane-anchored, PC1 tail is sufficient for activating STAT3 (Fig. 1), suggesting that no upstream regions of PC1 are required. Consistent with this, JAK2 interacts with the membrane-proximal half of the PC1 tail (Fig. 2D). Membrane anchorage is required for STAT3 activation because the soluble PC1 tail fails to increase pY-STAT3 (Fig. 3F), even though it still interacts with JAK2 (Fig. 2D). The mechanism of STAT3 activation by PC1 resembles that of cytokine receptors because it depends on JAK2 functionally and involves physical interaction with JAK2. This may suggest that STAT3 activation by PC1 is regulated by ligand binding similar to cytokine receptor regulation but no physiologically relevant extracellular ligands have clearly been identified to date.
The mechanisms underlying the observed accumulation of the cleaved, nuclear PC1 tail in ADPKD (5) (Fig. 3) are unclear. Renal cyst growth in ADPKD is thought to involve a genetic second-hit mechanism (23). However, instead of lack of PC1 protein expression, we and others have consistently observed increased immunoreactivity for PC1 in renal cysts in ADPKD (5, 24, 25) and nonorthologous mouse models (17). This suggests that (mutated) PC1 is overexpressed in renal cysts and we hypothesize that this may lead to degradation of defective protein and accumulation of proteolytic fragments corresponding to the cytoplasmic tail. A large fraction of PKD1 germline mutations are truncating mutations (3) that are not expected to lead to expression of the cytoplasmic tail. It is possible that the observed PC1 tail expression in cysts is often due to expression from the unaffected or somatically affected allele. Because cysts in ADPKD are thought to be clonal, and each with a distinct genotype, it is possible that the expression of the PC1 tail in individual cysts leads to a growth advantage and positive selection. Overexpression of normal PC1 in transgenic mice leads to a renal cystic phenotype (26). Furthermore, genetic inactivation of the Pkd1 gene in adult mice does not lead to an apparent renal cystic phenotype for several months (22). Altogether, these findings suggest that renal cyst growth in ADPKD may be associated with overexpression rather than lack of PC1. Similarly, renal cyst growth in other ciliopathies in which PC1 is intact may involve excessive PC1 expression, tail cleavage, and STAT signaling. This is consistent with the finding that the nuclear PC1 tail is overexpressed in renal cysts in a PKD mouse model caused by inactivation of the ciliary motor protein Kif3A (17).
We have previously shown that PC1 undergoes tail cleavage in vitro, resulting in a nuclear-targeted, C-terminal fragment of ∼15 kDa, suggesting a cleavage site in the middle of the tail (5). Chauvet et al. (17) independently also observed PC1 tail cleavage but reported a larger fragment of >30 kDa, suggesting a cleavage site in a transmembrane domain. We now report two prominent bands of ∼15 kDa (P15) and ∼30 kDa (P30), respectively, that are both overexpressed in ADPKD renal tissue (Fig. 3). This suggests that at least two stable cleavage products exist, which correspond to the full-length cytoplasmic tail and the C-terminal half of the tail of PC1, respectively.
On the basis of our findings, PC1 cleavage dramatically alters its regulation of STAT activity. Because only membrane-anchored PC1 has the ability to activate STAT3, tail cleavage is expected to terminate this signal. This is supported by the inability of PC1 constructs lacking the C-terminal coiled-coil domain to activate STAT3 (Fig. 1C). On the other hand, the P30 cleavage product now has the ability to coactivate STAT1, -3 (Fig. 3), and -6 (5) after STAT activation. P15 loses the ability to coactivate STAT1/3 (Fig. 3) but not STAT6 (5). The cleaved forms of the PC1 tail are not capable of increasing activation by tyrosine phosphorylation of any of these three STATs but appear to act purely by coactivation.
Therefore, depending on the cleavage status of PC1, renal epithelial cells may be directed toward different biological responses to growth factor and cytokine signaling. Furthermore, because ciliary STAT6 localization (5) and PC1 tail cleavage (2) were shown to be regulated by apical/luminal fluid flow, this suggests that PC1 can integrate mechanical (cilia bending) and chemical (cytokines/growth factors) signals.
Our finding that PC1 can both activate and coactivate STAT3 suggests that STAT3 regulation by PC1 may be relevant to renal cyst growth. STAT3 activation occurs in many forms of cancer. Whereas STAT3 activation often results in enhanced proliferation, and inhibition of apoptosis, these effects are cell-type specific and STAT3 activity can also be associated with growth arrest (27, 28). We find that STAT3 is highly active during renal growth in normal 7-d-old mice but becomes inactive before day 14 when renal growth is completed (Fig. 5B). A developmental switch occurs in mouse kidneys between postnatal days 13 and 14 that signals the end of the terminal renal maturation process (22). In highly proliferative cyst-lining cells in human ADPKD and several different PKD mouse models, STAT3 is constitutively active (Fig. 5). Altogether, these results strongly suggest that STAT3 signaling is a driving factor for renal epithelial proliferation during normal renal development and during cyst growth.
Several STAT3-activating growth factors/cytokines are relevant to the kidney. Hepatocyte growth factor (HGF) was found in cyst fluids in ADPKD (29). Transgenic mice overexpressing HGF develop renal cysts (30). STAT3 is required for HGF-induced branching tubulogenesis in MDCK cells (31). Activation of EGF-receptor signaling is well established in PKD (3). STAT3 is activated in tubule cells after acute kidney injury, probably via IL6 transsignaling and may aid in tissue preservation and repair (32). This may further support the idea that cyst growth in PKD is due to the aberrant activation of a normally dormant injury repair program (2).
Altogether, these results suggest that STAT3 is regulated by PC1 and that aberrant STAT3 activation contributes to proliferation in renal epithelial cells during development, cystic disease, and the response to renal injury. STAT3 may therefore be a promising drug target for treatment of ADPKD.
Materials and Methods
Plasmids.
Several PC1 tail constructs cloned into pCDNA4/TO-myc-His have been described (5). All other PC1 constructs were made by PCR and cloned into the same vector. The PC1 T3049V construct (9) was a gift from Gregory Germino (National Institutes of Health, Bethesda, MD). STAT1/3 luciferase reporter, containing four gamma interferon activation site (GAS) elements upstream of the luciferase gene, was a gift from Tom Hamilton (Cleveland Clinic, Cleveland, OH). The JAK2 plasmid was a gift from Olli Silvenoinen (University of Tampere, Tampere, Finland). The JAK2-DN and FLM4Y/F-PC1 constructs were made by site-directed mutagenesis (Stratagene). The STAT3-DN and STAT1-DN plasmids were obtained from Addgene (10, 11).
Antibodies and Reagents.
Anti-PC1 antibodies have been described (5). Total STAT3, pY-STAT3, and pY-STAT1 antibodies are from Cell Signaling Technology. Total STAT1 antibody is from Santa Cruz Biotechnology and anti-myc, from Upstate. The pY-STAT3 used for immunostaining is from Abcam. All cytokines are from R&D Systems, control rabbit IgG, from Sigma, and pyridone 6, from Calbiochem.
Luciferase Assays.
HEK293T cells were seeded on 12-well plates and transfected with 250 ng of reporter, 10 ng of β-galactosidase, and 250 ng of gene of interest using Lipofectamine 2000 in Opti-MEM. Plasmids were balanced with pEGFP (Clontech). PC1-null MEFs were transfected using Amaxa MEF Nucleofector Kit 2. Cytokines were added posttransfection for at least 16 h. Approximately 24 h posttransfection, cell lysates were assayed for luciferase and β-galactosidase activity. Luciferase units were normalized using β-galactosidase. Experimental conditions were assayed in triplicate; each bar represents average mean fold induction with respect to control; error bars represent SEM. Experiments are representative of at least three independent assays.
Human Samples.
Tissue samples from anonymous ADPKD patients and normal controls were obtained from the Cleveland Clinic or National Disease Research Interchange (NDRI) as per institutional guidelines. Samples were frozen in liquid nitrogen, fine shavings were ground with a micro tissue grinder on dry ice, dissolved in SDS/PAGE sample buffer, and used for Western blot and mass spectrometry.
SI Materials and Methods provides full descriptions of other methods.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants DK62338 (to T. Weimbs) and DK076017 (to T. Watnick), and American Heart Association Grant 0630335N (to J.M.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103816108/-/DCSupplemental.
References
- 1.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weimbs T. Polycystic kidney disease and renal injury repair: Common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol. 2007;293:F1423–F1432. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 3.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:118–130. doi: 10.1053/j.ackd.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low SH, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Bhunia AK, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J. Polycystins and primary cilia: Primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 8.Qian F, et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci USA. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei W, Hackmann K, Xu H, Germino G, Qian F. Characterization of cis-autoproteolysis of polycystin-1, the product of human polycystic kidney disease 1 gene. J Biol Chem. 2007;282:21729–21737. doi: 10.1074/jbc.M703218200. [DOI] [PubMed] [Google Scholar]
- 10.Wen Z, Darnell JE., Jr. Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 1997;25:2062–2067. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen Z, Zhong Z, Darnell JE., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 12.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 13.Reddy EP, Korapati A, Chaturvedi P, Rane S. IL-3 signaling and the role of Src kinases, JAKs and STATs: A covert liaison unveiled. Oncogene. 2000;19:2532–2547. doi: 10.1038/sj.onc.1203594. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JE, et al. Photochemical preparation of a pyridone containing tetracycle: A Jak protein kinase inhibitor. Bioorg Med Chem Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 15.Pedranzini L, et al. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66:9714–9721. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- 16.Basavanna U, et al. The isolated polycystin-1 COOH-terminal can activate or block polycystin-1 signaling. Biochem Biophys Res Commun. 2007;359:367–372. doi: 10.1016/j.bbrc.2007.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauvet V, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogswell C, et al. Positional cloning of jcpk/bpk locus of the mouse. Mamm Genome. 2003;14:242–249. doi: 10.1007/s00335-002-2241-0. [DOI] [PubMed] [Google Scholar]
- 19.Nauta J, Ozawa Y, Sweeney WE, Jr., Rutledge JC, Avner ED. Renal and biliary abnormalities in a new murine model of autosomal recessive polycystic kidney disease. Pediatr Nephrol. 1993;7:163–172. doi: 10.1007/BF00864387. [DOI] [PubMed] [Google Scholar]
- 20.Brown NE, Murcia NS. Delayed cystogenesis and increased ciliogenesis associated with the re-expression of polaris in Tg737 mutant mice. Kidney Int. 2003;63:1220–1229. doi: 10.1046/j.1523-1755.2003.00863.x. [DOI] [PubMed] [Google Scholar]
- 21.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol. 2010;21:489–497. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutters M, Germino GG. Autosomal dominant polycystic kidney disease: Molecular genetics and pathophysiology. J Lab Clin Med. 2003;141:91–101. doi: 10.1067/mlc.2003.13. [DOI] [PubMed] [Google Scholar]
- 24.Lanoix J, D'Agati V, Szabolcs M, Trudel M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD) Oncogene. 1996;13:1153–1160. [PubMed] [Google Scholar]
- 25.Ward CJ, et al. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc Natl Acad Sci USA. 1996;93:1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard L, et al. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet. 2000;9:2617–2627. doi: 10.1093/hmg/9.18.2617. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 28.Yue P, Turkson J. Targeting STAT3 in cancer: How successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horie S, et al. Mediation of renal cyst formation by hepatocyte growth factor. Lancet. 1994;344:789–791. doi: 10.1016/s0140-6736(94)92344-2. [DOI] [PubMed] [Google Scholar]
- 30.Takayama H, LaRochelle WJ, Sabnis SG, Otsuka T, Merlino G. Renal tubular hyperplasia, polycystic disease, and glomerulosclerosis in transgenic mice overexpressing hepatocyte growth factor/scatter factor. Lab Invest. 1997;77:131–138. [PubMed] [Google Scholar]
- 31.Boccaccio C, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 32.Nechemia-Arbely Y, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.