Abstract
Individuals with mood disorders exhibit alterations in the fibroblast growth factor system, including reduced hippocampal fibroblast growth factor-2 (FGF2). It is difficult, however, to pinpoint whether these alterations are a cause or consequence of the disorder. The present study asks whether FGF2 administered the day after birth has long-lasting effects on hippocampal development and emotionality. We show that early-life FGF2 shifts the pace of neurogenesis, with an early acceleration around weaning followed by a deceleration in adulthood. This, in turn, results in a denser dentate gyrus with more neurons. To assess the impact of early-life FGF2 on emotionality, we use rats selectively bred for differences in locomotor response to novelty. Selectively bred low-responder (bLR) rats show low levels of novelty-induced locomotion and exhibit high levels of anxiety- and depression-like behavior compared with their selectively bred high-responder counterparts. Early-life FGF2 decreased anxiety-like behavior in highly anxious bLRs without altering other behaviors and without affecting high-responder rats. Laser capture microscopy of the dentate gyrus followed by microarray analysis revealed genes that were differentially expressed in bLRs exposed to early-life FGF2 vs. vehicle-treated bLRs. Some of the differentially expressed genes that have been positively associated with anxiety were down-regulated, whereas genes that promote cell survival were up-regulated. Overall, these results show a key role for FGF2 in the developmental trajectory of the hippocampus as well as the modulation of anxiety-like behavior in adulthood, and they point to potential downstream targets for the treatment of anxiety disorders.
Keywords: elevated plus maze, gene expression microarray
Human postmortem studies suggest a role for the fibroblast growth factor (FGF) system in the pathophysiology of mood disorders. The expression of several members of the FGF family was altered in the forebrain of patients with major depressive disorder (MDD). Specifically, FGF2 mRNA expression was down-regulated in cortical areas and the hippocampus in MDD compared with controls (1, 2). The hippocampus seems to be particularly affected in MDD (3). A potential role for FGF2 in depression and anxiety disorders was first suggested by pharmacological studies in rodents, which showed that administration of antidepressant and anxiolytic drugs can increase FGF2 expression in the hippocampus (3–6). We then asked whether FGF2 is a modulator of emotionality in rodents and discovered that repeated administration of FGF2 was both antidepressant and anxiolytic (7, 8).
Using rats selectively bred for differences in novelty-seeking, we found that hippocampal expression of FGF2 mRNA was higher in high responders to novelty (bHRs) than in the low responders to novelty (bLRs). The bHRs have higher FGF2 levels and exhibit less anxiety-like behavior, whereas the bLRs exhibit more anxiety-like behavior. FGF2 seems to be a protective factor against anxiety-like behavior in the adult (7). These results suggest that FGF2 could mediate the genetic influences on anxiety-like behavior and led us to ask whether it plays a causative role during early development.
We focused on the hippocampus because of its critical role in processing emotionally salient information and controlling behavior (9–11). Although the present study focuses on the impact of FGF2 treatment on the hippocampus, this does not exclude the possibility of its effects on other brain regions. Indeed, other studies have shown how this manipulation results in increased cerebellar growth (12). FGF2 also has known organizational effects in the hippocampus, including increased cell number, volume, and survival in the dentate gyrus 3 wk after administration on the second day of life (13, 14). Neurogenesis peaks during the first week of life, and FGF2 treatment during this time window has been previously shown to cross the blood–brain barrier (13–15). However, the long-term effects of early-life FGF2 have not been described. Moreover, the consequences of this treatment on adult behavior have not yet been elucidated.
Here, we ask if a brief exposure to increased levels of FGF2 early in life can alter the trajectory of hippocampal development and affect anxiety-like behavior. Would this effect be powerful enough to rescue rats that are genetically predisposed to high levels of anxiety-like behavior and reverse their phenotype? If so, what are the molecular changes that accompany this behavioral modification?
To this end, we assessed hippocampal neurogenesis of weanling and adult rats exposed to one injection of FGF2 during early life. We then assessed the effect of early-life FGF2 on anxiety-like behavior in rats selectively bred to differ in this behavior and characterized the alterations in hippocampal gene expression in the adult rats most impacted by this treatment.
Results
FGF2 Treatment Boosts Cell Proliferation and Survival in the Dentate Gyrus 3 Wk Later.
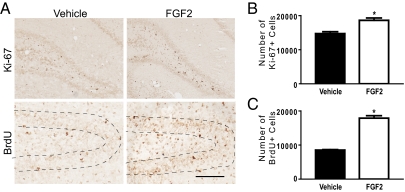
We first assessed the effect of FGF2 or vehicle on cell proliferation and survival in the dentate gyrus 3 wk after treatment [postnatal day 23 (PND23)] on the day after birth (PND2). Fig. 1A illustrates BrdU and Ki-67 immunoreactivities in the dentate gyrus at PND23. There was a significant increase in the number of Ki-67–labeled cells in the subgranular zone of the dentate gyrus in FGF2 rats compared with vehicle rats 3 wk after treatment, which is indicative of increased cell proliferation (Fig. 1B). There was also a significant increase in the number of BrdU-labeled cells in the dentate gyrus 3 wk after injection in FGF2 rats compared with vehicle rats, which is indicative of increased cell survival (Fig. 1C). These results suggest that FGF2 can boost cell proliferation and cell survival in the dentate gyrus during the first 3 wk of life.
Fig. 1.
FGF2 increased cell proliferation and cell survival in the dentate gyrus 3 wk later (PND23). (A) Ki-67 immunoreactivity (Upper) in the subgranular zone of the dentate gyrus and BrdU immunoreactivity (Lower) in the granule cell layer of the dentate gyrus in vehicle (Left) and FGF2 (Right) rats. Sections were stained with the monoclonal antibody anti–Ki-67 or the monoclonal antibody anti-BrdU. (Scale bar: 50 μm.) (B) FGF2 (n = 3) increased cell proliferation in the subgranular zone of the dentate gyrus compared with vehicle (n = 3) rats (t(4) = −4.25, P < 0.05). (C) FGF2 (n = 3) increased cell survival in the granule cell layer of the dentate gyrus compared with vehicle (n = 3) rats (t(4) = −12.50, P < 0.001). All values are mean ± SEM.
FGF2 Treatment Alters Neurogenesis in the Adult Dentate Gyrus.
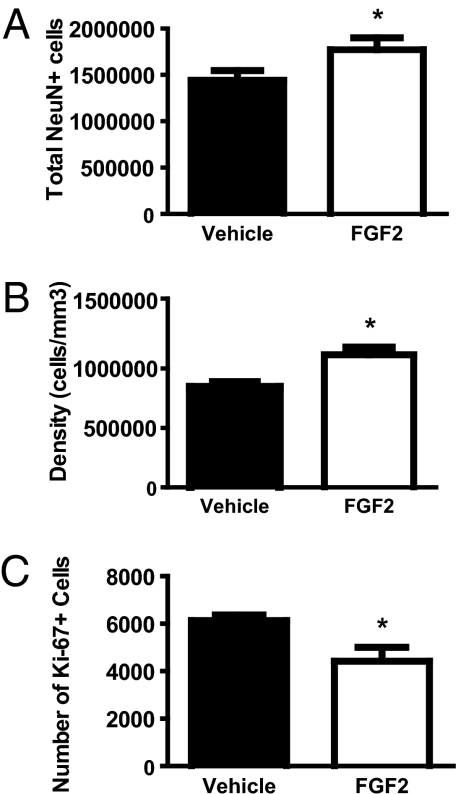
Next, we determined whether FGF2 altered the adult hippocampus. We assessed differences in cell proliferation in the subgranular zone of the dentate gyrus and the composition of the granule cell layer in adult rats (PND60). NeuN was used to distinguish neurons. As adults, there was a significant increase in the total number of neurons in FGF2-exposed rats compared with vehicle rats in the dentate gyrus (Fig. 2A). Although volume (Fig. S1A) and total cell numbers (Fig. S1B) were not different between the two groups in adulthood, FGF2 rats had a denser dentate gyrus than vehicle controls (Fig. 2B). Interestingly, FGF2 rats exhibited significantly less cell proliferation than vehicle controls, showing a clear inversion of the pattern seen in development (Fig. 2C). These results suggest that a single FGF2 injection leads to permanent alterations in the cellular composition of the adult hippocampus. This is exemplified by a densely packed neuronal population and significantly decreased cell proliferation in the adult dentate gyrus. Thus, FGF2 altered cell proliferation and resulted in long-term changes in hippocampal structure in adulthood.
Fig. 2.
FGF2 increased neuron number and cell density and decreased cell proliferation in the adult dentate gyrus (PND60). (A) FGF2 (n = 4) increased the total number of neurons in the granule cell layer of the dentate gyrus compared with vehicle (n = 4) rats (t(6) = 2.89, P < 0.05). (B) FGF2 (n = 4) increased the density of cells in the granule cell layer of the dentate gyrus compared with vehicle (n = 4) rats (t(6) = 2.63, P < 0.05). (C) FGF2 (n = 4) decreased cell proliferation in the subgranular zone of the dentate gyrus compared with vehicle (n = 4) rats (t(6) = 2.48, P < 0.05). All values are mean ± SEM.
Basal FGF2 Gene Expression in the Dentate Gyrus Is Higher in bHR Relative to bLR.
We then used a model with individual differences in emotional reactivity to assess basal FGF2 gene expression (16). Fig. S2A illustrates FGF2 gene expression in the hippocampus of bLR and bHR rats from the 21st generation of our breeding colony. As shown in Fig. S2B, adult bHR rats exhibited significantly higher FGF2 gene expression in the dentate gyrus than adult bLR rats. Moreover, FGF2 gene expression in the dentate gyrus was positively correlated with locomotor activity in response to novelty (Fig. S3). However, FGF2 gene expression was unchanged in other hippocampal subfields (Fig. S4). Thus, higher basal levels of FGF2 in the dentate gyrus in adulthood were correlated with increased locomotor activity in response to novelty.
FGF2 Reduces Anxiety-Like Behavior in bLRs Without Altering Other Behaviors.
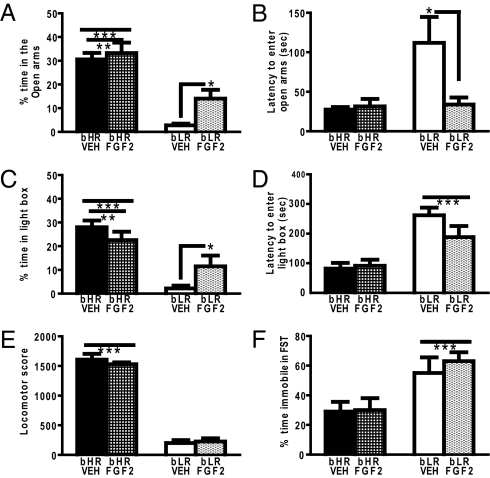
Because bHR adults exhibited increased FGF2 expression compared with bLRs, we determined whether FGF2 would alter anxiety-like behavior in either strain. bHR rats spent more time in the open arms of the elevated plus maze (EPM) than bLR, and FGF2 rats spent more time in the open arms compared with vehicle rats, indicating reduced anxiety-like behavior (Fig. 3A). Although there was no significant phenotype by FGF2 treatment interaction, a one-way ANOVA revealed that FGF2 reduced anxiety-like behavior in bLR. Although FGF2 reduced anxiety-like behavior in bLRs, it is important to note that it did not completely reverse their phenotypic behavior. FGF2 also impacted the latency of bLRs to enter the open arms of the EPM, with FGF2 rats exhibiting shorter latency to initially enter the open arms; again, this suggests reduced anxiety-like behavior (Fig. 3B).
Fig. 3.
FGF2 altered anxiety-like behavior in bLR rats in adulthood. (A) FGF2 resulted in increased time spent in the open arms of the EPM. Specifically, anxiety-like behavior was decreased in bLR/FGF2 compared with bLR/VEH rats (two-way ANOVA: group, F(1,29) = 50.75, P < 0.001; treatment, F(1,29) = 4.48, P < 0.05; one-way ANOVA: group, F(3,29) = 5.38, P < 0.05). *P < 0.05; Fisher's least significant difference (LSD) posthoc test in bLR/VEH (n = 8) vs. bLR/FGF2 (n = 8). **P < 0.005; Fisher's LSD posthoc test in bHR/FGF2 (n = 9) vs. bLR/FGF2 (n = 8) and bHR/VEH (n = 8) vs. bLR/FGF2 (n = 8). ***P < 0.001; Fisher's LSD posthoc test in bHR/FGF2 (n = 9) vs. bLR/VEH (n = 8) and bHR/VEH (n = 8) vs. bLR/VEH. (B) FGF2 resulted in a decreased latency to enter the open arms of the EPM. Specifically, anxiety-like behavior was decreased in bLR/FGF2 rats compared with bLR/VEH rats (two-way ANOVA: group, F(1,29) = 6.26, P < 0.05; treatment, F(1,29) = 4.55, P < 0.05; group × treatment interaction, F(1,29) = 5.64, P < 0.05). *P < 0.005; Fisher's LSD posthoc test in bLR/FGF2 (n = 8) vs. bLR/VEH (n = 8), bLR/FGF2 (n = 8) vs. bHR/FGF2 (n = 9), and bLR/FGF2 (n = 8) vs. bHR/VEH (n = 8). (C) FGF2 resulted in an increase in time spent in the light compartment of the light–dark box, suggesting decreased anxiety-like behavior in bLR/FGF2 rats compared with bLR/VEH rats (two-way ANOVA: group, F(1,46) = 29.65, P < 0.0001; group × treatment interaction, F(1,46) = 6.01, P < 0.05). *P < 0.05; Fisher's LSD posthoc test in bLR/FGF2 (n = 11) vs. bHR/FGF2 (n = 14) and bLR/FGF2 (n = 11) vs. bHR/VEH (n = 14). **P < 0.005; Fisher's LSD posthoc test in bLR/VEH (n = 11) vs. bHR/FGF2 (n = 14) and bLR/VEH (n = 11) vs. bHR/VEH (n = 14). ***P < 0.001; Fisher's LSD posthoc test in bLR/FGF2 (n = 11) vs. bLR/VEH (n = 11). (D) FGF2 did not alter the latency to enter the light in bLR rats (two-way ANOVA: group, F(1,46) = 29.35). ***P < 0.0001. Number of rats: bLR/VEH (n = 11), bLR/FGF2 (n = 11), bHR/FGF2 (n = 14), and bHR/VEH (n = 14). (E) FGF2 did not alter locomotor activity in bLR rats (two-way ANOVA: group, F(1,29) = 541.53). ***P < 0.0001. Number of rats: bLR/VEH (n = 8), bLR/FGF2 (n = 8), bHR/FGF2 (n = 9), and bHR/VEH (n = 8). (F) FGF2 also did not alter depression-like behavior in bLR rats (two-way ANOVA: group, F(1,29) = 15.80). ***P < 0.0005. Number of rats: bLR/VEH (n = 8), bLR/FGF2 (n = 8), bHR/FGF2 (n = 9), and bHR/VEH (n = 8). All values are mean ± SEM.
In the light–dark box, bHRs spent more time in the light box than bLRs (Fig. 3C) and exhibited a shorter latency to enter the light than bLRs (Fig. 3D), indicating reduced anxiety-like behavior. Although there was no effect of FGF2, there was a significant phenotype by treatment interaction. bLRs/FGF2 spent more time in the light box compared with bLRs/vehicle (VEH).
In terms of locomotor activity, all bHR rats exhibited significantly more locomotor activity than bLRs (Fig. 3E). FGF2 had no effect on locomotor activity, and there was no interaction. In the forced swim test, there was a main effect of phenotype, because all bLRs exhibited more immobility compared with bHRs (Fig. 3F). However, there was no effect of treatment and no phenotype by treatment interaction. Thus, FGF2 had no effect on novelty-induced locomotor activity or depression-like behavior.
FGF2 Alters Gene Expression in the Adult Dentate Gyrus of bLR Rats.
We next sought to determine the genes that were altered between bLR/FGF2 and bLR/VEH rats, which might contribute to their differences in anxiety-like behavior. Microarray analysis identified a number of transcripts (n = 226) that were significantly different between bLR/FGF2 rats compared with bLR/VEH rats in adulthood (Dataset S1). Interestingly, an equal number of genes were significantly up-regulated (49.6%) and down-regulated (50.4%). Table S1 shows the specific transcripts that were significantly above threshold in the top five functions based on ingenuity analysis. Based on this analysis, the majority of the transcripts were related to cellular functions, including cell morphology, cellular assembly, organization, and movement.
FGF2 Increases Neurotrophic Tyrosine Kinase Receptor Type 3 Gene Expression in the Adult Dentate Gyrus of bLR Rats.
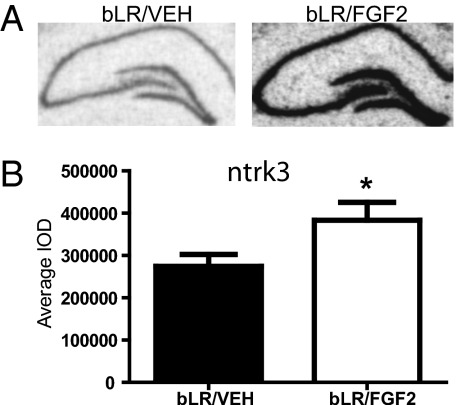
Glutamic acid decarboxylase 1 (gad1) and neurotrophic tyrosine kinase receptor type 3 (ntrk3) were selected for confirmation by mRNA in situ hybridization. Vimentin (vim) and bcl2-like 2 (bcl2l2) were selected for confirmation by quantitative RT-PCR (qRT-PCR). Although ntrk3 and bcl2l2 were not included in the top five ingenuity functions (Table S1), these genes were of interest for other reasons. The selection of genes for confirmation from Dataset S1 was based primarily on previous associations with anxiety or neurogenesis. Because we can predict the direction of change based on the microarray results, we performed one-tailed t tests. As illustrated in Fig. 4A, ntrk3 gene expression seemed elevated in various subfields of the hippocampus of bLR/FGF2 rats compared with bLR/VEH rats. In Fig. 4B, ntrk3 gene expression was significantly increased in the dentate gyrus in bLR/FGF2 compared with bLR/VEH rats. However, no effect of FGF2 treatment on bLRs was observed in the subiculum, suggesting regional selectivity of the effect (Fig. S5). There was no difference in gad1 gene expression in the dentate gyrus of bLR/FGF2 compared with bLR/VEH rats (Fig. S6).
Fig. 4.
FGF2 altered gene expression in the dentate gyrus by mRNA in situ hybridization in bLR rats in adulthood. (A) Representative images of ntrk3 gene expression in the hippocampus of bLR/VEH (Left) and bLR/FGF2 (Right) rats. (B) bLR/FGF2 (n = 4) rats exhibited increased ntrk3 gene expression compared with bLR/VEH (n = 4) rats (t(6) = −2.15, *P < 0.05). All values are mean ± SEM.
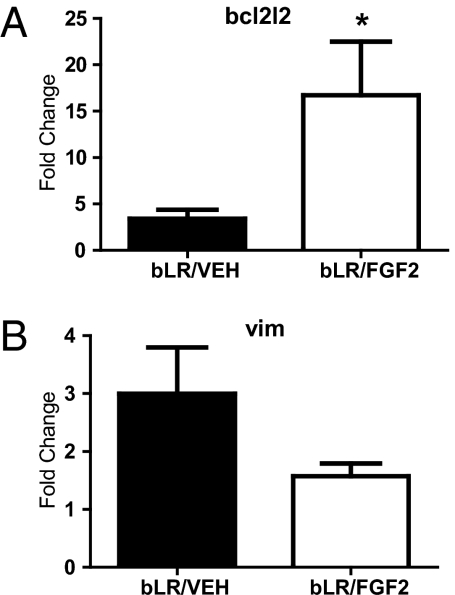
FGF2 Increases bcl2l2 Expression in the Adult Dentate Gyrus of bLR Rats.
As shown in Fig. 5A, bcl2l2 was significantly increased in the dentate gyrus of bLR/FGF2 compared with bLR/VEH rats. There was also a nonsignificant trend for vimentin to be decreased in the dentate gyrus of bLR/FGF2 compared with bLR/VEH rats (Fig. 5B). Overall, the validation experiments suggest that ntrk3 and bcl2l2 might be involved in the differences in anxiety-like behavior observed between bLR/FGF2 and bLR/VEH rats.
Fig. 5.
FGF2 altered gene expression in the dentate gyrus by qRT-PCR in bLR rats in adulthood. (A) bLR/FGF2 (n = 4) rats exhibited increased bcl2l2 gene expression compared with bLR/VEH (n = 4) rats (t(6) = −2.26, *P < 0.05). (B) There was a nonsignificant trend for bLR/FGF2 rats (n = 4) to exhibit decreased vimentin gene expression compared with bLR/VEH rats (n = 4; t(6) = 1.73, P = 0.07). All values are mean ± SEM.
Discussion
This study manipulated FGF2 and characterized the resulting long-term alterations on hippocampal neurogenesis and gene expression as well as the consequences on emotional behavior. Based on our data, we draw four conclusions. (i) FGF2 levels early in life can determine the developmental trajectory of the dentate gyrus throughout life. (ii) Elevated levels of FGF2 early in life increase neuronal number and cell density in the adult dentate gyrus. (iii) Early-life exposure to FGF2 selectively rescues the high anxiety-like behavior phenotype of the bLR rats that have low FGF2 levels. (iv) The reduction in anxiety in bLR rats is associated with altered expression of genes involved in cell survival and anxiety. Remarkably, a single FGF2 injection on the second day of life resulted in lasting changes in the adult dentate gyrus and decreased anxiety-like behavior in rats genetically bred to express this phenotype.
Early-life FGF2 altered the hippocampus of juvenile rats, with FGF2 weanling rats exhibiting more cell proliferation and potentially, more cell survival in the dentate gyrus relative to controls. Because BrdU was administered 6 h after the initial injection of FGF2 and assessed 3 wk later, we cannot state for certain that cell survival was altered without other effects. It is important to note that FGF2 may have affected the proliferation rate or rate of cell migration into the dentate gyrus as well. Early-life FGF2 also resulted in profound long-term alterations in the adult hippocampus. FGF2 rats exhibited an increased number of neurons in the dentate gyrus without a significant change in hippocampal volume, resulting in increased neuronal density. However, it should be noted that any negative finding should be interpreted with caution given the small sample sizes used in these studies. However, these results extend previous findings on the effects of FGF2 in early development (12–14) and are consistent with our finding that FGF2 administration in adulthood increased the survival of neurons in the dentate gyrus (7). Overall, FGF2 treatment produced an initial acceleration of neurogenesis early in life, which led to long-term alterations in the hippocampus. This subsequently caused a shift to a mostly neuronal phenotype in the dentate gyrus, and it is consistent with the fact that FGF2 promotes neurogenesis before gliogenesis during early development (17, 18).
The above-mentioned effects may result in subtle but functionally relevant changes in behavioral interactions with the environment. Recent findings in humans have shown that the developmental trajectory of change in the morphology of the cerebral cortex, rather than the final cortical morphology per se, is predictive of level of intelligence in adults (19). Interestingly, differences in hippocampal volume have been reported in patients with affective disorders (20). However, whether the trajectory of hippocampal development is altered in humans vulnerable to anxiety has not been studied.
The anxious phenotype in bLRs was rescued by FGF2, and the rats exhibited anxiety-like behavior within the normal range. These results establish a link between FGF2 around birth and subsequent vulnerability to anxiety. Here, we present evidence that FGF2 in the dentate gyrus is correlated with novelty-induced locomotion, a behavior that is also correlated with measures of anxiety-like behavior. We have previously shown that the anxious phenotype is associated with neurogenesis in adult bLR and bHR rats (7). Moreover, we have recent evidence showing that decreased FGF2 gene expression in the dentate gyrus is associated with increased anxiety-like behavior in outbred rats (21).
The microarray studies were focused on investigating changes in the dentate gyrus of those rats that profited from the FGF2 treatment and thus, compared bLR/FGF2 with bLR/VEH. However, genes altered in the dentate gyrus could also be altered in other areas. Several genes involved in cellular functions were altered by early-life FGF2, and these findings were consonant with our observations in the neurogenesis studies. The increased ntrk3 expression was confirmed in bLR/FGF2 vs. bLR/VEH rats by mRNA in situ hybridization. Interestingly, there is evidence that ntrk3 promotes the survival of postmitotic neurons (22). Moreover, recent studies have linked variations in this gene, SNPs, to anxiety disorders (23–25). Taken together, these results suggest that ntrk3, which showed a lifelong change in gene expression after FGF2, is involved in cell survival in early hippocampal development and associated with anxiety-like behavior.
Also noteworthy was the finding of increased bcl2l2 in bLR/FGF2 compared with bLR/VEH rats. Structurally, bcl2l2 is a member of the bcl2 family, and bcl2 is known to enhance survival of newborn neurons in the hippocampus (26). Similarly, bcl2l2 has been shown to play a role in cell survival in cell lines (27). Similar to ntrk3, an SNP in bcl2 has been found to be associated with generalized anxiety disorder (28). These results suggest that the bcl2 family may both regulate cell survival and play a role in anxiety disorders. In support of this view, bcl2 overexpression in mice resulted in decreased fear behavior, and mice with a targeted disruption of bcl2 exhibited increased anxiety-like behavior (29, 30). These findings are in agreement with our bcl2l2 results. Indeed, the presence of SNPs associated with anxiety disorders in the two genes confirmed in this study is additional support for a developmental role of ntrk3 and bcl2l2 in anxiety disorders. However, studies that manipulate these genes in adulthood by a viral vector approach should be performed to provide functional validation.
In conclusion, a single dose of FGF2 early in life resulted in a long-term decrease in anxiety-like behavior in rats that exhibit high anxiety-like behavior (bLR). This finding suggests that the FGF system likely plays a role in the development of mood disorders. Early-life FGF2 also permanently altered the developmental trajectory of the hippocampus, producing a shift in the pattern of neurogenesis as well as lasting changes in hippocampal composition and gene expression. Thus, the developmental timing of FGF2 expression may represent a key molecular mechanism involved in the genesis of anxiety disorders and provide a possible point of therapeutic intervention.
Materials and Methods
Nonselectively Bred Rats (Outbred).
On the day after birth (PND2), litters were culled to four males and four females and injected with either FGF2 (20 ng/g in 50 μL 0.1 M PBS with 1% BSA s.c. into the axillary space; Sigma) or vehicle (0.1 M PBS with 1% BSA s.c.). FGF2 is at near maximal levels 24 h later (14). A subset of rats received a BrdU injection (100 mg/kg in 50 μL 0.9% saline s.c.; Sigma) 6 h after the PND2 FGF2 injection.
Selectively Bred Rats (bHR and bLR).
High anxiety-like behavior (bLR) and low anxiety-like behavior (bHR) rats were generated from our in-house breeding colony, where the bLR/bHR lines have been maintained for several generations (16). Adult males were tested for locomotor activity on PND60. They were tested using the EPM and light–dark box on PND62 and the forced swim test on PND64–PND65.
Locomotor Activity.
Selectively bred bHR and bLR rats from the 21st generation of our breeding colony were screened for locomotor activity as previously described (7).
EPM.
Selectively bred bHR and bLR rats were assessed in the EPM over 5 min as previously described (7).
Forced Swim Test.
Selectively bred bHR and bLR rats were assessed in the forced swim test (FST) over 2 d. Testing was performed as previously described, with the exception that none of the rats received injections between the two test days (8).
Light–Dark Box.
In a separate experiment, bLR and bHR pups from the 26th generation were tested in the light–dark box.
Immunohistochemistry.
Perfused brains were sliced at −20 °C, and a series of every 12 sections was cut at 30 μm throughout the dentate gyrus of the hippocampus. Sections were stained for BrdU, Ki-67, and NeuN.
Cell Counting.
Every 12th section was selected for cell-counting analysis. Volume estimation and the total number of cells or neurons were determined as previously described, with the following exception: the final magnification was set at 630× for the optical fractionator technique (31). For Ki-67 and BrdU analyses, total cell counts were determined as previously described (7). For each brain, the total number of BrdU- and Ki-67–labeled cells was estimated by multiplying the number of cells counted by 12 (15, 32).
Laser Capture Microdissection.
Brains from bLR/VEH and bLR/FGF2 were selected for capture. Fresh frozen brains were cryosectioned at −20 °C throughout the hippocampus at 10 μm, mounted on Superfrost Plus slides (Fisher Scientific), and stored at −80 °C until use. Sections from these series were also used for mRNA in situ hybridization. The cresyl violet step was omitted to preserve RNA quality (33). Care was taken to ensure that the same level of the dentate gyrus (Bregma = −3.30) was taken from each rat (34).
RNA Isolation and Amplification.
Total RNA from the adherent dentate cells was extracted and isolated from the laser capture microdissection (LCM) caps using a PicoPure RNA Isolation Kit (Arcturus). The isolated RNA was then amplified. The final biotin-labeled cRNA was generated from the bioarray high-yield RNA transcription kit (ENZO Life Sciences), and it resulted in 1–2 μg/μL.
Microarray Analysis.
Equal amounts (750 ng) of amplified total RNA sample from each rat were hybridized to RatRef −12 Expression BeadChips and scanned on the BeadStation system (Illumina) following the manufacturer's instructions.
qRT-PCR.
Starting from the last amplification, cDNA was diluted 1:10 and quantified with the Quant-iT PicoGreen cDNA Quantification kit (Invitrogen). cDNA (30 ng) was used in combination with ABI TaqMan Low Density Arrays custom designed using predesigned inventoried assays (Applied Biosystems). The gene expression fold-change results were calculated by the 2-ΔΔCt method (35). The sample mean Ct of the internal control (GAPDH) was subtracted from the sample mean Ct of the gene of interest (ΔCt). For each gene, the sample with the absolute highest mean ΔCt was selected as the calibrator and subtracted from the mean ΔCt of each sample (ΔΔCt) (36).
mRNA in Situ Hybridization.
An initial in situ hybridization study assessed baseline FGF2 mRNA levels in bLR vs. bHR rats. Two later studies evaluated gad1 and ntrk3 as a follow-up to the microarray experiments (Table S1). Our mRNA in situ hybridization methodology has been previously described (8). Sections were taken every 200 μm.
Statistical Analyses.
Immunohistochemistry, mRNA in situ hybridization, and qRT-PCR were analyzed by a Student t test. Anxiety-like behavior was analyzed by ANOVAs followed by Fisher's posthoc comparisons. All data are presented as mean ± SEM, and statistical significance was assumed at P < 0.05.
Supplementary Material
Acknowledgments
We are grateful to Sharon Burke, Jennifer Fitzpatrick, Mary Hoversten, James Stewart, James Beals, Suzanne Smith, Stephanie Cooke, and Ellen Pedersen for their technical support. The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund LLC. This research was supported by National Institute of Mental Health (NIMH) Grant R36MH078694, National Institutes of Health (NIH) Grant 5P01MH42251, Office of Naval Research Grant N00014-02-1-0879, Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC Grant UL1RR024986, and the Rachel Upjohn Clinical Scholars Award.
Footnotes
Conflict of interest statement: The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund LLC. A shared intellectual property agreement exists between this philanthropic fund and University of Michigan, University of California, Stanford University, Hudson-Alpha Institute, and Cornell University to encourage the development of appropriate findings for research and clinical applications.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103732108/-/DCSupplemental.
References
- 1.Evans SJ, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Pinilla F, Dao L, Choi J, Ryba EA. Diazepam induces FGF-2 mRNA in the hippocampus and striatum. Brain Res Bull. 2000;53:283–289. doi: 10.1016/s0361-9230(00)00342-7. [DOI] [PubMed] [Google Scholar]
- 5.Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA. Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol Psychiatry. 2004;55:1095–1102. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- 7.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 10.Bannerman DM, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Roullet P, Lassalle JM. Genetic variation, hippocampal mossy fibres distribution, novelty reactions and spatial representation in mice. Behav Brain Res. 1990;41:61–70. doi: 10.1016/0166-4328(90)90054-i. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Tao Y, Black IB, DiCicco-Bloom E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J Neurobiol. 2001;46:220–229. doi: 10.1002/1097-4695(20010215)46:3<220::aid-neu1004>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Black IB, DiCicco-Bloom E. Hippocampal granule neuron production and population size are regulated by levels of bFGF. Eur J Neurosci. 2002;15:3–12. doi: 10.1046/j.0953-816x.2001.01832.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 16.Stead JD, et al. Selective breeding for divergence in novelty-seeking traits: Heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- 17.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 20.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eren-Koçak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry. 2011;69:534–540. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minichiello L, Klein R. TrkB and TrkC neurotrophin receptors cooperate in promoting survival of hippocampal and cerebellar granule neurons. Genes Dev. 1996;10:2849–2858. doi: 10.1101/gad.10.22.2849. [DOI] [PubMed] [Google Scholar]
- 23.Alonso P, et al. Genetic susceptibility to obsessive-compulsive hoarding: The contribution of neurotrophic tyrosine kinase receptor type 3 gene. Genes Brain Behav. 2008;7:778–785. doi: 10.1111/j.1601-183X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Muiños-Gimeno M, et al. Allele variants in functional MicroRNA target sites of the neurotrophin-3 receptor gene (NTRK3) as susceptibility factors for anxiety disorders. Hum Mutat. 2009;30:1062–1071. doi: 10.1002/humu.21005. [DOI] [PubMed] [Google Scholar]
- 25.Armengol L, et al. 5′ UTR-region SNP in the NTRK3 gene is associated with panic disorder. Mol Psychiatry. 2002;7:928–930. doi: 10.1038/sj.mp.4001134. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, et al. Bcl2 enhances survival of newborn neurons in the normal and ischemic hippocampus. J Neurosci Res. 2006;84:1187–1196. doi: 10.1002/jnr.21036. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki T, et al. BCL2L2 is a probable target for novel 14q11.2 amplification detected in a non-small cell lung cancer cell line. Cancer Sci. 2007;98:1070–1077. doi: 10.1111/j.1349-7006.2007.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sipilä T, et al. An association analysis of circadian genes in anxiety disorders. Biol Psychiatry. 2010;67:1163–1170. doi: 10.1016/j.biopsych.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: Further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res. 2005;165:172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Rondi-Reig L, et al. Fear decrease in transgenic mice overexpressing bcl-2 in neurons. Neuroreport. 1997;8:2429–2432. doi: 10.1097/00001756-199707280-00004. [DOI] [PubMed] [Google Scholar]
- 31.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 32.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 33.Kerman IA, Buck BJ, Evans SJ, Akil H, Watson SJ. Combining laser capture microdissection with quantitative real-time PCR: Effects of tissue manipulation on RNA quality and gene expression. J Neurosci Methods. 2006;153:71–85. doi: 10.1016/j.jneumeth.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. Stereotaxic Brain Atlas. New York: Academic; 1998. [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Frank MG, Der-Avakian A, Bland ST, Watkins LR, Maier SF. Stress-induced glucocorticoids suppress the antisense molecular regulation of FGF-2 expression. Psychoneuroendocrinology. 2007;32:376–384. doi: 10.1016/j.psyneuen.2007.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.