Abstract
We describe a role for ECM as a biosensor for inflammatory microenvironments that plays a critical role in peripheral immune tolerance. We show that hyaluronan (HA) promotes induction of Foxp3- IL-10–producing regulatory T cells (TR1) from conventional T-cell precursors in both murine and human systems. This is, to our knowledge, the first description of an ECM component inducing regulatory T cells. Intact HA, characteristic of healing tissues, promotes induction of TR1 capable of abrogating disease in an IL-10–dependent mouse colitis model whereas fragmentary HA, typical of inflamed tissues, does not, indicating a decisive role for tissue integrity in this system. The TR1 precursor cells in this system are CD4+CD62L−FoxP3−, suggesting that effector memory cells assume a regulatory phenotype when they encounter their cognate antigen in the context of intact HA. Matrix integrity cues might thereby play a central role in maintaining peripheral tolerance. This TR1 induction is mediated by CD44 cross-linking and signaling through p38 and ERK1/2. This induction is suppressed, also in a CD44-dependent manner, by osteopontin, a component of chronically inflamed ECM, indicating that CD44 signaling serves as a nexus for fate decisions regarding TR1 induction. Finally, we demonstrate that TR1 induction signals can be recapitulated using synthetic matrices. These results reveal important roles for the matrix microenvironment in immune regulation and suggest unique strategies for immunomodulation.
The tissue microenvironment undergoes major changes during inflammation and its resolution. We have studied the role of the ECM as a communications bridge to the adaptive immune system, informing infiltrating lymphocytes regarding the tissue status and guiding subsequent responses. In particular we have examined the interplay between the TR1 regulatory T cell subset and hyaluronan (HA), a component of ECM.
TR1 cells are CD4+FOXP3− regulatory cells that play a crucial role in resolving inflammation and maintaining peripheral immune tolerance (1). TR1 mediate contact-independent immune tolerance through the secretion of substantial amounts of IL-10 (2). Although diverse experimental conditions have been used in TR1 induction (1, 3–8), the specific progenitor population and governing factors in vivo are unclear (1).
HA is a long, highly charged disaccharide with prominent roles in structural biology, wound healing, and immunology. The size of HA in a wound environment is known to correlate with the stage of injury and its resolution (9). Low molecular weight HA (LMW-HA; <15 saccharides; <3 kDa) predominate during acute and persistent inflammation and have been demonstrated to be proinflammatory and proangiogenic. Conversely, intact high molecular weight HA (HMW-HA) predominates in noninflamed or healing tissues and is thought to be inert or anti-inflammatory (9) We previously identified a role for HMW-HA in promoting the persistence and function of established FoxP3+ natural T regulatory cells (nTregs) (10–12). nTregs are another regulatory T cell subset that are thought to primarily arise in the thymus (13). HA was recently reported to promote IL-10 production in intestinal biopsies upon oral administration (14). However, to our knowledge, ECM components have not been implicated in the induction of regulatory T cells and there are no described roles for HA or CD44, the primary HA receptor, in TR1 biology.
Given that TR1 cells are induced in peripheral tissues, presumably in response to local environmental cues, we hypothesized that HMW-HA may promote TR1 induction. Here we evaluate this hypothesis and the role of CD44 signaling as a nexus for fate decisions regarding TR1 induction. Finally, we use synthetic matrices to recapitulate matrix integrity cues and promote TR1 induction.
Results
Intact HA Promotes TR1 Induction from Effector Memory T-Cell Precursors.
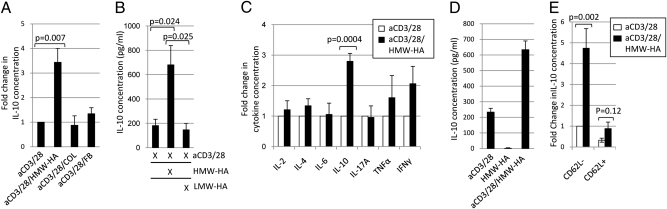
To ascertain the contribution of ECM components to TR1 induction, we devised an in vitro activation assay using immobilized plate-bound ECM components (Fig. S1A). To exclude FOXP3+ nTregs, we used GFP/FOXP3 knock-in mice and depleted the CD4+ T cells isolated from these animals of GFP/FOXP3+ cells. HMW-HA had a capacity to promote IL-10 whereas other ECM molecules did not (Fig. 1A). HMW-HA. but not LMW-HA generated from the same HMW-HA, promoted production of IL-10 protein (Fig. 1B) and mRNA (Fig. S1B), implicating a decisive role for HA integrity in this system. Significant enhancement of other TH1, TH2, or TH17 cytokines tested was not observed (Fig. 1C). IFN-γ and TNF-α were increased but not significantly (P = 0.079 and P = 0.504, respectively). Blocking antibodies directed against IFN-γ or TNF-α did not diminish HA-mediated IL-10 production (Fig. S1C). TGF-β was not significantly increased (Fig. S1D). By using tissues from GFP/IL-10 knock-in mice, we found that, whereas a fraction of induced GFP/IL-10+ cells produced IFN- γ+, induced TR1 cells were otherwise negative for TNF-α, IL-2, IL-4, and IL-17 production (Fig. S1E). The effect of HMW-HA was dependent on TCR ligation (Fig. 1D). Cells induced to express IL-10 by HMW-HA costimulation retained this property even after being washed and restimulated with PMA/ionomycin (Fig. S1F).
Fig. 1.
HMW-HA costimulation induces a TR1-like phenotype in CD4+GFP/FoxP3−CD62L− effector memory T cells. (A) IL-10 production upon activation with aCD3/28 alone or in conjunction with the ECM components HMW-HA, COL, and fibrinogen (FB). (B) Effects of HMW-HA or LMW-HA treatment on IL-10 production. (C) Fold change in TH1, TH2, and TH17 cytokines levels upon HMW-HA costimulation (n ≥ 5 independent experiments each for A–C). (D) IL-10 production upon activation with aCD3/28, HMW-HA alone, or the two reagents in combination. Each condition was performed in triplicate; the image is representative of three experiments. (E) Fold change in IL-10 concentration in cell culture supernatants taken from mouse CD4+GFP/FoxP3− T cells sorted on the basis of CD62L expression and activated with or without HMW-HA (n = 6). For A, C, and E, data were normalized to the aCD3/28 condition, with this value equaling 1 for each experiment.
HMW-HA disproportionately promoted IL-10 production in the effector memory CD62L− fraction of CD4+ T cells (Fig. 1E). CD62L− T cells are known to express CD44 at high levels (15), a phenotypic characteristic likely to be important in interactions with HMW-HA. In contrast, the CD62L+ T cell fraction after activation produced similar amounts of IL-10 with or without HMW-HA, at levels comparable to anti-CD3 (aCD3)/28/HMW-HA treatment of freshly isolated CD62L− cells (Fig. S1G).
HA-Induced TR1 Cells Are Functional.
The RAG.1−/− mouse colitis model is a well established system for evaluating IL-10–dependent regulatory T-cell effects. The infusion of CD4+CD45RBhi naive effector T cells typically causes colitis in these animals whereas the coinfusion of regulatory T cells abrogates colitis in an IL-10–dependent manner (9, 17). We used this model system to evaluate the regulatory capacity of TR1 cells induced with HMW-HA. Mice that received CD4+FoxP3-depleted T cells activated with aCD3/28/HMW-HA exhibited significantly improved survival relative to animals that received the same cells activated with aCD3/28 alone. Freshly isolated CD4+GFP/FOXP3+ nTreg cells completely abrogated disease whereas infusion of PBS solution alone in conjunction with the CD4+CD45RBhi naive effector T cells led to the demise of 90% of the animals in that experimental group (Fig. 2A). These effects on survival occurred in conjunction with diminished colitis (Fig. 2B). Representative colonic sections clearly indicate the presence of healthy tissue with substantial numbers of goblet cells in animals treated with CD4+GFP/FOXP3+ nTregs or CD4+GFP/FOXP3− cells treated with aCD3/28/HMW-HA. However, in mice that received CD4+GFP/FOXP3− cells treated with aCD3/28 alone or PBS solution, pathologic features consistent with colitis are seen (Fig. 2C).
Fig. 2.
Generated TR1 can suppress the development of colitis. (A) Impact of putative TR1 and controls on survival in an IL-10–dependent mouse colitis model (average n = 12 mice per group). (B) Histology scores for the colitis seen in the mice in A. (C) Representative histology of colon sections taken from the mice in A demonstrates goblet cell depletion, inflammatory infiltrate, epithelial shedding, and crypt microabscesses only in mice receiving aCD3/28-treated T cells or PBS solution.
HA Induction of TR1 Cells Is CD44-Dependent.
CD44 is the primary cell-surface receptor for HA (17). We found that CD44−/− mice exhibited significant impairment of IL-10 up-regulation in response to HMW-HA (Fig. 3A). This indicates that CD44 is necessary for HMW-HA–mediated induction of IL-10. Consistent with this, costimulation of WT CD4+GFP/FOXP− T cells with plate-bound aCD44 robustly induced IL-10 production at the level of protein (Fig. 3B) and mRNA (Fig. S2A). However, soluble aCD44 did not up-regulate IL-10 production, revealing a requirement for CD44 cross-linking (Fig. 3B). The increase in IL-10 upon CD44 cross-linking was still observed following normalization to proliferation (Fig. 3C), dispelling the possibility that the IL-10 increase was an artifact of enhanced proliferation. Antibodies directed at ICOS-1, a costimulatory molecule with roles in T-cell activation (18), were included as a control. Unlike the cytokine profile observed upon HMW-HA treatment, CD44 cross-linking also significantly increased TNF-α and IFN-γ (Fig. S2B). Neither WT nor CD44−/− mice had detectable CD4+IL-10+ splenocytes directly ex vivo (Fig. S2C).
Fig. 3.
CD44 cross-linking promotes MAP kinase-dependent IL-10 production. (A) HMW-HA–induced IL-10 production using WT or CD44−/− precursor T cells (n = 7). (B) IL-10 production upon costimulation with plate-bound or soluble aCD44 (n = 5). (C) Effects of CD44 cross-linking on IL-10 production, normalized to proliferation. Data are expressed as pg/mL of IL-10 produced on a per-cpm basis. (D) Suppression assay using TR1 cells induced with aCD44 costimulation. C and D are each representative of two experiments. Representative intracellular staining (E) and fold change in IL-10 production (F) following treatment with aCD44 and selective inhibitors of p38 (SB202190), ERK1/2 (UO126), and MEK (PD98059; n = 5). (G) Fold change in IL-10 concentration in cell culture supernatants taken from mouse CD4+GFP/FoxP3− T cells sorted on the basis of CD62L expression and activated with or without aCD44 Ab. Data were normalized to the aCD3/28 condition of the CD62L− population, with this value equaling 1 for each experiment (n = 4).
TR1 cells induced with CD44 cross-linking were functional, as demonstrated in vitro (Fig. 3D). However, this suppressive function was lost when T cells from IL-10−/− mice were used as a source of TR1 cells. Of note, CD44 costimulation of mouse CD4+GFP/FoxP3− cells did not induce FoxP3 expression (Table S1).
Although CD44 facilitates signaling through numerous pathways, IL-10 production is reported to be primarily the product of signaling through MAP kinases, particularly those involving p38 and ERK1/2 (19). Intracellular staining for IL-10 after CD44 costimulation identified enhanced IL-10 production that was lost upon addition of specific small-molecule inhibitors of ERK1/2 and p38 signaling but not upon inhibition of MEK1 (Fig. 3E and F). This indicates that CD44 cross-linking promotes IL-10 production via a MAP kinase-dependent pathway. Consistent with this, we found that treatment with aCD44 together with a cross-linking antibody led to enhanced phosphorylation of both p38 and ERK1/2, which peaked 10 min after activation (Fig. S3 A and B). If either the cross-linking Ab or the aCD44 Ab was left out, enhanced phosphorylation of p38 and ERK1/2 was not seen. Exogenous IL-2 had a negligible impact on p38 and ERK/1/2 phosphorylation (Fig. S3 C and D). The experiments in Fig. S3 A–D were performed using human CD4+CD25− T cells; similar results were seen with mouse cells (Fig. S3 E and F). As with HMW-HA, CD44 cross-linking disproportionately promoted IL-10 production in the CD62L−, effector memory population (Fig. 3G).
Intact HA Promotes Induction of Human TR1.
Costimulation of human CD4+CD25− T cells with either HMW-HA or anti-CD44 antibodies significantly increased IL-10 production (Fig. S4A) and generated functional TR1 cells (Fig. S4B) but did not promote induction of Foxp3 (Fig. S4C). We therefore conclude that HMW-HA and CD44 also promote TR1 induction from human conventional T cells.
Exogenous IL-2 Boosts HMW-HA Induced IL-10 Production.
Exogenous IL-2 significantly increased IL-10 production in the presence of plate-bound HMW-HA (Fig. S5). However, the enhanced IL-10 production seen upon IL-2 addition to the aCD3/28 condition did not reach statistical significance, leading us to suspect that the beneficial effect on IL-10 production previously reported for IL-2 (20) may not be relevant at the low levels of TCR activation used in our system.
Osteopontin Abrogates HA TR1 Induction in a CD44-Dependent Manner.
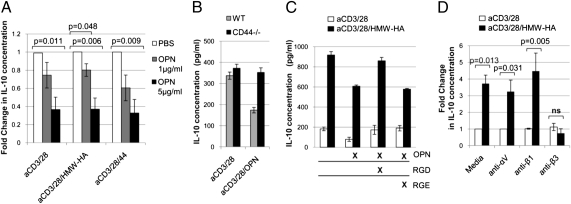
In healing tissues, HA typically exists in the context of a complex ECM. We therefore asked whether other ECM components that are also CD44 ligands might impact the HA-mediated effect on TR1 induction described here. Osteopontin (OPN) is a matrix glycoprotein found in abundance in chronic inflammation and known to exacerbate autoimmunity (21, 22). Given that OPN is known to impact IL-10 production and is a CD44 ligand (23, 24), we explored the hypothesis that OPN inhibits HA-mediated TR1 induction. OPN decreased basal levels of IL-10 production seen upon aCD3/28 activation alone and negated the increase in IL-10 production seen upon HMW-HA costimulation in a dose-dependent manner (Fig. 4A). This was also the case for mRNA expression (Fig. S6A). OPN inhibited HMW-HA–mediated IL-10 production to an equivalent extent irrespective of IL-2 supplementation (Fig. S6B), indicating that OPN acts distal to or independent of STAT5 signaling.
Fig. 4.
OPN abrogates HMW-HA–mediated IL-10 production. (A) Effects of OPN concentration on IL-10 production (n = 3). (B) Effect of OPN on IL-10 production by WT and CD44−/− T cells. (C) Effect of RGD or RGE peptides on OPN-mediated suppression of IL-10 production. Data for B and C are each representative of three experiments. (D) Effects of integrin antibodies on HMW-HA–mediated IL-10 production (n = 5).
OPN effects are known to be mediated by interactions with both CD44, as well as to the αVβ3 integrin receptor, to which it binds via an RGD motif (23). We observed that the decrease in basal IL-10 production upon OPN treatment was lost in CD44−/− mice (Fig. 4B), implicating CD44 in OPN effects on IL-10. However, addition of RGD peptide, but not RGE control peptide (Fig. 4C) or addition of an agonist antibody directed at the β3 integrin receptor subunit (Fig. 4D), blocked the suppression of IL-10 production by OPN. These data implicate roles for both CD44 and β3 in OPN effects on HA-mediated IL-10 production and indicate a nexus for regulatory control of the TR1 pathway by ECM components.
Synthetic ECM Hydrogel Promotes IL-10 Production.
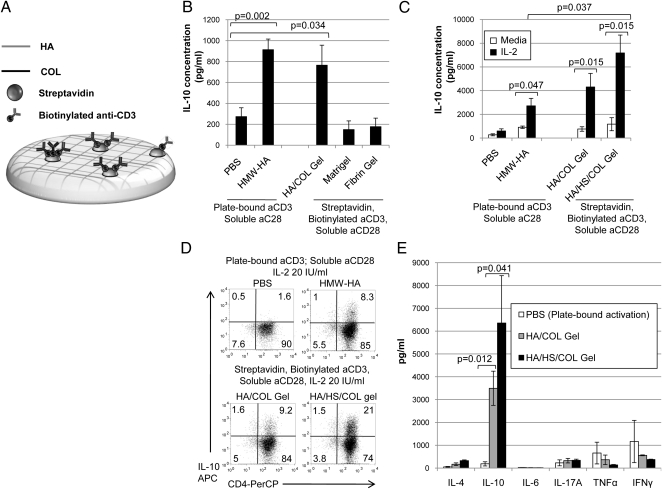
We explored the potential of biomimetics of HA-containing matrix to induce IL-10 production. Extracel is a commercially available HMW-HA and collagen (COL)-based hydrogel preparation (25), which we modified to deliver a polyclonal antigenic stimulus through the addition of streptavidin and biotinylated aCD3 before polymerization, referred to henceforth as an HA/COL gel. A schematic of this design is shown (Fig. 5A). CD4+GFP/FOXP3− T cells activated using this platform produced IL-10 in comparable quantities to that seen with plate-bound activation. Matrigel or a fibrin hydrogel did not promote IL-10 induction (Fig. 5B).
Fig. 5.
A synthetic matrix promotes TR1 induction. (A) Schematic of a hydrogel that delivers a set of stimuli capable of inducing TR1 from conventional T-cell precursors. (B) IL-10 production following plate-based or hydrogel-based activation (n = 5). (C) The impact of supplemental IL-2 on IL-10 production in the setting of plate-based or HA/COL (Extracel) or HA/COL/HS (Extracel-HP) hydrogels (n = 5). (D) Representative intracellular staining for IL-10 under these same conditions. (E) Levels of TH1, TH2, and TH17 cytokines upon hydrogel-based activation (n = 3).
In tissues, IL-2 is associated with sulfated proteoglycans such as heparan sulfate (HS), possibly prolonging its half-life and function (26, 27). We therefore asked whether a synthetic matrix containing HS could be used to deliver IL-2 in conjunction with the other signals necessary for TR1 induction. Extracel-HP, a hydrogel preparation that incorporates HS in addition to collagen and HMW-HA (henceforth referred to as HA/HS/COL gel), engendered equivalent IL-10 production in the absence of exogenous IL-2. Upon IL-2 supplementation the amount of IL-10 produced was significantly increased in the setting of HA/HS/COL gel (P = 0.037) but not HA/COL gel (P = 0.23) relative to plate-bound HMW-HA (Fig. 5C). This is consistent with reports of enhanced functionality of cytokines in HS-bound form (28, 29). Representative staining data are shown in Fig. 5D. This enhancement of TR1 induction was not associated with increases in other Th1, Th2, or TH17 cytokines (Fig. 5E). We confirmed that HA/HS/COL gel retains IL-2 (Fig. S7).
Discussion
The local inflammatory milieu and the ECM in particular are underappreciated partners of the adaptive immune response. Herein we provide evidence of ECM modulation and control of IL-10 production, a key immunoregulatory cytokine in peripheral tissues. By using both mouse and human cells, we show that intact HA promotes induction of Foxp3− IL-10–producing T cells with regulatory properties (i.e., TR1 cells) and that these function in vivo.
The TR1 cells described here differ from TR1 cells described previously in that the TR1 progenitor cells in this system are CD4+CD62L− effector memory cells rather than naive cells (1). This suggests that these cells have previously encountered their cognate antigens and assume a regulatory phenotype when they do so again in the context of HMW-HA. HA-induced TR1 may function in multiple stages of inflammation, as has been proposed for other TR1 (1). Our colitis data suggest that HA-induced TR1 can prevent inflammation. Conversely, a role for HA-induced TR1 in the resolution of inflammation is raised by our report that TH1 cytokines promote HA production by dendritic cells (DC) (30). Matrix integrity cues might thereby play a central role in maintaining peripheral tolerance to self antigens.
The exclusivity with which HMW-HA treatment, and particularly HMW-HA–based hydrogels, promoted IL-10 production is also noteworthy and differs from some other described TR1 cells (1). In particular, we did not observe significantly enhanced production of IFN-γ or other cytokines variably associated with TR1 cells (1). Nor did we observe induction of FoxP3, a signaling molecule associated with Tregs. As we previously reported that HMW-HA promotes Foxp3 expression by mature Tregs (10–12), this suggests differences between HMW-HA-mediated modulation of different regulatory T-cell types. Another mechanistic distinction is that intact HA promotes TGF-β production by Treg cells (11) but not TR1. Given that CD44v isoforms possess diverse ECM ligand specificities (31), it is also possible that other ECM components may differentially interact with specific regulatory subsets (30) in a highly contextual and specific manner.
We identify two levels of regulation that modulate the capability of HA to induce TR1 regulatory cells. The first is the size of HA in the system. Although HMW-HA, characteristic of healing or uninjured tissues, promotes IL-10 production, fragmentary LMW-HA, indicative of active tissue injury, does not, indicating a decisive role for HA integrity in TR1 induction. It is known that the length of HA chains dictates their ability to cross-link multiple CD44 receptors on the cell surface (32), and this cross-linking is critical to a number of CD44-mediated functions (33, 34). The requirement for CD44 cross-linking in this system provides a potential mechanistic explanation tying TR1 induction to the inflammatory milieu in vivo.
A second level of control over HMW-HA induction of TR1 cells is the influence of OPN, a matrix glycoprotein found in abundance in many settings of chronic inflammation and autoimmunity (21, 35). Our data indicate that OPN overrides HMW-HA–mediated TR1 induction in a dose-dependent manner via interactions with both CD44 and integrin receptors. These data point to a central role for CD44 in fate decisions regarding of TR1 induction. Furthermore, we demonstrate that the effect of OPN can be replicated with an antibody directed against β3 integrin. These data indicate a nexus for regulatory control of the TR1 pathway by ECM components. Given the requirement in HA-mediated TR1 induction for a TCR stimulus, DCs and other antigen-presenting cells (APCs) may serve as an important source of HA. It was recently reported that IFN-γ receptor engagement drives DC-mediated TR1 induction and inhibition of OPN production (36). We recently showed that DC production of HA was likewise promoted by IFN-γ and that HA is found at the immune synapse (30). We are currently investigating this HA/OPN/IFN-γ axis in DC-mediated TR1 induction.
Building upon our findings, we have used commercial HA-based hydrogels as platforms to provide the necessary cues for TR1 induction. These capitalize on the shared capacity of intact HA and an HA-based hydrogels to induce TR1. In this context, the hydrogel can be regarded as a synthetic biomimetic of intact ECM. The use of synthetic matrices to induce TR1 points toward novel strategies for immunomodulation.
Materials and Methods
Induction of TR1 Cells and Controls.
CD4+GFP/FoxP3+ T cells (2 × 105) were activated for 96 h on plates initially coated with aCD3 (0.5 μg/mL in 50 μL) with or without CD44 antibody (1 μg/mL), washed, and then subsequently coated with 0.2 mg/mL BSA-conjugated HMW-HA or 10% BSA. After 96 h, cells and supernatants were collected for analysis. For TR1 cell induction using hydrogels, Extracel and Extracel-HP (Glycosan Biosystems) were used per the manufacturer's instructions. Biotinylated anti-CD28 Ab (1.0 μg/mL), aCD3 Ab (10 μg/mL; BD Biosciences), and streptavidin (10 μg/mL; Sigma-Aldrich) were added before cross-linking. Cells (2 × 105) were layered on gels of 25 μL volume following cross-linking. Additional information is included in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Zarembinski, Prestwich, and Buckner for their advice and comments. This study was supported by National Institutes of Health Grants DK046635 (to G.T.N.), HL018645 (to T.N.W.), DK080178-03 (to P.L.B.), JDRF 25-2010-648 (to T.N.W. and P.L.B.), and R-03 DK089128-01 (to P.L.B.) and by the Juvenile Diabetes Research Foundation (Center for Translational Research, Benaroya Research Institute at Virginia Mason).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017360108/-/DCSupplemental.
References
- 1.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 2.Vieira PL, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 3.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 4.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen AE, Gad M, Walter MR, Claesson MH. Induction of regulatory dendritic cells by dexamethasone and 1alpha,25-dihydroxyvitamin D(3) Immunol Lett. 2004;91:63–69. doi: 10.1016/j.imlet.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Kemper C, et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 7.Levings MK, et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 8.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 9.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: An information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Bollyky PL, et al. Cutting edge: High molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 11.Bollyky PL, et al. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol. 2009;183:2232–2241. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollyky PL, et al. Intact extracellular matrix and the maintenance of immune tolerance: High molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 14.Asari A, Kanemitsu T, Kurihara H. Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via Toll-like receptor 4 in the intestinal epithelium. J Biol Chem. 2010;285:24751–24758. doi: 10.1074/jbc.M110.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budd RC, et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 16.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 18.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 19.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji-Takayama K, et al. The production of IL-10 by human regulatory T cells is enhanced by IL-2 through a STAT5-responsive intronic enhancer in the IL-10 locus. J Immunol. 2008;181:3897–3905. doi: 10.4049/jimmunol.181.6.3897. [DOI] [PubMed] [Google Scholar]
- 21.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: The inside story. Nat Rev Immunol. 2009;9:137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hur EM, et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 23.Ashkar S, et al. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 24.Heilmann K, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2008;13:1162–1174. doi: 10.1111/j.1582-4934.2008.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prestwich GD, et al. Injectable synthetic extracellular matrices for tissue engineering and repair. Adv Exp Med Biol. 2006;585:125–133. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 26.Najjam S, Gibbs RV, Gordon MY, Rider CC. Characterization of human recombinant interleukin 2 binding to heparin and heparan sulfate using an ELISA approach. Cytokine. 1997;9:1013–1022. doi: 10.1006/cyto.1997.0246. [DOI] [PubMed] [Google Scholar]
- 27.Wrenshall LE, Platt JL, Stevens ET, Wight TN, Miller JD. Propagation and control of T cell responses by heparan sulfate-bound IL-2. J Immunol. 2003;170:5470–5474. doi: 10.4049/jimmunol.170.11.5470. [DOI] [PubMed] [Google Scholar]
- 28.Schönherr E, Hausser HJ. Extracellular matrix and cytokines: A functional unit. Dev Immunol. 2000;7:89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lortat-Jacob H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol. 2009;19:543–548. doi: 10.1016/j.sbi.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Bollyky PL, et al. Th1 cytokines promote T-cell binding to antigen-presenting cells via enhanced hyaluronan production and accumulation at the immune synapse. Cell Mol Immunol. 2010;7:211–220. doi: 10.1038/cmi.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puré E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 32.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 33.Fujii Y, Fujii K, Nakano K, Tanaka Y. Crosslinking of CD44 on human osteoblastic cells upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003;539:45–50. doi: 10.1016/s0014-5793(03)00182-0. [DOI] [PubMed] [Google Scholar]
- 34.Huet S, et al. CD44 contributes to T cell activation. J Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- 35.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.