Abstract
The steroid hormone signaling axis is thought to play a central role in initiation and progression of many hormonally regulated epithelial tumors. It is unclear whether all cancer-initiating signals depend on an intact hormone receptor signaling machinery. To ascertain whether cell autonomous androgen receptor (AR) is essential for initiation of prostate intraepithelial neoplasia (PIN), the response of AR-null prostate epithelia to paracrine and cell autonomous oncogenic signals was assessed in vivo by using the prostate regeneration model system. Epithelial-specific loss of AR blocked paracrine FGF10-induced PIN, whereas the add back of exogenous AR restored this response. In contrast, PIN initiated by cell-autonomous, chronic-activated AKT developed independent of epithelial AR signaling. Our findings demonstrate a selective role for AR in the initiation of PIN, dependent on the signaling pathways driving tumor formation. Insights into the role of hormone receptor signaling in the initiation of epithelial tumors may help define this axis as a target for chemoprevention of carcinomas.
Keywords: tissue recombination, tumor promoter, tumor suppressor
Pharmacologic agents targeting hormone receptor signaling are widely used in therapy of carcinomas arising from the breast (estrogen receptor; ER), prostate (androgen receptor; AR) and endometrium (ER, and progesterone receptor; PR) (reviewed in refs. 1 and 2). It is unclear whether the hormone receptor signaling axis plays a causative role in initiation of these malignancies or whether it can be bypassed by oncogenic signals. Loss of PR has a protective effect in initiation of mammary tumors caused by 7,12-dimethylbenz(a) anthracene (DMBA) in mice (3). These results suggest that initiation of breast cancer via DMBA depends on PR signaling. Conversely, estrogen receptor knockout mice developed mammary tumors similar to controls in response to Wnt-1 expression (4), suggesting that initiation of these tumors is independent of ER signaling. The benefits of androgen ablation in treating prostate cancer were demonstrated decades ago (5; reviewed in ref. 6). However, the roles of autonomous and extrinsic AR in initiation of prostate cancer remain unknown.
The prostate is a hormonally regulated gland. During development, androgen-mediated effects on prostatic epithelium are thought to be driven by stromal AR (7, 8). This model is derived from murine embryonic urogenital tissue fragment recombination studies (8). With the inductive effects of wild-type (WT) stroma, functionally AR-null epithelia from testicular feminization (Tfm) mice gave rise to prostate-like glands (8). In contrast, prostate glands did not develop when Tfm stroma were combined with WT epithelia (8). Inductive effects of stroma on the epithelium are proposed to occur via secretion of “andromedins” (9–12). The andromedin hypothesis suggests that binding of circulating androgens to stromal AR induces the release of soluble factors that orchestrate growth and differentiation of the prostate by binding to cognate epithelial receptors (9–11). An andromedin is defined as a paracrine stromal factor expressed in an androgen-responsive tissue whose expression must be regulated by androgens (13). Among implicated andromedins are fibroblast growth factor (FGF) 7, FGF10, and Insulin growth factor 1 (10–12). Because none of these are androgen regulated, a true andromedin remains unidentified.
The transformation of a normal prostate cell to cancer is thought to occur with a switch from paracrine to cell-autonomous AR signaling (14, 15). Correlational studies demonstrate high levels of epithelial AR expression in prostate cancer metastases but low levels of AR in tumor stroma (15). In vivo studies demonstrate efficient growth of prostate cancer xenografts implanted in AR-null host mice (14, 15). These results demonstrate that continuous growth of established tumors relies on epithelial AR (14, 15), but do not address its role in cancer initiation.
The role of cell autonomous AR in the formation of prostate cancer has been investigated (16). When probasin promoter-driven prostate epithelial AR deletion was achieved in a transgenic adenocarcinoma of the mouse prostate (TRAMP) model, a more aggressive and metastatic cancer was seen compared with controls (16). In these studies, epithelial AR functions as a tumor suppressor (16). The prostate epithelium is predominantly composed of luminal and basal cells. Despite deletion of AR in most prostate epithelia over time, in this model, AR was still expressed in 50% of the basal cells at 12 wk of age (16). Therefore, conclusions may be limited by the remaining AR-positive basal cells, which are potential prostate stem cells and efficient targets for prostate carcinogenesis (17–19).
Prostatic intraepithelial neoplasia (PIN) is a well-established precursor lesion to prostate cancer (20; reviewed in ref. 21). To assess the role of cell autonomous AR in prostate cancer, we used two signals that could initiate PIN, paracrine signaling by FGF10 (22) and cell autonomous signaling by myristoylated AKT (23, 24). There are important differences between the FGF10 and AKT cancer-initiating signals. Paracrine FGF10 primarily exerts its effects by binding to prostate epithelial fibroblast growth factor receptor 1 (FGFR1) (22). Signaling by FGF10-FGFR1 leads to activation of multiple targets including AKT (25) and is subject to several levels of regulation. In response to growth factor receptor signaling, AKT is recruited to the plasma membrane, where it is activated by phosphorylation but it is subject to inactivation by phosphatases (reviewed in ref. 26). The FGFR signaling axis is regulated at other levels, including receptor internalization and degradation (25, 27). Conversely, myristoylation of AKT (23, 24) results in chronic signaling due to plasma membrane localization and constitutive activation of its serine/threonine protein kinase (28–30). Our previous studies demonstrated that PIN lesions initiated by paracrine FGF10 had high levels of epithelial AR (22), whereas AKT-mediated lesions had normal epithelial AR expression (24). These observations led to the current study hypothesis: Cell-autonomous signaling by AR could play a central role in the initiation of FGF10 but not AKT-induced prostate neoplasia. We chose a regeneration system by using dissociated prostate epithelia and stroma (31), advantageous for its ability to work with populations of prostate epithelia that are AR-null from the onset of exposure to growth-promoting signals. We assessed the role of epithelial AR in initiating PIN via paracrine (FGF10) and cell-autonomous (AKT) oncogenic signals. We demonstrate a selective role for autonomous AR signaling in initiation of prostate neoplasia that depends on the specific growth promoting signal.
Results
Tfm Urogenital Sinus Epithelia Cannot Form PIN in the Presence of Strong Paracrine FGF10 Signaling.
We first used embryonic tissue from a functionally AR-null mutant mouse to ask whether signaling through AR was essential for the initiation of FGF10-induced neoplasia. The Tfm mouse harbors a mutation in AR rendering the receptor unable to bind to ligand, unstable and inactive (32, 33). As a result, adult male Tfm mice have agenesis of the prostate gland (32). Therefore, in these experiments, the embryonic tissue that gives rise to the prostate gland, the urogenital sinus epithelium (UGSE), was used. We first demonstrated our ability to grow dissociated populations of embryonic WT UGSE and WT urogenital sinus mesenchyme (UGSM) in the in vivo regeneration assay (Fig. S1A).
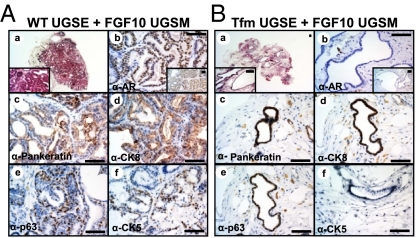
To assess the role of AR in initiation of FGF10-induced neoplasia, dissociated suspensions of Tfm or WT UGSE were recombined with FGF10-expressing UGSM (Fig. S1B). Recombination of WT UGSE with FGF10-UGSM resulted in the formation of high-grade PIN and adenocarcinoma (Fig. 1A). Regenerated tubules expressed high levels of nuclear AR (Fig. 1A, b). In striking contrast, recombination of Tfm UGSE with FGF10-UGSM resulted in the formation of atrophic tubular structures one layer thick without hallmarks of hyperplasia (Fig. 1B). Expression of AR was absent in Tfm regenerated tubules, whereas nuclear AR was detected in stromal cells of these grafts (Fig. 1B, b and Fig. S1C). The significant differences in the two grafts are not due to differences in expression of FGF10. All FGF10-UGSM were subjected to Q-PCR to confirm overexpression (Fig. S2A) and tracked with the GFP marker (Fig. S2B). Our results demonstrate that Tfm epithelia do not form hyperplasia despite exposure to paracrine FGF10. Both WT and Tfm-regenerated tubules were epithelial based on expression of pan-keratin and CK8 (Fig. 1A, c and d, and B, c and d). Tfm-regenerated tubules lacked secretions and had an intermediate phenotype expressing the luminal marker CK8 and basal marker p63 (Fig. 1B, d and e), but not CK5 (Fig. 1B, f).
Fig. 1.
Tfm epithelia did not become hyperplastic despite paracrine FGF10. The 4 × 104 WT (A) or Tfm (B) dissociated UGSE were recombined with 1 × 105 FGF10-UGSM and regenerated in vivo. (A) Paracrine FGF10 resulted in formation of hyperplastic and abnormal glands with WT UGSE (a, c). High levels of epithelial AR were detected in tubules regenerated from WT UGSE (b). There was an expansion of both luminal (d) and basal cells (e and f) in response to paracrine FGF10. (B) Tfm UGSE formed single-layered glandular structures, devoid of hyperplasia (a). Epithelial AR was absent in tubules regenerated from Tfm UGSE (b). Tfm regenerated tubules were epithelial (c), expressed luminal marker CK8 (d) and basal marker p63 (e) but not CK5 (f). (Scale bars: 100 μm.)
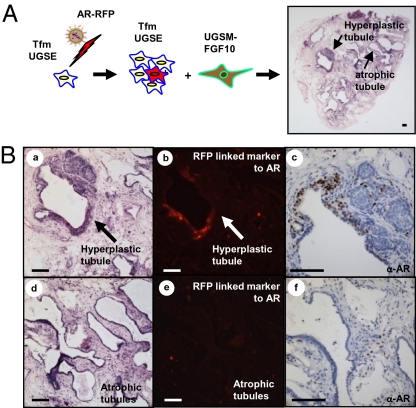
We hypothesized that the add back of AR to Tfm UGSE could restore response to paracrine FGF10 signaling. UGSE harvested from Tfm male mice were infected with a vector expressing WT AR with cis-linked red fluorescent protein (RFP) (24), combined with FGF10 UGSM and placed in the regeneration system (Fig. 2A). Areas of hyperplasia adjacent to single-layered atrophic tubules were observed (Fig. 2 A and B). Staining for AR revealed expression of AR and RFP in the hyperplastic areas (Fig. 2B, b and c) and absence of AR or RFP in the atrophic regions (Fig. 2B, e and f). The add back of AR caused Tfm epithelia to form areas of prostate hyperplasia, with increased number of basal cells (Fig. S3, a and c vs. b and d), similar to the response of WT epithelia to paracrine FGF10 (22).
Fig. 2.
Add back of AR to Tfm UGSE restored formation of hyperplasia in response to paracrine FGF10. (A) The 1.5 x 105 Tfm UGSE were infected with lentivirus-expressing AR and combined with 3 × 105 FGF10-UGSM. In regenerated glands, areas of hyperplasia were seen adjacent to single-layered atrophic tubules. (B) After add back of AR, hyperplastic Tfm tubules (a), expressed AR by immunohistochemistry (c), and RFP cis-linked marker from the viral delivery vector (b). Adjacent nonhyperplastic tubules in the same graft (d) lacked expression of epithelial AR (f) and did not express RFP (e). (Scale bars: 100 μm.)
These results suggest that the loss of autonomous AR may block response of embryonic prostate epithelia to paracrine FGF10 signals. Two possibilities may explain these findings. Autonomous AR signaling may be essential for response of prostate epithelia to FGF10 signals. Alternatively, loss of epithelial AR could hamper development and differentiation of embryonic prostate epithelia that normally respond to paracrine FGF10 signaling.
Decreased Levels of AR in Adult Prostate Epithelium Diminished Response to Paracrine FGF10 and an Add Back of AR Could Restore This Response.
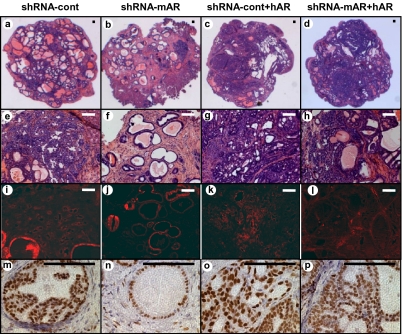
Prostate cancer predominantly affects adult males. In the next set of experiments, endogenous levels of AR were dramatically reduced in adult prostate epithelium by using shRNA knockdown. Importantly, basal levels of AR expression were maintained to promote the differentiation of regenerated prostate epithelium. The shRNA targeting murine AR (34) or a control scrambled shRNA were cloned into a lentiviral vector tagged with RFP (Fig. S4A). shRNA-mediated knockdown of AR protein was confirmed (Fig. S4B). Dissociated WT prostate epithelia were infected with either construct, combined with FGF10 UGSM and placed in the in vivo regeneration assay. Knockdown of AR expression resulted in a decrease in the percentage area of tumor-regenerated tissue (Fig. 3 A vs. B and Fig. S4C). Expression of the shRNA-mAR in prostate epithelia resulted in the regeneration of some RFP-positive tubules (Fig. 3J) that had relatively lower levels of AR (Fig. 3 N vs. M) and were less hyperplastic compared with controls (Fig. 3F and Fig. S4D). Some tubules appeared completely normal in histology with prostate secretions (Fig. 3F). To ask if the add back of AR in adult epithelium could rescue the response to paracrine FGF10, human AR was expressed from the same vector as the shRNA-mAR (Fig. S4A) and add back of AR protein by this vector was confirmed (Fig. S4B). Because of the sequence specificity of shRNA-mAR to murine AR, expression of human AR should not be affected. Add back of AR to adult prostate epithelia restored response to paracrine FGF10 demonstrated by histology (Fig. 3B vs. Fig. 3D) and percentage area of tumor-regenerated tissue (Fig. S4C).
Fig. 3.
Decreased levels of epithelial AR attenuated response to paracrine FGF10, whereas add back of AR restored this response in adult epithelium. To knock down AR in WT murine adult epithelium, an shRNA against mouse AR was used (shRNA-mAR). Simultaneous with knockdown of murine AR, add back of AR was achieved by using a human AR in the same lentiviral construct (shRNA-mAR+hAR). As control, a scramble shRNA construct was used with or without human AR (shRNA-control and shRNA-control+hAR). The 2 × 105 prostate epithelia were infected with each lentivirus and combined with 4 × 105 FGF10-UGSM cells. The predominance of regenerated glands in shRNA-mAR grafts were normal, in contrast to regeneration of predominantly hyperplastic glands in the control-shRNA grafts (A and E vs. B and F). Expression of RFP marked the site of lentiviral infection (I–L). Although residual levels of AR were detected in all shRNA-mAR–regenerated tubules, these glands were predominantly normal with decreased levels of epithelial AR compared with control glands (N vs. M). Add back of hAR concomitant with loss of mAR resulted in the formation of predominantly hyperplastic glands histologically similar to shRNA-control+hAR (C and G vs. D and H). All regenerated glands with AR add back had high levels of nuclear AR (O, P). (Scale bars: 100 μm.)
Collectively these results demonstrate that decreased levels of AR in adult prostate epithelium diminished response to paracrine FGF10, and add back of androgen receptor could restore this response.
Autonomous AR Signaling Functions as a Tumor Promoter in Adult Prostate Epithelia Exposed to Paracrine FGF10.
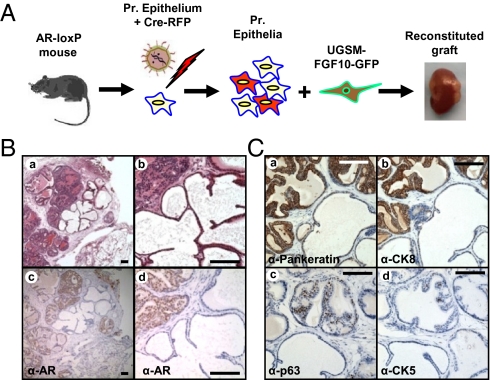
Cross talk between epithelium and stroma may result in selective pressures in the microenvironment that can influence tumor progression (35, 36). Differential response of WT and AR-null prostate epithelium to paracrine FGF10 could be caused by alteration in the FGF10-induced tumor microenvironment resulting from loss of epithelial AR. To address this question, a mixture of WT and AR-null prostate epithelial cells were exposed to paracrine FGF10 and regenerated in the same graft. Prostate epithelium was harvested from mice where AR is floxed by loxP sites (37). Dissociated cells were infected with a Cre-recombinase expressing lentivirus FU-CreRFP (Fig. S5A), resulting in loss of epithelial AR in subsets of prostate epithelia (Fig. 4A). These cells were combined with FGF10-UGSM and placed in the prostate regeneration system (Fig. 4A). Resulting grafts contained areas of hyperplasia with secretions adjacent to atrophic tubules lacking prostatic secretions (Fig. 4B, a and b). Hyperplastic regions expressed high levels of epithelial AR, whereas immunodetection of AR was minimal to absent in the atrophic tubules (Fig. 4B, c and d). Given that all tissue (AR WT and AR-null) were regenerated in the same graft, the mosaic phenotype is likely a result of loss of AR in a subset of the prostate epithelia and not selective pressures induced in the tumor microenvironment. Atrophic tubules expressed pan-keratin, low levels of CK8, and few p63-positive cells (Fig. 4C and Fig. S5D). Although CK5 was expressed in hyperplastic areas, it was not detected in regenerated atrophic tubules (Fig. 4C, d).
Fig. 4.
Loss of cell autonomous AR in adult prostate epithelium did not alter the tumorigenic capacity of FGF10-overexperssing stromal microenvironment. (A) The 105 dissociated prostate epithelia harvested from ARflox/Y mice were infected with a Cre lentiviral vector resulting in the loss of AR in a subset of epithelia. These epithelia were combined with 2 × 105 FGF10-UGSM and were regenerated. (B) Areas of hyperplasia were seen adjacent to atrophic appearing glands within the same regenerated grafts (a and b). Immunohistochemistry revealed high levels of AR in the hyperplastic glands and absence of AR detection in the atrophic prostate glands (c and d). The normal prostatic secretions seen in the hyperplastic glands was absent in the atrophic tubules. (C) AR-null regenerated tubules were epithelial because they expressed pancytokeratin (a). These atrophic glands expressed low levels of prostate luminal marker CK8 (b) and had a diminution in number of basal cells compared with adjacent control/hyperplastic glands (c and d). (Scale bars: 100 μm.)
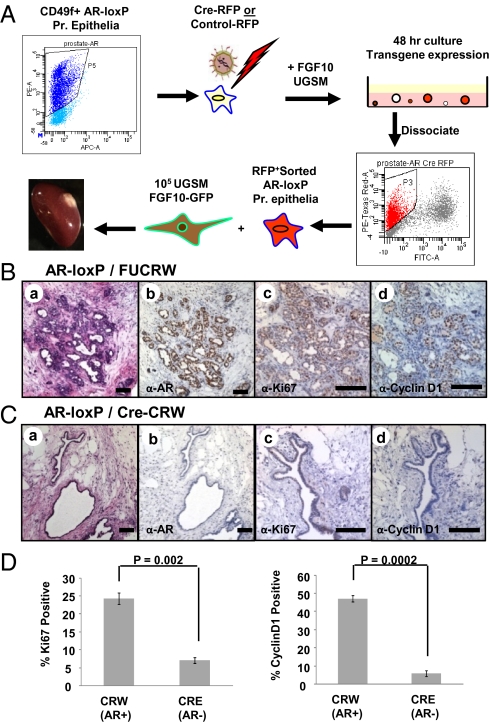
We then wanted to compare the proliferation of AR-null vs. WT adult prostate epithelia exposed to paracrine FGF10. Given that autonomous loss of AR did not affect the tumor microenvironment, the percentage of adult AR-null prostate epithelia was enriched in each regenerated graft. CD49f-positive prostate epithelial cells (38) from adult ARflox/Y mice were fluorescence-activated cell sorted (FACS) and infected with FU-Cre-CRW (Fig. S5B) or the RFP control lentivirus. Cells were combined with FGF10 UGSM and cultured in vitro to allow for viral integration and transgene expression. After 48 h, RFP-positive cells were isolated by FACS (Fig. 5A), combined with FGF10-UGSM and placed in the prostate regeneration assay. There was a diminution in prostate epithelia growth with loss of AR (Cre-expressing) compared with control (Fig. S6A, a vs. Fig. S6B, a). These results suggest that epithelial AR may be important for regeneration of prostate tubules even in the presence of WT AR-expressing stroma. The majority of tubules regenerated in the Cre-expressing grafts were atrophic, single-layered, negative for AR, and positive for RFP (Fig. 5C, a and b and Fig. S6B, a and b). In contrast, the tubules regenerated in the control grafts, were hyperplastic and expressed high levels of nuclear AR (Fig. 5B, a and b and Fig. S6A, a). Cre was detected by PCR in DNA extracted from LCM dissected tissue, with its identity authenticated by sequencing (Fig. S6C). The proliferation index of AR null and WT prostate epithelia was quantified by measuring the percentage of Ki67-positive cells in AR null and WT FGF10 grafts (Fig. 5D and Fig. 5B, c vs. Fig. 5C, c). To ascertain the number of prostate epithelia transitioning from G1 to S phase, cyclin D1 expression was also measured (Fig. 5D and Fig. 5B, d vs. Fig. 5C, d). Despite paracrine FGF10 signaling, a significant decrease in proliferating (Ki67-positive) and cycling (cyclin D1-expressing) prostate epithelia was noted with loss of epithelial AR (Fig. 5D). These results demonstrate that initiation of PIN via paracrine FGF10 relies on intact epithelial AR signaling machinery, which promotes growth of prostate epithelia in response to FGF10.
Fig. 5.
Epithelial AR functions as a tumor promoter in response to paracrine FGF10 signaling. (A) CD49f-positive, FACS-sorted ARflox/Y prostate epithelia were infected with FU-Cre-CRW or FUCRW lentiviruses marked with RFP, combined with FGF10 UGSM and placed into in vitro culture. After 48 h, RFP-positive FACS-sorted cells were combined with FGF10-UGSM and placed in the regeneration assay. (B) Control regenerated tissue was hyperplastic (a) and expressed high levels of epithelial AR in response to FGF10 (b). Hyperplastic tissues in these grafts were Ki67-positive (c) and expressed cyclin D1 (d). (C) With the loss of AR, regenerated tubules were predominantly atrophic (a). Regenerated atrophic tubules were AR negative (b). Only few cells in these glands expressed Ki67 (c) or cyclin D1 (d) (D) The percentage of Ki67-expressing and cyclin D1-expressing cells were significantly lower in the AR null tubules compared with WT AR-regenerated glands. Results are expressed as the average % positive ± 1 SD. (Scale bars: 100 μm.)
AKT-Initiated PIN Developed Independent of Cell Autonomous AR Signaling.
Loss of PTEN, resulting in cell-autonomous activation of AKT, is a common genetic alteration associated with prostate adenocarcinoma (39, 40). Mutations in the pleckstrin homology domain of AKT are also reported in prostate cancers, resulting in AKT activation independent of other alterations in the PI3Kinase pathway (41). Cell-autonomous activation of AKT is sufficient for initiation of high-grade PIN in naïve adult prostate epithelia (23, 24). We asked whether PIN induced by activation of AKT would depend on cell-autonomous signaling by AR.
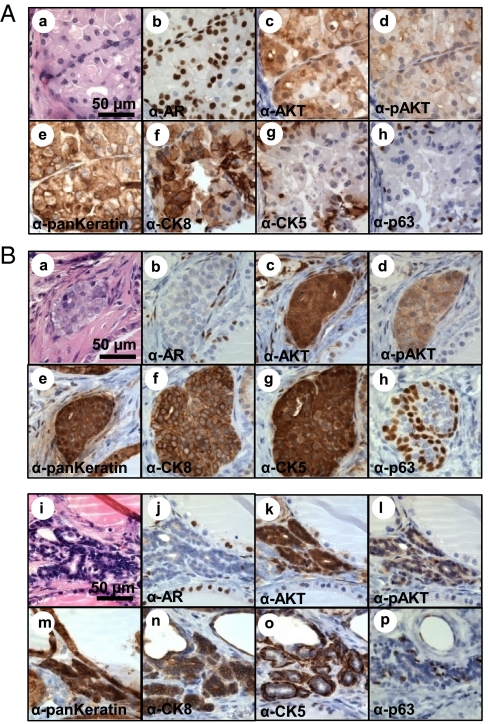
To achieve concomitant loss of AR and AKT activation, dissociated prostate epithelia from adult ARflox/Y mice were infected with a lentivirus-expressing Cre and myristoylated AKT (myrAKT) or a lentivirus expressing myrAKT alone (Fig. S7A). These cells were combined with WT stroma and placed in the prostate in vivo regeneration assay. RFP expression was lower in Cre-infected grafts, suggesting that loss of AR in prostate epithelia could diminish tubule regeneration (Fig. S8A). Expression of myrAKT in the context of epithelial AR resulted in increased proliferation of epithelial cells that formed solid nests (Fig. 6A). These cells were large with abundant cytoplasm and enlarged hyperchromatic nuclei (Fig. 6A). The borders of the nests were smooth and regular without evidence of invasion (Fig. 6A). These features are consistent with a diagnosis of mPIN, a conclusion supported by immunohistochemical staining showing the presence of p63+/CK5+ near the periphery and CK8+ cells in the center of the nests (Fig. 6A, f–h).
Fig. 6.
Cell-autonomous activation of AKT in prostate epithelia resulted in formation of PIN independent of epithelial AR signaling. (A) Cell-autonomous expression of AKT in the context of WT AR (b) resulted in the formation of epithelial nests (e) with large abundant cytoplasm, enlarged and hyperchromatic nuclei, and nuclear pleomorphism (a). Overexpression of AKT (c) and expression of phosphorylated AKT (d) was confirmed in hyperplastic epithelia. Basal cells expressing p63 (h) and CK5 (g) were detected in the periphery of CK8-expressing luminal cells (f). (B) Despite efficient Cre-mediated deletion of AR (b and j) nests of hyperplastic prostate epithelium formed (a, e, and i, m) with cell autonomous activation of AKT demonstrated by over expression of AKT (c and k) and expression of p-AKT (d and l). Some hyperplastic regions contained regions with expansion of transient amplifying cells with foci that were double positive for luminal (f) and basal (g and h) markers. Other tubules had characteristics of mPIN with a hyperplastic phenotype and glands composed of basal cells (o and p) surrounding luminal cells (n).
When AR was deleted in the context of AKT activation (FUCRW-mAKT-Cre grafts), we observed nests of proliferating epithelial cells with architectural and cytologic features identical to controls with the exception that the nests were much smaller in size (Fig. 6B). Immunohistochemical staining showed the presence of p63+ cells at nest peripheries (Fig. 6B, h and p) but in contrast to the FUCRW-mAKT grafts, some cells were double positive for CK5 and CK8 (Fig. 6B, f and g), a feature that has been described for prostate transient-amplifying cells.
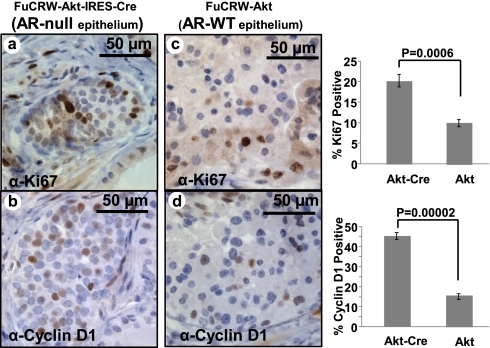
Contrary to observations in FGF10-regenerated grafts, activation of AKT in the context of epithelial AR loss resulted in increased percentages of Ki67 and cyclin D1-positive cells compared with controls (Fig. 7). These results suggest that despite the loss of AR, prostate epithelia underwent active proliferation in response to a cell autonomous mitogenic signal such as AKT activation. Despite active proliferation of AR-null–AKT-initiated PIN lesions, their overall size was smaller compared with WT AR–AKT-initiated PIN (Fig. 6A compared with Fig. 6B). These differences could not be accounted for by increased apoptosis. Quantification of apoptotic cells using the TUNEL assay revealed no significant difference in apoptosis rates of AR WT and AR-null PIN lesions initiated by AKT (Fig. S8B).
Fig. 7.
Loss of AR in prostate epithelia resulted in an increased number of proliferating and cycling cells in the context of AKT activation. To compare the percentage of proliferating cells, histologic sections of regenerated grafts expressing activated AKT were stained with Ki67 (A and C) and cyclin D1 (B and D). With cell autonomous activation of AKT an increase in the percentage of proliferating (Ki67 positive) and cycling (cyclin D1 positive) prostate epithelial was detected in AR-null epithelium compared with WT AR glands.
These results suggest that AKT-initiated PIN occurred independent of autonomous AR signaling. Loss of epithelial AR, in the context of AKT activation, led to increased cycling and proliferation in prostate epithelia; however, the overall size of PIN lesions were smaller compared with controls.
Discussion
We provide evidence demonstrating that epithelial AR can function either as a tumor promoter or a tumor suppressor, and its function in prostate tumorigenesis depends on the cancer initiating signal. A major advantage of our experimental approach compared with previously published models (16) is the ability to achieve loss of AR in all prostate epithelia (basal and luminal) simultaneous with exposure to cancer-initiating signals (autonomous AKT or paracrine FGF10). A potential drawback of our approach is the possible diminution in development and differentiation of cancer-initiating cells due to loss of epithelial AR. To address this concern in FGF10-initiated tumors, partial loss of AR was achieved with an shRNA allowing basal levels of AR expression allowing differentiation of prostate epithelia. Our results with all three experimental approaches used in the FGF10 model (Tfm, AR-knockdown, and AR-loxP) point to a critical role for AR in mediating response to this paracrine growth promoting signal. Similar to tumors initiated in the AR-knockout (ARKO) TRAMP mice (16), cell autonomous AKT could initiate PIN independent of epithelial AR. Upon epithelial loss of AR, AKT-initiated tumors resembled the histology of WT tumors but were smaller in size despite a higher proliferation index and number of actively cycling cells. Some AR-null AKT-initiated foci had characteristics of transient amplifying cells, expressing both luminal and basal cell markers. It is possible that initiation of PIN by AKT signaling may be independent of AR, whereas cell-autonomous AR signaling is required to establish differentiated lineages in the neoplastic lesion. Given that signaling by ER α may also have an important role in prostate cancer initiation (reviewed in ref. 42), its expression was examined. Expression of ER α was low in FGF10 grafts both in hyperplastic AR-WT areas and in AR-null atrophic regions (Fig. S9A). Conversely, expression of epithelial ER α was heterogeneous in both WT AR and AR-null AKT-initiated hyperplastic lesions (Fig. S9B). We propose that the differential dependence on cell-autonomous AR in each of these hyperplastic lesions is due to differences in the growth-promoting signals.
The tumor microenvironment can play an important role in initiation of carcinomas; therefore, stromal AR may be equally important in the initiation and progression of prostate cancer. Tumors that developed in prostate epithelial-specific ARKO TRAMP mice were larger with a higher proliferation index compared with ARKO TRAMP tumors where AR is lost in both epithelium and stroma (43). These results suggest that signaling through stromal AR may have a tumor-promoting effect, but do not answer this important question experimentally. The in vivo prostate regeneration model system could be an ideal platform for testing the role of stromal AR in initiation and progression of prostate cancer.
Given that prostate cancer affects 1 of 6 men (44), chemoprevention for this disease should be explored particularly in patients at high risk for developing prostate neoplasia. In two randomized prospective trials, administration of 5-α-reductase inhibitors (blocking conversion of testosterone to dihydrotestosterone, a more potent ligand for the AR) demonstrated a relative risk reduction of ≈20% in incidence of prostate cancer (45, 46). These clinical trials demonstrate that chemoprevention for prostate cancer is achievable. The natural biology of prostate cancer, which typically has a protracted clinical course starting with a preneoplastic lesion (PIN), also provides a unique opportunity for chemoprevention. A better understanding of genetic predisposing factors and the molecular profile of preneoplastic lesions likely to progress to invasive cancer can provide valuable insights into identifying patients that may benefit from chemoprevention. Despite the conventional belief that AR plays a central role in driving prostate tumorigenesis, administration of antiandrogens may need to be individualized to achieve clinical benefits. Understanding the compartment specific role of AR in response to specific growth promoting signals during prostate tumorigenesis will ultimately have important implications for chemoprevention and therapy of this prevalent hormonally regulated tumor.
Materials and Methods
Experimental Animals.
Mice were maintained in accordance with University of California, Los Angeles (UCLA) Department of Laboratory Animal Medicine-approved protocols and animal experiments were performed under UCLA Animal Research Committee approval. Mating and genotyping are described in Table S1 and SI Materials and Methods.
Plasmids.
MSCV-IRES-GFP and MSCV-FGF10-IRES-GFP plasmids were described (22). Details of vector construction are described in SI Materials and Methods.
Regeneration of the Prostate and Viral Infection of Prostate and Stromal Cells.
Regeneration and viral infection of prostate epithelia and stromal cells have been described (22, 31). Dissection and dissociation of UGSE and UGSM was based on published protocols (8, 31). To generate FGF10 UGSM, WT UGSM was infected with a retrovirus expressing FGF10 and the color marker green fluorescent protein (GFP) (22).
Immunohistochemistry.
Fixation of tissue specimens was performed in 10% buffered formalin and then ethanol as described (31). Immunostaining details are described in SI Materials and Methods.
Laser Capture Microdissection.
LCM was performed by using the Arcturus PixCell IIe machine. Cre detection from LCM tissue is detailed in SI Materials and Methods.
FACS.
Dissociated prostate cells were suspended in DMEM/10% FBS, and cell sorting was performed on the BD FACS Aria II. For CD49f sorting, cells were stained with PE-conjugated anti-mouse CD49f (BD Pharmingen 555736) for 30 min at 4 °C.
Quantification of Proliferation and Apoptosis.
Percentages of Cyclin D1, Ki67, or TUNEL-positive epithelia were counted on three separate grafts per condition (minimum of 300 total cells) and averaged. Error bars correspond to ±SD. A two-tailed t test was used to determine statistical significance.
Supplementary Material
Acknowledgments
We thank Ms. Shirley Quan for her help in arranging mice and Drs. John T. Isaacs, Hong Wu, and David Mulholland for helpful discussions and providing some reagents. S.M. is supported by a PCF Young Investigators Award, Building Interdisciplinary Research Careers in Women's Health Grant 5 K12 HD001400 (National Institutes of Health/National Institute of Child Health and Human Development), Liz Tilberis Scholars Program from the Ovarian Cancer Research Fund, UCLA Jonsson Comprehensive Cancer Center Foundation Seed Grant, The Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research Award, and The Scholars in Translational Medicine gift. H.C. was supported by US Army Medical Research: Department of Defense/Prostate Cancer Research Program Prostate Cancer Training Award X81XWH-08-1-0329. R.L. was supported by California Institute for Regenerative Medicine Training Grant T1-00005. J.H. is supported by American Cancer Society Grant RSG-07-092-01-TBE, Department of Defense Prostate Cancer Research Program PC061456, a Development Research Award from University of California, Los Angeles Prostate Cancer Science Prize for Online Resources in Education [principal investigator (PI): R. Reiter], and a Creativity Award from the Prostate Cancer Research Foundation (PI: M. Rettig). L.X. is supported by an R00CA125937 Grant. This work is supported in part by award from the Prostate Cancer Foundation (to O.N.W. and J.H.). O.N.W. is an Investigator of The Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105243108/-/DCSupplemental.
References
- 1.Rice LW. Hormone prevention strategies for breast, endometrial and ovarian cancers. Gynecol Oncol. 2010;118:202–207. doi: 10.1016/j.ygyno.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28:4038–4044. doi: 10.1200/JCO.2009.27.4290. [DOI] [PubMed] [Google Scholar]
- 3.Lydon JP, Ge G, Kittrell FS, Medina D, O'Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59:4276–4284. [PubMed] [Google Scholar]
- 4.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 5.Huggins C, Sr, Hoges CV. Studies on prostatic cancer II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 6.Kohli M, Tindall DJ. New developments in the medical management of prostate cancer. Mayo Clin Proc. 2010;85:77–86. doi: 10.4065/mcp.2009.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha GR. Mesenchymal-epithelial interactions: Past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 8.Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 9.Tenniswood M. Role of epithelial-stromal interactions in the control of gene expression in the prostate: an hypothesis. Prostate. 1986;9:375–385. doi: 10.1002/pros.2990090407. [DOI] [PubMed] [Google Scholar]
- 10.Le H, Arnold JT, McFann KK, Blackman MR. DHT and testosterone, but not DHEA or E2, differentially modulate IGF-I, IGFBP-2, and IGFBP-3 in human prostatic stromal cells. Am J Physiol Endocrinol Metab. 2006;290:E952–E960. doi: 10.1152/ajpendo.00451.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- 12.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–2128. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- 13.Thomson AA. Mesenchymal mechanisms in prostate organogenesis. Differentiation. 2008;76:587–598. doi: 10.1111/j.1432-0436.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 15.Vander Griend DJ, et al. Cell-autonomous intracellular androgen receptor signaling drives the growth of human prostate cancer initiating cells. Prostate. 2010;70:90–99. doi: 10.1002/pros.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu Y, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci USA. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson DA, et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci USA. 2010;107:2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulholland DJ, et al. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeal JE, Bostwick DG. Intraductal dysplasia: A premalignant lesion of the prostate. Hum Pathol. 1986;17:64–71. doi: 10.1016/s0046-8177(86)80156-3. [DOI] [PubMed] [Google Scholar]
- 21.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 22.Memarzadeh S, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin L, et al. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci USA. 2006;103:7789–7794. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 27.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed NN, et al. The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 29.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 30.Andjelković M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 31.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 33.He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19:2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao J, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 35.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Bierie B, Moses HL. Under pressure: stromal fibroblasts change their ways. Cell. 2005;123:985–987. doi: 10.1016/j.cell.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 37.De Gendt K, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 40.Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- 41.Boormans JL, et al. E17K substitution in AKT1 in prostate cancer. Br J Cancer. 2010;102:1491–1494. doi: 10.1038/sj.bjc.6605673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellem SJ, Risbridger GP. Treating prostate cancer: A rationale for targeting local oestrogens. Nat Rev Cancer. 2007;7:621–627. doi: 10.1038/nrc2174. [DOI] [PubMed] [Google Scholar]
- 43.Niu Y, et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci USA. 2008;105:12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 45.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 46.Andriole GL, et al. REDUCE Study Group Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.