Abstract
Extrafollicular (EF) B-cell responses are increasingly being recognized as an alternative pathway of B-cell activation, particularly in autoimmunity. Critical cellular interactions required for the EF B-cell response are unclear. A key question in autoimmunity, in which Toll-like receptor (TLR) signals are costimulatory and could be sufficient for B-cell activation, is whether T cells are required for the response. This is pivotal, because autoreactive B cells are considered antigen-presenting cells for autoreactive T cells, but where such interactions occur has not been identified. Here, using AM14 site-directed transgenic rheumatoid factor (RF) mice, we report that B cells can be activated, differentiate, and isotype-switch independent of antigen-specific T-cell help, αβ T cells, CD40L signaling, and IL-21 signaling to B cells. However, T cells do dramatically enhance the response, and this occurs via CD40L and IL-21 signals. Surprisingly, the response is completely inducible T-cell costimulator ligand independent. These results establish that, although not required, T cells substantially amplify EF autoantibody production and thereby implicate T-independent autoreactive B cells as a potential vector for breaking T-cell tolerance. We suggest that these findings explain why autoreactivity first focuses on self-components for which B cells carry TLR ligands, because these will uniquely be able to activate B cells independently of T cells, with subsequent T–B interactions activating autoreactive T cells, resulting in chronic autoimmunity.
Keywords: systemic lupus, autoantibodies
Although much attention has been recently focused on the germinal center (GC) reaction—which creates long-lived memory and plasma cells—some B-cell responses engage primarily the extrafollicular (EF) pathway of activation (1). This response occurs predominantly in the marginal sinus bridging channels at the border of the T-cell zone and red pulp, spilling into the adjacent red pulp areas. These locations are rich in dendritic cells and other myeloid cells but lack follicular dendritic cells. EF responses rapidly generate large numbers of short-lived plasmablasts. The location and cell types inherent in the EF response suggest that it may be regulated quite differently from the GC response and may generate different cellular products with different biological implications. The EF pathway is seen transiently in T cell-dependent responses to protein antigens (Ags) (1) and more recently has been appreciated as a major and sometimes sustained component of pathogen responses, particularly to bacteria (2, 3).
Despite its likely importance in immunity, many aspects of the EF response remain poorly defined. For example, the roles of T cells in the EF response are not clear. The EF response is thought to undergo minimal, if any, somatic hypermutation of Ig variable regions in responding cells and similarly to have little isotype switch (1). Mutation and switch are often thought to rely on T-cell signals, yet it remains poorly defined whether these processes are influenced by T cells in the EF response. In this connection, immunization with T-independent Ags induces a robust but transient and largely unswitched EF response, suggesting that T cells are not required (4).
Recently, it has become apparent that autoantibody responses also proceed through the EF pathway (5, 6). Evidence for this comes from multiple mouse systems and can be inferred from the production of plasmablasts and short-lived autoantibody responses in humans (5, 7–9). It seems that a prominent requirement for systemic autoimmune EF responses is that the self-Ag contain a Toll-like receptor (TLR) ligand that is recognized by a B cell-intrinsic TLR. Indeed, targets of autoantibodies such as DNA, RNA, and self-IgG [in this case as immune complexes (ICs) with IgG antinuclear Abs] do contain TLR ligands. Conversely, when an artificial Ag is provided with a TLR9 ligand, it engages a predominantly EF response (10).
The identification of a TLR-driven EF-based activation of autoreactive B cells has broad implications for understanding the mechanisms that initiate and propagate B-cell autoimmunity, most of which have yet to be fully explored. For example, activation of autoreactive B cells at EF sites promotes disease by generating pathogenic autoantibodies as well as activated B cells that in turn can mediate damage by Ab-independent mechanisms, in particular by promoting autoreactive T-cell activation (11). Therefore, it is critical to understand the cellular interactions and signals that are required for and control EF responses in autoimmunity, because any of these could be therapeutic targets.
Given the pivotal nature of breaching T-cell tolerance in the establishment of chronic autoimmunity, it is important to determine whether EF responses in autoimmunity involve T cells, and if so, to understand how these interactions take place. We have been using AM14 B-cell receptor (BCR) transgenic (Tg) mice (AM14), specific for self-IgG2a of the “a” allotype [rheumatoid factor (RF) specificity], to study spontaneous and induced RF responses that both use the EF pathway (5, 12). AM14 B cells are activated by IgG2a antichromatin in vitro and in vivo in a TLR-dependent fashion (13, 14). In vitro and presumably in vivo the antichromatin Abs complex with ubiquitous chromatin released from dying cells, forming antigenic chromatin-containing ICs. In vivo, IgG2a antichromatin stimulates a robust EF response without evident GCs that depends on a combination of TLR7 and TLR9 and requires MyD88 expression in the B cell (14).
When studying only IgM responses in conventional IgM BCR Tg mice, we found that induced plasmablast responses were of normal magnitude in a T cell-deficient MRL/lpr background (14). However, larger numbers of somatic mutation in T-sufficient contexts suggested that T cells could influence the response. Nonetheless, direct evidence for T–B collaboration in this system was lacking, and the signals involved were unknown. To determine whether T cells do indeed affect the response, and if so how, we have used a new AM14 BCR site-directed (sd)-Tg mouse model, which can undergo isotype switch, as well as a BALB/c background to eliminate confounding influences of the autoimmune milieu (15). Using a combination of genetic and inhibitor approaches, we have found that the TLR-dependent EF autoantibody response in this model can indeed proceed to completion in the absence of T cells, including plasmablast differentiation and isotype switch. However, we also found that Ag-specific T cells can greatly augment the response. This occurs in a CD40L and IL-21–dependent fashion but is inducible T-cell costimulator ligand (ICOSL) independent. This indicates that the response can initiate without T cells but that the EF response is nonetheless an important site for T–B collaboration in the generation of classic autoantibodies. This has important implications for understanding how autoimmunity initiates, as well as for how to block it.
Results
Effects of T Cells Restricted to an Irrelevant TCR on the EF RF Response.
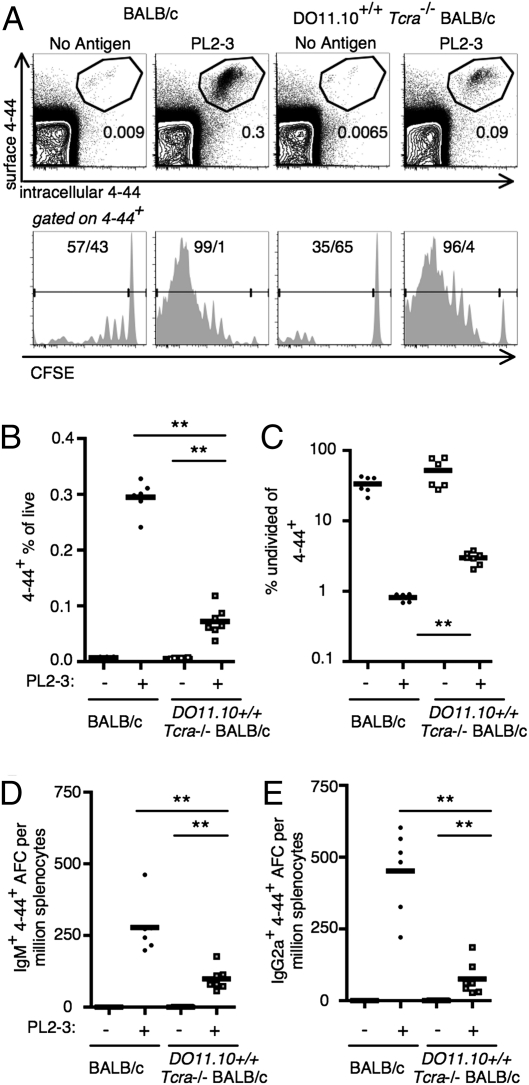
T cells can provide help to B cells in an Ag-specific or nonspecific manner (16). We tested the requirement for Ag-specific T-cell help by transferring AM14 sd-Tg BALB/c B cells into DO11.10+/+ TCRα−/− BALB/c mice. In these experiments, nearly all T cells in the mice were specific only for an ovalbumin peptide and thus should not be able to help AM14 B cells in their response to antichromatin Abs. The presence of T cells of an irrelevant specificity preserved lymphoid architecture and controlled for nonspecific effects of the absence of T cells. In the AM14 sd-Tg mice a small subset of B cells expressing certain Vκ8 family L chains recognizes self-IgG2a, and we identify these cells using the 4–44 antiidiotype Ab (5, 17). In both DO11.10+/+ TCRα−/− BALB/c and WT recipients, the transferred 4–44+ AM14 B-cell population expanded in response to the IgG2a antichromatin Ab, PL2-3, at day 6, as detected by flow cytometry, but not if Ag was not given. However, PL2-3–induced expansion of 4–44+ AM14 B cells was decreased approximately fourfold in the DO11.10 BALB/c recipients compared with WT recipients (Fig. 1A, Upper, and 1B). Antigen-specific division of 4–44+ cells in both DO11.10 and WT recipients in response to PL2-3 was detectable using dilution of carboxyfluorescein succinimidyl ester (CFSE). However, proliferation of 4–44+ cells was decreased in DO11.10 recipients compared with WT recipients, as shown by an increased fraction of undivided 4–44+ cells in the PL2-3–treated DO11.10 mice (Fig. 1A, Lower, and 1C). Similarly, both WT and DO11.10 recipients were permissive for 4–44+ Ab-forming cell (AFC) differentiation. However, the generation of both IgM and IgG2a 4–44+ AFC populations was reduced in DO11.10 recipients compared with WT recipients (Fig. 1 D and E), with IgG2a being somewhat more affected than IgM. DO11.10 mice have a stable, albeit smaller, CD4 T-cell compartment (Fig. S1A). This provided normal lymphoid architecture in DO11.10 recipients to host EF plasmablast responses similar to those found in WT recipients (Fig. S1B). Thus, by all parameters analyzed, lack of specific T-cell help does not eliminate the ability of 4–44+ cells to respond. However, specific T-cell help has a role in optimizing 4–44+ B-cell proliferation and differentiation.
Fig. 1.
Specific T-cell help is not required for but enhances the RF B-cell response to IgG2a antichromatin Abs. BALB/c or DO11.10 TCR Tg,TCRα-deficient BALB/c mice were killed on day 6, and splenocytes were analyzed after transfer of purified AM14 sd-Tg B cells and administration of PL2-3 as indicated in Materials and Methods. (A) Representative flow cytometry plots are shown for mice in treatment groups, as indicated for both rows. Upper: Live events. Lower: 4–44+ events. (A, Upper, and B) 4–44+ surface and intracellular double-positive cells were quantitated. (A, Lower, and C) Proliferation was assessed using CFSE dilution with 4–44+CFSEhi cells considered to be undivided. (D and E) Splenic 4–44+ AFCs of IgM (D) or IgG2a (E) isotype. Data are compiled from two independent experiments. **P < 0.01, Mann-Whitney two-tailed test.
Effects of T-Cell Deficiency on the EF RF Response.
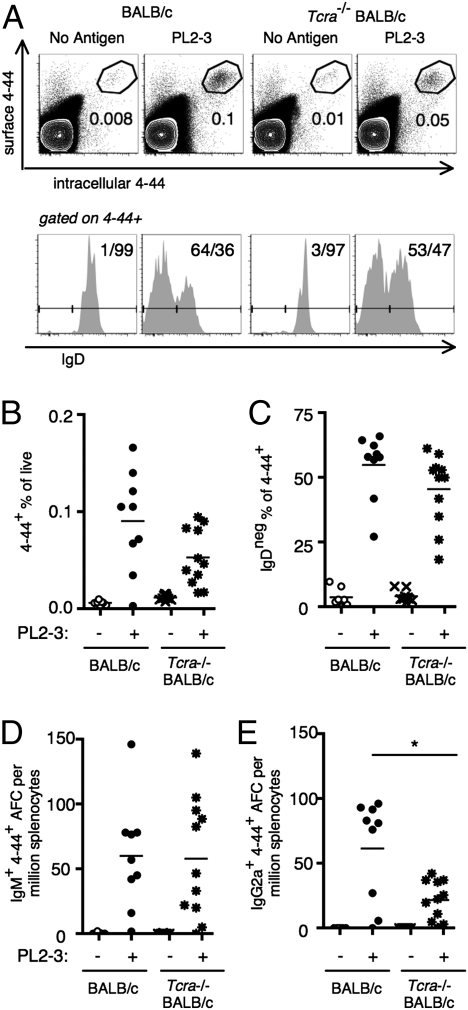
Although indicating a role for Ag-specific T-cell help, the prior experiment does not eliminate the possibility that T cells could also be helping B cells to initiate the response in a nonspecific manner. If T cells also had a nonspecific role, elimination of all T cells would abrogate the response. To test this, we transferred AM14 sd-Tg BALB/c B cells into TCRα−/− BALB/c mice, which lack all αβ T cells, or into control BALB/c mice. We then activated the AM14 B cells with PL2-3 and analyzed the response at day 5. The expansion of 4–44+ B cells was comparable in TCRα−/− mice compared with WT controls (Fig. 2A, Upper, and 2B). Similarly, IgD down-regulation was comparable between the two groups of mice (Fig. 2A, Lower, and 2C). The 4–44+ IgM AFC numbers were also not different between WT and TCRα-deficient mice (Fig. 2D). In contrast, numbers of 4–44+ IgG2a AFCs were reduced ≈2.5-fold in TCRα−/− mice, although still substantial in quantity compared with the unimmunized TCRα−/−controls (Fig. 2E). These results indicate that αβ T-cell help is not required for initiating the AM14 B-cell response to PL2-3, but such T cells optimize the differentiation of IgG2a AFCs. There is therefore no evidence of a nonspecific effect of T-cell help. If T cells were providing help nonspecifically, we would have found a lower response in the fully αβ T cell-deficient animals; if anything, the response is more robust in the mice lacking such cells compared with the T cell-restricted animals.
Fig. 2.
αβ T cells are not required for but enhance the RF B-cell response to IgG2a antichromatin Abs. BALB/c or TCRα-deficient BALB/c mice were killed on day 5, and splenocytes were analyzed after cell transfer of purified AM14 sd-Tg B cells and administration of PL2-3. (A) Representative flow cytometry plots are shown for treatment groups as indicated and are gated as described in Fig. 1A. (A, Upper, and B) 4–44+ surface and intracellular double-positive cells. (A, Lower, and C) Fraction of 4–44+ cells that had lost expression of IgD. (D and E) Splenic 4–44+ AFCs of IgM (D) or IgG2a (E) isotype. Data are compiled from four independent experiments. *P < 0.05, Mann-Whitney two-tailed test.
These results clearly show that Ag-specific T cells are not required to initiate the EF AM14 B-cell response to antichromatin Abs in vivo. Proliferation and differentiation were both observed in the absence of Ag-specific T-cell help. However, T cells do seem to have a role in supporting the amplification of the EF B-cell response because the response was quantitatively diminished in the absence of T cells. Further, we infer that Ag-specific T-cell help is required for an optimal response, because an environment containing nonspecific T-cell help (Fig. 1) did not augment the AM14 B-cell response at all compared with an environment lacking αβ T cells (Fig. 2). The magnitude of the control responses was somewhat smaller in these experiments compared with those in Fig. 1, which we attribute to a different lot of Ag as well as a 1-d difference in the duration of the response. It is not clear why the reduction of AM14 B-cell response to antichromatin antibodies in DO11.10 recipients was more robust compared with the reduction seen in αβ T cell-deficient mice. DO11.10 mice contain Treg (Fig. S1A), and these may regulate the AM14 B-cell response in an Ag-nonspecific way.
Molecular Mechanisms of T-Cell Help for the EF Response.
To investigate mechanisms by which Ag-specific T cells help EF B cells, we tested three components of T-cell help: CD40L signaling, IL-21 secretion, and ICOS signaling. All of these are known to be important for the generation and maintenance of the GC pathway (18, 19), but none has been tested in vivo in this type of autoreactive EF response.
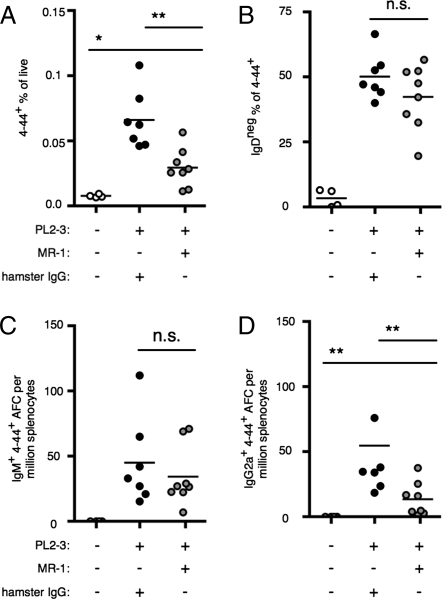
To test the role of CD40L in PL2-3 induction of AM14 B-cell activation, we used the blocking Ab MR-1 (20), comparing it with a hamster IgG control. CD40L was blocked before and during PL2-3 activation of transferred AM14 sd-Tg BALB/c B cells in BALB/c mice, and the response was observed at day 5 after activation. Expansion of the 4–44+ population was observed in both groups but was suboptimal in mice treated with MR-1 (Fig. 3A). Within the 4–44+ population, a similar proportion of cells had down-regulated IgD in both groups (Fig. 3B). The 4–44+ IgM AFC population was also comparable in both groups (Fig. 3C). In contrast, CD40L-blockade reduced the frequency of 4–44+ IgG2a AFCs that developed in response to PL2-3 (Fig. 3D). Thus, CD40L interactions are important for optimizing the AM14 B-cell response to PL2-3.
Fig. 3.
Blocking CD40L only partially inhibits the RF B-cell response to IgG2a antichromatin Abs. BALB/c mice were killed on day 5 after cell transfer with CD40L blocking and PL2-3 as indicated. (A) 4–44+ surface and intracellular double-positive cells. (B) Fraction of 4–44+ cells that had lost expression of IgD. (C and D) Splenic 4–44+ AFCs of IgM (C) or IgG2a (D) isotype. Data are compiled from three independent experiments. *P < 0.05 and **P < 0.01, Mann-Whitney two-tailed test.
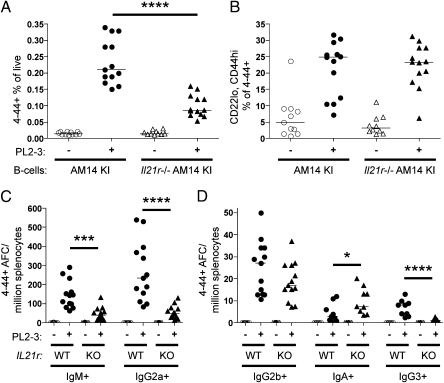
IL-21 secreted by T follicular helper (TFH) cells has been shown to support GC responses (18). Most pertinent to the AM14 response to antichromatin Ab, a subset of T cells that resembles TFH has been identified by FACS and histology to be located in the EF regions of diseased MRL/lpr and other autoimmune-prone mice (21). These EF T cells express ICOS and have down-regulated PSGL1, much like TFH cells, but express CXCR4, unlike TFH. Notably, they secrete IL-21, which can in turn promote in vitro B-cell isotype switch. Given the evidence for a role of Ag-specific T cells in promoting the EF response to antichromatin, we wanted to determine whether IL-21 signaling in the B cell was important for the antichromatin-driven EF response. To test this, we transferred IL-21R−/− AM14 sd-Tg BALB/c B cells or WT AM14 sd-Tg BALB/c B cells into WT BALB/c recipients and administered PL2-3. Although both types of AM14 B cells expanded in response, IL-21 receptor deficiency on the transferred AM14 B cells resulted in a 2.5-fold less expansion compared with the WT AM14 B cells (Fig. 4A). Cells differentiating toward the plasmablast fate can be identified as CD22lo and CD44hi (5). The proportion of the 4–44+ cells that became CD22lo and CD44hi in response to PL2-3 was equal in both WT and IL-21R–deficient AM14 B cells (Fig. 4B), indicating that IL-21 signals in the B cell were not required for initial differentiation toward the plasmablast lineage. We observed 4–44+ AFCs that were IgM and IgG2a in both groups. However, the AFC response was not as large when AM14 B cells lacked the IL-21R, with approximately a sixfold decrease in the frequency of IgG2a AFCs and a fourfold decrease in the frequency of IgM AFCs (Fig. 4C). Interestingly, in both cases there were comparable populations of 4–44+ IgG2b AFCs, whereas the 4–44+ IgA AFCs were increased, and the 4–44+ IgG3 AFCs were decreased (Fig. 4D), showing that IL-21 affects only some isotypes. Thus, IL-21 has a direct influence on the later stages of B-cell differentiation, affecting isotype switch and plasmablast maturation to the AFC stage.
Fig. 4.
Deficiency of IL-21R reduces class switch to IgG2a. On day 0, 8 million AM14 sd-Tg or AM14 sd-Tg.IL-21R KO B cells were purified (88–90% purity) and transferred into CD45.1 BALB/c recipients. Mice were either left untreated or injected with PL2-3. On day 6, splenocytes were assayed by flow cytometry to detect the 4–44+ population (A) and CD22lo CD44hi plasmablasts within the 4–44+ population (B). (C) Splenic 4–44+ AFCs of IgM or IgG2a; and (D) IgG2b, IgA, or IgG3 isotypes. Data are compiled from three independent experiments. *P < 0.05; ***P < 0.001; ****P < 0.0001, Mann-Whitney two-tailed test.
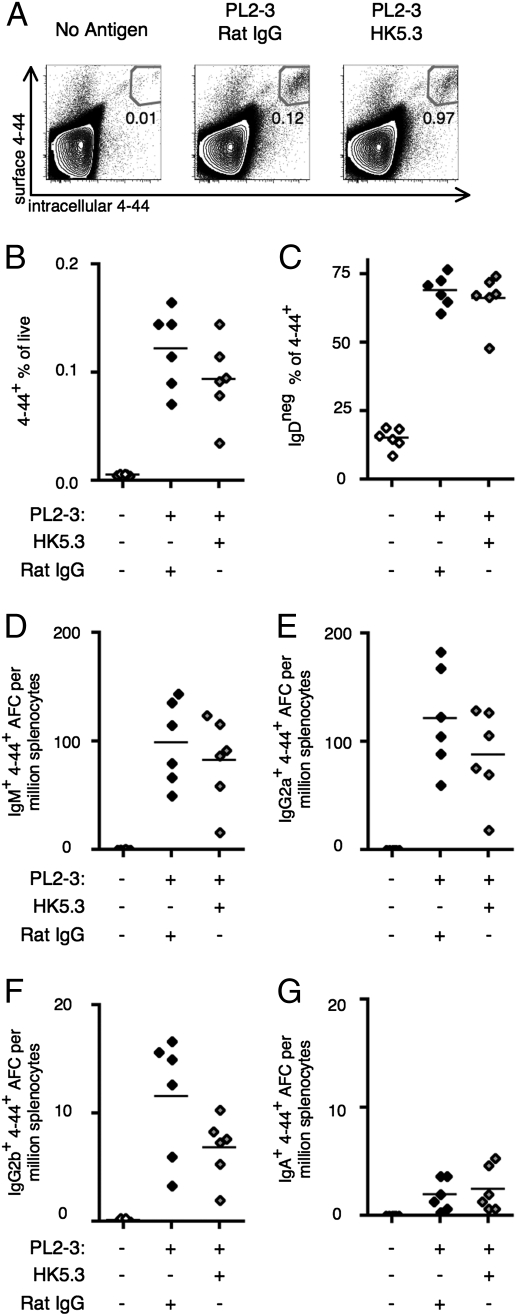
Because ICOS is up-regulated on TFH cells, it is important to know whether T cells that augment the EF response require ICOS signaling to function. To assess this, B cells were isolated from AM14 sd-Tg BALB/c mice and transferred to BALB/c recipients and activated with PL2-3. These mice were injected with HK5.3 antibody to block ICOSL (22). Because ICOS is not up-regulated until after T-cell activation, the first injection was given on day 2. The 4–44hi population increased in both PL2-3–treated groups of mice regardless of ICOSL blocking (Fig. 5 A and B), a reflection of Ag-driven activation. The proportion of the 4–44+ population that had down-regulated IgD was also equivalent in both groups (Fig. 5C). We examined four different isotypes of 4–44+ AFC in response to PL2-3: IgM, IgG2a, IgG2b, and IgA. Across all isotypes, there was no change in 4–44+ AFC production (Fig. 5 D–G). Equivalent 4–44+ plasmablast and AFC responses were also observed in ICOSL Ab or control-treated intact AM14 sd-Tg BALB/c mice (Fig. S2). ICOSL blocking capability was validated because we observed a substantial reduction of GCs in response to NP-chicken gamma globulin (NP-CGG) in alum both by flow cytometry and immunofluorescence histology (Fig. S3). These data show that ICOS signaling is not required for initial EF RF B-cell activation and differentiation.
Fig. 5.
Blocking ICOSL does not inhibit the RF B-cell response to IgG2a antichromatin Abs. BALB/c mice were killed on day 6 after transfer of AM14 sd-Tg B cells and administration of PL2-3 along with ICOSL blocking or control Ab. Representative flow cytometry plots are shown, gated as described in Fig. 1A. (A and B) 4–44+ surface and intracellular double-positive cells. (C) Fraction of 4–44+ cells that had lost expression of IgD. (D–G) Splenic 4–44+ AFCs of IgM (D), IgG2a (E), IgG2b (F), or anti-IgA (G) isotype. Data are compiled from two independent experiments.
Discussion
T cells have been observed at the EF site (23, 24), but their role in defined EF B-cell responses has not been clear. Our data show, using multiple systems and approaches to block T cells, that they are not required for the complete maturation of the EF response. However, at a quantitative level, T cells contribute substantially, enhancing the response, for example by augmenting the 4–44+ IgG2a+ AFC response on the order of sixfold (Fig. 1E). Over the course of time in a setting of spontaneous and chronic autoreactive B-cell activation, the enhancement provided by T cells would be quite substantial. Indeed, disease is reduced in lupus-prone mice deprived of T cells from birth or treated chronically with T-depleting Abs (25, 26) (although it should be noted that these mice do make some autoantibodies). It seems likely that B cells activated by TLR ligand-containing self-Ag must interact with and activate T cells to fully expand and maintain the response. Once such a T–B collaborative amplifying loop is established, the response could become self-sustaining, leading to chronic autoimmunity (27).
The experiments described here provide important insights into the influence of T cells on the development of an autoreactive B-cell response. First, we found that specific T cells are required; AM14 B cells responded similarly whether T-cell help was of an irrelevant specificity or completely absent. Second, we observed that CD40L is an important ligand in the process, further implicating T cells and strongly suggesting the need for a cognate interaction between T and B cells. Third, IL-21, although not essential—as with other T-cell signals—influences the response in both quantitative and qualitative fashions (promoting IgG2a and IgG3 switching) via its direct action on responding B cells. IL-21 has previously been implicated in contributing to murine models of autoimmune disease, presumably via multiple effects on T and B cells (28); here we define a specific influence directly on the autoreactive B cell. Taken together, we conclude that Ag-specific T cells optimize the AM14 B-cell response to PL2-3 in vivo through CD40L and IL-21 but are not required for either initiation or completion of differentiation to the plasmablast stage (Fig. S4).
This work also provides an in vivo link to T EF helper cells first characterized by Odegard et al. (21) that produce IL-21 and can promote plasmablast expansion and switching in vitro. Although ICOS is required for the development of T EF helper cells in chronically autoimmune mice, our data support an ICOS-independent mechanism in helping B cells during their initial activation and differentiation. This supports earlier work implicating a requirement for ICOS in B-cell responses to protein Ag but not polysaccharide Ag (29). Our data support the idea that the requirements for effective T-cell help adapt depending on the nature and the persistence of the Ag. In systemic autoimmunity, Ag is constitutively generated from dying cells. This may allow synergy of BCR with TLR signaling to bypass the requirement for ICOS.
The requirement for specific T cells does raise an important unanswered question: what is the nature of the auto-Ag(s) for these T cells? There is no obvious foreign Ag being introduced when PL2-3, a syngeneic IgG2a purified from syngeneic animals, is introduced. Presumably T cells are activated that recognize self-Ag in the chromatin-containing IC and/or the self-IgG. Despite expectations of self-tolerance, several groups have shown that chromatin or splicesome protein-specific T cells do exist in the periphery of normal animals and that they can be activated, particularly when specific B cells present Ags (30, 31). Similarly, T cells specific for self-IgG have been described (32). It will be of great interest to identify the specificities and types of T cells that are providing help to the pathogenic EF response.
In general, the emphasis on “T-dependent” vs. “T-independent” responses in classical model systems may have clouded the issue of what happens in a more physiological setting with biologically relevant Ags. In our case, we have used an Ag relevant to the production of both RF and antichromatin Abs in vivo. This Ag has some characteristics of a T-independent type 1 Ag, with a TLR ligand and possibly an enhanced ability to crosslink the BCR given the multivalent nature of ICs and also of chromatin itself (14). Notably, pathogens, such as bacteria and viruses, would share many of these properties, although whether they are able to activate B cells in an analogous way remains unclear (33). With such Ags, the simple concepts of “T-dependent” or “T-independent” are inadequate to describe the in vivo interactions that control the EF B-cell response, and this is where the present work provides important insights. Our data demonstrate that all aspects of the response can and do happen in the absence of T cells, including expansion, differentiation, and isotype switch [mutation was also previously demonstrated (14)]. The significant implication of this observation is that self-reactive B cells can be activated all of the way to effector function without the need to break T-cell tolerance. We propose, therefore, that expression of nucleic acid-specific TLRs by B cells is a weak link in the systems that prevent autoreactivity but has been evolved as a result of the benefit from enhanced responses to microbial pathogens. Self-Ags that provide a TLR ligand will have a differentially greater ability to activate B cells in the absence of T-cell help, and this program of activation leads much further than previously thought. We suggest that this TLR requirement explains why antinuclear Abs are a final common outcome of many immune dysregulatory disorders, and indeed, the EF pathway of activation is being identified in a variety of systems with B-cell autoimmunity (8, 27, 34).
Materials and Methods
Mice.
AM14 sd-Tg BALB/c mice were generated as previously described (15). BALB/c mice were purchased from Jackson Laboratories. DO11.10 Tg TCRα−/− and TCRα−/− BALB/c mice were obtained from Dr. Kim Bottomly (Yale University, New Haven, CT) (35). IL-21R−/− BALB/c mice (36) were obtained from Dr. Warren Leonard (National Institutes of Health, Bethesda, MD). CD45.1 BALB/c mice were obtained from Dr. Hyam Levitsky (Johns Hopkins School of Medicine, Baltimore, MD). All mice were maintained at Yale University School of Medicine under specific pathogen free conditions and used according to Institutional Animal Care and Use Committee-approved protocols.
B-Cell Isolation and Adoptive Transfer.
Splenic B cells were prepared using the EasySep Mouse B Cell Enrichment kit (Stemcell Technologies) according to the manufacturer's instructions, resulting in purity of 95% or greater as determined by FACS, unless otherwise indicated. Three million B cells in PBS were injected i.v. per mouse, unless otherwise indicated.
Ascites Preparation and Immunization.
PL2-3 hybridoma was grown and Ab was obtained as previously described (12). PL2-3 (500 μg) was administered to animals i.p. on days 0, 2, and 4.
CFSE Labeling.
Primary cells were cultured at 37° for 10 min in the dark with 10 μM CFSE (Invitrogen) in PBS with 0.1% BSA.
Blocking Antibodies.
To block CD40L, MR-1 mAb was purified from serum-free tissue culture supernatant using Protein G sepaharose (Pharmacia) as previously described (17). Two hundred micrograms in sterile PBS was injected i.p on days −1 and 2. To block ICOSL, 200 μg HK5.3 mAb (22) in sterile PBS was injected i.p. on days 2 and 4.
Flow Cytometry.
Cell preparation and staining was performed as previously described (17). Dead cells were excluded using ethidium monoazide staining. Abs were made in the laboratory as previously described (17) or purchased from vendors as indicated. The following staining reagents were used for these experiments: 4–44 (AM14 anti-Id)-biotin, 4–44 Alexa-647, anti-CD4 Pacific Blue (GK1.5), anti-CD22 PE (Cy34.1; BD), anti-CD25 PE (7D4; Southern Biotech), anti-CD44 Alexa 488 (IM7), anti-CD45 Alexa 488 (6B2), anti-CD95 PE (Jo2; BD), anti-FoxP3 APC (FJK-16s; eBioscience), anti-IgD FITC (11-26c.2a; Biolegend), anti-TCR β biotin (H57-597; BD), PNA biotin (Vector Laboratories), and streptavidin (SA) PE-Cy7 (BD).
Samples were analyzed on an LSRII (BD). Data were analyzed with FlowJo software.
ELISpot Assay.
ELISpot assay was performed and analyzed as previously described (12). Goat anti-Ig isotype coating Abs were purchased from Southern Biotech.
Supplementary Material
Acknowledgments
We thank the Yale Animal Resources Center for excellent animal husbandry; Dr. Joe Craft for help and advice on the inducible T-cell costimulator ligand blocking experiments; and Dr. Warren Leonard, National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), for providing the IL-21R–deficient BALB/c mice and for critical reading of the manuscript. This work was supported by NIH Grant R01-AI43603 (to M.J.S.), an Arthritis Foundation Postdoctoral Fellowship (to M.L.O.), and NIH Kirschstein National Research Service Award Predoctoral Fellowship 1F31AI071694-01 (to R.A.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018571108/-/DCSupplemental.
References
- 1.MacLennan IC, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 2.Racine R, Chatterjee M, Winslow GM. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J Immunol. 2008;181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham AF, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 4.Fagarasan S, Honjo T. T-Independent immune response: New aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 5.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: Differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 6.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferraro AJ, Drayson MT, Savage CO, MacLennan IC. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer BF, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobi AM, et al. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:305–308. doi: 10.1136/ard.2008.096495. [DOI] [PubMed] [Google Scholar]
- 10.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–3977. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- 11.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 12.Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 14.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, et al. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity. 2010;43:607–618. doi: 10.3109/08916930903567500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 17.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 18.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 19.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 20.Noelle RJ, et al. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai H, et al. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J Immunol. 2002;169:4332–4339. doi: 10.4049/jimmunol.169.8.4332. [DOI] [PubMed] [Google Scholar]
- 23.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 24.William J, Euler C, Leadbetter E, Marshak-Rothstein A, Shlomchik MJ. Visualizing the onset and evolution of an autoantibody response in systemic autoimmunity. J Immunol. 2005;174:6872–6878. doi: 10.4049/jimmunol.174.11.6872. [DOI] [PubMed] [Google Scholar]
- 25.Wofsy D, Seaman WE. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987;138:3247–3253. [PubMed] [Google Scholar]
- 26.Peng SL, et al. Murine lupus in the absence of alpha beta T cells. J Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 27.Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, et al. A novel ICOS-independent, but CD28- and SAP-dependent, pathway of T cell-dependent, polysaccharide-specific humoral immunity in response to intact Streptococcus pneumoniae versus pneumococcal conjugate vaccine. J Immunol. 2008;181:8258–8266. doi: 10.4049/jimmunol.181.12.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumortier H, et al. B and T cell responses to the spliceosomal heterogeneous nuclear ribonucleoproteins A2 and B1 in normal and lupus mice. J Immunol. 2000;165:2297–2305. doi: 10.4049/jimmunol.165.4.2297. [DOI] [PubMed] [Google Scholar]
- 31.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: Escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 32.Granucci F, Rescigno M, Marconi G, Foti M, Ricciardi-Castagnoli P. Ig-specific T cell receptor-transgenic T cells are not deleted in the thymus and are functional in vivo. J Exp Med. 1996;183:203–213. doi: 10.1084/jem.183.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehr T, et al. Role of repetitive antigen patterns for induction of antibodies against antibodies. J Exp Med. 1997;185:1785–1792. doi: 10.1084/jem.185.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dittrich AM, et al. A new mechanism for inhalational priming: IL-4 bypasses innate immune signals. J Immunol. 2008;181:7307–7315. doi: 10.4049/jimmunol.181.10.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.