Abstract
We have established an HLA-A2.1 transgenic rabbit /cottontail rabbit papillomavirus (CRPV) infection model. Using this novel transgenic animal model, we reported earlier that a multivalent epitope DNA vaccine (CRPVE1ep1-5) containing five HLA-A2.1 restricted epitopes from CRPVE1 (42-50, 149-157, 161-169, 245-253 and 303-311) was successful in providing strong and specific protective and therapeutic immunity. Among these five epitopes, two (161-169 and 303-311) have been proven to stimulate strong immunity in both HLA-A2.1 transgenic mouse and rabbit models. In the current study, we further identified the remaining three epitopes (CRPVE1/42-50,149-157, 245-253) in both animal models. CRPVE1/149-157 was able to induce specific CTL responses in HLA-A2.1 transgenic mice by DNA immunization but undetectable by peptide immunization. CRPVE1/42-50 and 245-253 failed to respond in HLA-A2.1 transgenic mice either by peptide or DNA immunization. All the three epitopes when administrated as DNA vaccines, however, were able to stimulate strong protective immunity in HLA-A2.1 transgenic rabbits in a dose dependent manner. Among the five epitopes, two (CRPVE1/ 303-311and CRPVE1/149-157) DNA vaccines also showed specific therapeutic effects in CRPV-infected HLA-A2.1 transgenic rabbits. Taken together, the HLA-A2.1 transgenic rabbit model recognized more epitopes than did the HLA-A2.1 transgenic mouse model. Our data demonstrate that the HLA-A2.1 transgenic rabbit model can complement the HLA-A2.1 transgenic mouse model for the development and testing of new HLA-A2.1 restricted prophylactic and therapeutic T cell based DNA vaccines.
Keywords: HLA-A2.1 transgenic mouse and rabbit, Epitope DNA vaccine, T cell mediated immune responses, Gene-gun, Protective and therapeutic immunity, CRPV
Introduction
HLA-A2.1 is a prevalent human MHCI molecule [1,2]. Many well characterized HLA-A2.1 restricted epitopes have been tested for their therapeutic effects for viral infection or tumor formation in the HLA-A2.1 transgenic mouse (HHD) model [3-6]. Mouse models, however, show limited susceptibility to certain human pathogens such as ocular HSV-1, HTLV-1, tuberculosis and syphilis for which rabbits are susceptible [7-9]. Our recently established HLA-A2.1 transgenic rabbit thus provides an excellent host to test the immunogenicity of different epitope vaccines from these pathogens to compensate for certain limitations of the HHD mouse model [10, 11].
Human papillomaviruses (HPVs) are small DNA tumor viruses, some of which induce malignancy in genital, anal, head and neck and also skin tissues [12]. The viruses show high species specificity and thus no animal model is available to study HPV infection in vivo [13-15]. In addition, no laboratory rodent papillomavirus model has been reported to date. We and others have used the cottontail rabbit papillomavirus (CRPV) / rabbit model as a surrogate model for high-risk HPV infections in the human population [15-18].
In previous studies, we used online MHCI epitope prediction software to identify and screen five HLA-A2.1 restricted epitopes from CRPVE1 and to generate a multivalent epitope DNA vaccine for in vivo testing [10]. This multivalent DNA vaccine provided complete protection and strong therapeutic effect against CRPV infection with a single booster immunization [19]. Our further studies also demonstrated that two (CRPVE1/161-169, 303-311) of these five epitopes could stimulate detectable specific immune responses in HHD mice upon peptide immunization and promoted strong protective and therapeutic immune responses in HLA-A2.1 transgenic rabbits [20].
In the current study, we further tested the remaining three epitopes (CRPVE1/42-50, 149-157, 245-253) using the two HLA-A2.1 transgenic models and summarized the studies from these five CRPVE1 epitopes for the immunogenicity and protective immunity following peptide or DNA vaccinations. Three (CRPVE1/161-169, 303-311 and 149-157) out of five epitopes were immunogenic when tested in HHD mice by epitope and DNA vaccination. However, all five epitope DNA vaccines provided strong and specific protective immunity in HLA-A2.1 transgenic rabbits. This latter finding indicates that some epitopes that showed specific protective immunity in rabbits would have been missed had we analyzed their responses using only the HHD mouse model for screening. Partial therapeutic immunity was also induced by CRPVE1/149-157 in addition to CRPVE1/303-311 epitope DNA vaccination in HLA-A2.1 transgenic rabbits which was impossible to test in the HHD mice [20]. Our data demonstrate that while a correlation between these two transgenic animal models was found, HLA-A2.1 transgenic rabbits are more advantageous for screening and testing new protective and therapeutic DNA vaccines in vivo in a natural papillomavirus/host model.
Material and Methods
Animals
Rabbits expressing the HLA-A2.1 transgene were either bred with New Zealand White rabbits purchased commercially (outbred background) or EIII/JC inbred rabbits (inbred background). The HLA-A2.1 transgenic (HHD) mice were a kind gift from Dr. Francois Lemonnier (the Institut Pasteur) [21] and bred in our animal core facility. All the animals were maintained in the animal facilities of the Pennsylvania State University College of Medicine. All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine. Human genomic HLA-A2.1 DNA (a generous gift from Dr. Victor Engelhard) with the human promoter was randomly integrated into rabbit chromosomes by microinjection to generate transgenic rabbits as described previously [10]. The expression of HLA-A2.1 on rabbit cell surface was confirmed with immunohistochemistry or fluorescent flow cytometry analysis. The HLA-A2.1 gene has been stably passed on to EIII/JC inbred offspring for ten generations without diminishing expression level [11].
Peptide and DNA immunization in HLA-A2.1 transgenic (HHD) mice
Five HLA-A2.1 restricted epitope peptides from CRPVE1 [42-50 (SLLDDTD QV), 149-157(ILNATARV), 161-169 (LLFRQAHSV), 245-253 (ALLSQLLGV) and 303-311 (MLQEKPFQL)], as well as HBV core T helper peptide (TPPAYRPPNAPIL) were synthesized in the core facility of Pennsylvania State University College of Medicine.
CRPVE1/42-50, 149-157, 161-169, 245-253 and303-311, and HPV16E7/82-90 epitope DNA vaccines were synthesized by GenScript (NJ, USA) and subsequently cloned into an expression vector PCX as described previously [10]. The ubiquitin motif A76 cloned into PCX (identified as Ub3) and HPV16E7/82-90 DNA vaccine were used as controls in some of the experiments [20].
For peptide immunization, the peptides were diluted into 1×PBS buffer (4 mg/ml containing 5% DMSO). HBV core T helper peptide was diluted into 1×PBS buffer (5.6 mg/ml containing 5% DMSO). Each peptide was mixed with HBV core T helper peptide and emulsified in incomplete Freund’s adjuvant (IFA) at a 1:1:2 (V/V/V) ratio [22]. 6-8 weeks old HHD mice were injected with 50 μl of emulsion on both sides of the base of the tail. Two mice were used for each peptide immunization. The mice were immunized twice with 2-week intervals between injections. Spleens were harvested one week after the booster immunization [20].
For DNA immunization, mice were anaesthetized with working solutions of ketamine (10μg/kg) and xylazine (1μg/kg). The abdomen was carefully shaved with clippers. Six shots of CRPVE1/ 42-50, 149-157 or 245-253 epitope DNA vaccines were applied using helium driven gene-gun system (400dpi) as described previously [10]. Each mouse was immunized twice within a two-week interval. Spleens were harvested one week after the booster immunization.
HLA-A2.1 restricted epitope DNA vaccines and vaccination in rabbits
HLA-A2.1 transgenic rabbits were immunized with CRPVE1/42-50, 149-157, 161-169, 245-253, 303-311and HPV16E7/82-90 epitope DNA vaccines by gene-gun delivery system as described previously [10]. In brief, the epitope DNA vaccines were purified with the QIAGEN MaxiPrep kit and adjusted to final concentration of 1μg/1μl in 1×TE buffer and then precipitated onto 1.6μm-diameter gold micro particles at a ratio of 1μg of DNA/0.5mg of gold particles as described by the manufacturer (Bio-Rad, Hercules, California). Inner ear skin sites were shaved and swabbed with 70% ethanol, and then DNA/gold particles were bombarded onto these sites by a gene gun at 400 lb/in [2, 23] when the animals were anesthetized with ketamine (40mg/kg) and xylazine (5mg/kg).
For protective vaccination, groups of rabbits were immunized twice with 24 shots of test E1 epitopes or a control DNA vaccine (Ub3 or HPV16E7/82-90) respectively according to our previously published methods [10]. Rabbits immunized twice with 12 shots of DNA vaccines were identified as having half-dose immunization. The immunized animals were subsequently challenged with Hershey CRPV DNA (identified as wild type CRPV, wtCRPV) and an E6/ E7 codon-modified DNA (identified as coCRPV) at four left and four right back sites respectively (5μg construct/ site) at one week after the booster immunization [19]. The rationale for the additional challenge with coCRPV genomes was that these genomes grow more rapidly and present a greater vaccine challenge for the immunized rabbits [19].
For therapeutic experiments, rabbits were challenged with wtCRPV DNA and a wtCRPV DNA with E8ATGko mutant at four left and four right back sites respectively (5μg construct/ site). The rationale for including the CRPVE8ATGko mutant [24] in this study was that this latter genome produced slow-growing, small papillomas that could represent a smaller tumor burden for the therapeutic vaccine because skin tumors are very difficult to resolve. Four weeks following viral DNA challenge, the rabbits were immunized with 20μg of test E1 epitopes or control vaccine respectively for three times at three-week intervals.
Bulk CTL generation in vitro
Spleen cells were harvested from either peptide immunized HHD mice or DNA vaccinated HLA-A2.1 transgenic rabbits. The splenocytes were stimulated in vitro weekly with gamma irradiated either corresponding peptide pulsed dendritic cells or autologous spleen or fibroblast cells for two times respectively as described in previous studies [10, 22]. The bulk CTLs were then used for tetramer binding assay, intracellular cytokine release assay and 51chromium release assay.
Tetramer binding assay
Cultured bulk CTLs from both HHD mice and HLA-A2.1 transgenic rabbits were labeled with species-appropriate FITC conjugated anti-mouse CD8 (eBioscience) or FITC conjugated anti-rabbit CD8 (Fitzgerald Inc.) respectively and then reacted with specific PE conjugated tetramers (synthesized by the tetramer core facility of the National Institute of Health). Two-color flow cytometry analysis was used for detecting specific tetramer binding CD8 T cells on FSCAN II (BD) at the core facility of Pennsylvania State University College of Medicine [10].
Intracellular cytokine assay
Because anti-rabbit interferon gamma is not available commercially, this assay was conducted only on HHD mouse CTLs. Bulk mouse CTLs were cultured in triplicate wells of a 96-well plate with 1μM peptide (either test E1 peptides or a reference peptide HIVGagP17/77-85) and 1μM Brefeldin A (Sigma) at 37°C for 3-4 hours. The cells were then labeled with FITC conjugated anti-mouse CD8 (eBioscience) and PE conjugated anti-mouse interferon gamma (eBioscience) and analyzed by two-color flow cytometry at the core facility of Pennsylvania State University College of Medicine as described previously [10].
51Chromium release assay
Specific killing by rabbit CTLs was examined by 51chromium release assay. T2 (a TAP-deficient HLA-A2.1 positive human cell line) cell cultures were labeled with Na51Cr (300 μCi) overnight before the assay and then pulsed with peptides (either test E1 peptides or a reference peptide) for 1 hour on the day of assay as target cells. In vitro stimulated mouse or rabbit spleen cells were harvested and divided into triplet wells using Effector /Target (E :T ) ratios of 30:1, 10:1. 3.3:1 and 1:1. Target cells were co-cultured with the spleen cells at 37°C for 4 hours. Bulk CTLs from CRPVE1/161-169 and CRPVE1/303-311 peptide immunized mice were used as positive control for this assay. Supernatant from target cells cultured alone and target cells lysed with 5%SDS were counted as minimum and maximum release respectively. The 51Cr release was counted by a Gamma-counter and the specific killing was calculated using standard formula [10, 25].
Viral DNA challenge on rabbits and statistical analysis
Rabbits were anesthetized with Ketamine (40mg/kg) and Xylazine (5mg/kg). Rabbit back skin was scarified as reported previously [26]. Three days later, rabbits were challenged with wild type CRPV or CRPVE8ATGko mutant DNA (5μg DNA/ site) [18, 23]. Beginning three weeks after DNA challenge, the rabbits were monitored weekly for papilloma development.
Papilloma size was determined by calculating the cubic root of the product of length × width × height of individual papillomas in millimeters to obtain a geometric mean diameter (GMD). Data were represented as the means (± SEMs) of the GMDs for all the papillomas in each test group. Each data contained the mean of all challenge sites from all the animals from one group at a certain time point. Statistical significance was determined by unpaired student t-test comparison (P<0.05 was considered significant) using Sigma Plot software. The frequency of sites without papillomas was calculated as tumor free sites (the number of sites without papillomas) /total challenged sites. Statistical significance was determined by Fisher’s exact test (P<0.05 was considered significant).
Results
CTL generation in HHD mice by peptide immunization
Our previous study demonstrated that a successful immune response could be stimulated by CRPVE1/161-169 and 303-311 peptide immunizations of HHD mice [20]. Using the same strategy, we immunized two mice with CRPVE1/245-253, 42-50 and 149-157 peptides twice with a two-week interval between immunizations respectively. Mice immunized with HPV16E7/82-90 peptide were used as positive control. The spleens were harvested one week after the booster immunization and cultured in vitro with gamma-irradiated peptide-pulsed mouse dendritic cells prepared as described [27]. After two in vitro stimulations, the cultured bulk CTLs from HLA-A2.1 transgenic mice were tested for the generation of specific CD8 T cells using synthesized tetramers. Consistent with previous studies, HPV16E7/82-90 peptide immunized mice generated specific tetramer positive CD8 T cells. However, all the test peptide immunized HHD mice failed to generate specific tetramer positive CD8 T cells (data not shown). Since we could not determine if the failure of tetramer binding was due to dysfunctional tetramers or unresponsive bulk CTLs for these two epitopes, we further conducted an IFNγ assay to examine these bulk CTLs.
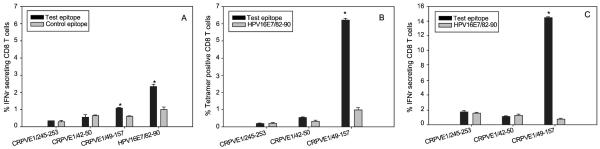
The bulk CTLs were then examined for intracellular interferon gamma (IFNγ) levels [22]. Significantly more IFNγ secreting CD8 T cells were found in the mice immunized with HPV16E7/82-90 peptide. Very low but significantly more IFNγ secreting CD8 T cells were generated in CRPVE1/149-157 peptide immunized mice (Figure 1A, P<0.05, unpaired student t test). No significant IFNγ secreting CD8 T cells were found in the mice immunized with the remaining two peptides (Figure 1A, P>0.05, unpaired student t test).
Figure 1.
Peptide and epitope DNA vaccine immunized HHD mice generated specific tetramer binding CD8 T cells that secreted intracellular interferon gamma (IFNγ) after in vitro culture. The spleen cells harvested from CRPVE1/149-157, 42-50, 245-253 and HPV16E7/82-90 peptide (A) or epitope DNA vaccinated mice (B and C) were stimulated with corresponding peptide pulsed mouse dendritic cells weekly twice. These bulk CTLs were then tested for A) Intracellular IFNγ labeling for peptide vaccination, B) tetramer binding and C) Intracellular IFNγ labeling for DNA vaccination. A) A very low but significant population of specific CD8 T cells secreting IFNγ in CRPVE1/149-157 peptide immunization was found (P<0.05, unpaired student t test) and no detectable IFNγ secreting CD8 T cells were found from either CRPVE1/42-50 or CRPVE1/245-253 peptide immunized HHD mice; B) Significantly more specific CD8 T cell binding to CRPVE1/149-157 and CRPVE1/42-50 tetramer was found in epitope DNA vaccinated HHD mice respectively (P<0.05, unpaired student t test). C) Significantly more IFNγ secreting CD8 T cells were also found in CRPVE1/149-157 epitope DNA vaccinated mice to corresponding peptide stimulation when compared with a reference peptide (P<0.05, unpaired student t test) but not in the other two epitope DNA vaccinated groups (N=2/ group).
CTL generation in HHD mice by DNA immunization
DNA vaccine delivered by gene-gun has been demonstrated to stimulate the most potent immune response in the mouse [28]. We therefore wanted to test whether DNA vaccination could augment the immune response of the three low or non-responsive epitopes in HHD mice. DNA vaccines for these epitopes were designed and synthesized as previously described [10]. Two immunizations with six shots for each HHD mouse were applied to abdomen skin sites and spleen cells were harvested one week after the booster immunization. The spleen cells were subsequently cultured as previously described [22]. After two in vitro stimulations, the splenocytes from HLA-A2.1 transgenic mice were tested for the generation of specific CD8 T cells using synthesized tetramers. Significantly more tetramer positive CD8 T cells specific to CRPVE1/149-157 and to CRPVE1/42-50 were found with much higher responses detected for CRPVE1/149-157; there was no response to CRPVE1/245-253 (Figure 1B, P<0.01, P<0.05 and P>0.05 respectively, unpaired student t test).
The bulk T cells were then examined for intracellular IFNγ levels. A significant population of IFNγ secreting CD8 T cells was found for the mice immunized with 149-157 epitope DNA when compared to control peptides (Figure 1C, P<0.01, unpaired student t test). No significant population of IFNγ secreting CD8 T cells was found in CRPVE1/42-50 and CRPVE1/245-253 epitope DNA immunized mice (Figure 1C, P>0.05, unpaired student t test). Therefore, DNA immunization stimulated a stronger immune response when compared with peptide immunization for CRPVE1/149-157 epitope but not for CRPVE1/42-50 and CRPVE1/245-253 epitopes.
CTL generation in the HLA-A2.1 transgenic rabbit after DNA immunization
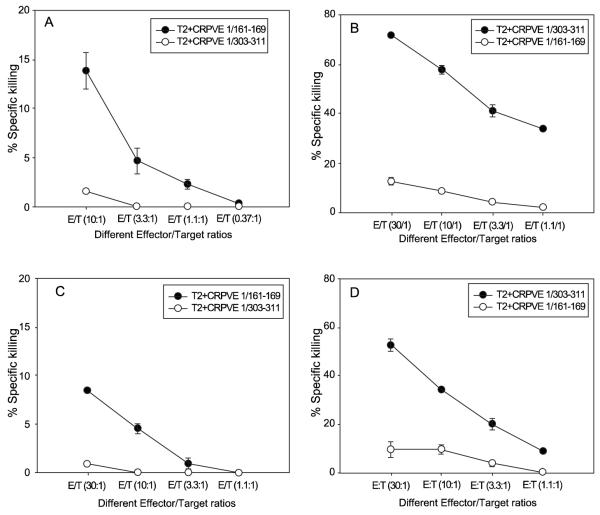
Next, we wanted to test whether any of the five epitopes could stimulate specific CTLs in HLA-A2.1 transgenic rabbits. Two HLA-A2.1 transgenic rabbits per epitope were immunized with CRPVE1/ 161-169, CRPVE1/303-311, CRPVE1/149-157, CRPVE1/42-50 or CRPVE1/245-253 epitope DNA vaccines twice with a three-week interval between the vaccinations [19]. The rabbit spleen cells and sera were harvested; and spleen cells were stimulated with autologous spleen or fibroblast cells pulsed with peptides. After two in vitro stimulations, the bulks CTLs were examined for tetramer binding. CTLs from CRPVE1/161-169 immunized HLA-A2.1 transgenic rabbits showed significant levels of specific tetramer binding while the others failed to show any responses (Data not shown). The specific killing by rabbit CTLs was conducted by 51chromium release assay because anti-rabbit IFNγ antibody was not available commercially to conduct the intracellular cytokine release assay. At the same time, CTLs cultured from CRPVE1/161-169 and CRPVE1/303 peptide immunized HHD mice were used as positive controls. These mouse CTLs have been demonstrated to generate significantly higher levels of tetramer and IFNγ specific CD8 T cells in our previous study [20]. Consistent with those data, both mouse CTLs were able to kill corresponding peptide pulsed target cells by 51chromium release assay (Figure 2A and 2B, P<0.05, unpaired student t test). These two epitope DNA vaccinated HLA-A2.1 transgenic rabbits also showed comparative specific killing to their specific peptide labeled target cells as detected by the chromium release assay (Figure 2C and D, P<0.05, unpaired student t test). Rabbits vaccinated with the other three DNA epitope vaccines failed to generate detectable specific CTLs (data not shown). Therefore, two (CRPVE1/161-169 and CRPVE1/303-311) out of the five HLA-A2.1 restricted E1 epitope DNA immunized HLA-A2.1 transgenic rabbits were capable of inducing specific cytotoxic T cells after in vitro stimulations.
Figure 2.
CRPVE1/161-169 and CRPVE1/303-311 peptide vaccinated HHD mice (A and B) and DNA vaccinated HLA-A2.1 transgenic rabbits (C and D) generated specific cytotoxic T cells. 51Chromium release assay were conducted on the bulk CTLs of these two epitope peptide immunized HHD mice or DNA vaccinated HLA-A2.1 transgenic rabbits in vitro as shown in materials and methods. A) Significantly higher levels of specific killing were found in cultures of HHD mice cells against T2 cells pulsed with CRPVE1/161-169 (A) peptides vs. T2 cells pulsed with CRPVE1/303-311 (P<0.05, unpaired student t test) following vaccinated with CRPVE1/161-169 or vice versa (B); Similar results were found from rabbit cells against T2 cells pulsed with CRPVE1/161-169 peptides vs. T2 cells pulsed with CRPVE1/303-311 (P<0.05, unpaired student t test) following vaccination with CRPVE1/161-169 epitope DNA vaccine (C) and vice versa (D). The data were representative of three individual rabbits. CRPVE1/303-311 epitope vaccinated animals generated relatively higher levels of specific killing CTLs when compared with CRPVE1/161-169 epitope vaccinated animals. Other three epitopes (CRPVE1/245-253, 42-50, 149-157) were unresponsive for this test (data not shown).
Protective immunity by epitope DNA vaccination in outbred HLA-A2.1 rabbits against CRPV DNA infection
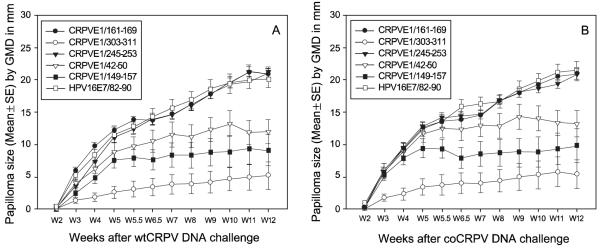
The CRPV/rabbit model is an in vivo infection model that allows us to test the host immunity against the development of CRPV-induced papillomas. Our previous study has demonstrated strong protective immunity in both CPRVE1/303-311 and CRPVE1/161-169 DNA vaccinated HLA-A2.1 transgenic rabbits [20]. Our next goal was to test if any or all of the three remaining epitope DNA vaccines could stimulate specific protective immunity in HLA-A2.1 transgenic rabbits. HLA-A2.1 transgenic rabbits were divided into four groups and vaccinated with the three DNA vaccines and a control vaccine (HPV16E7/82-90) respectively (Table 1). After a single booster immunization, the rabbits were challenged with wtCRPV and a more vigorous mutant CRPV with E6 /E7 codon modified CRPV (coCRPV) at left and right back sites respectively. Tumor development was monitored weekly until week 12. Three out of four rabbits from the CRPVE1/245-253 and CRPVE1/42-50 groups and, and three out of three rabbits from the CRPVE1/149-157 group were protected from both wt and coCRPV DNA challenge while no rabbits from the control group (HPV16E7/82-90) were protected (Table 1). Taken together, all three together with previous identified two epitope DNA vaccines provided partial to complete protective immunity in HLA-A2.1 transgenic rabbits (Figure 3, Table 1, P<0.05, Fisher’s exact test).
Table 1.
Protective immunity induced by full-dose (24 shots) epitope DNA vaccination in outbred HLA-A2.1 transgenic rabbits.
| Vaccine (rabbit numbers) |
Papilloma sites | Protected sites | Protection rate |
|---|---|---|---|
| CRPVE1/161-169 (N=8) |
24 | 32 | 24/32 (75%)a |
| CRPVE1/303-311 (N=5) |
20 | 20 | 20/20 (100%)a |
| CRPVE1/245-253 (N=4) |
4 | 12 | 12/16 (75%)a |
| CRPVE1/42-50 (N=4) |
4 | 12 | 12/16 (75%)a |
| CRPVE1/149-157 (N=3) |
0 | 12 | 12/12 (100%)b |
| HPV16E7/82-90 (N=4) |
14 | 2 | 2/16 (12.5%) |
P=0.02
P=0.01 vs. HPV16E7/82-90 group, Fisher’s exact test
Figure 3.
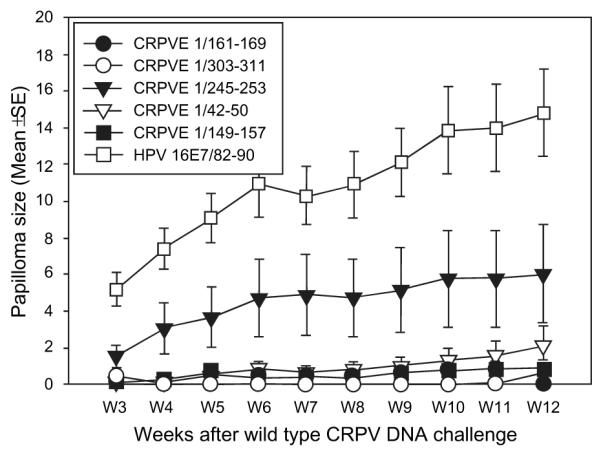
Papilloma outgrowth in HLA-A2.1 transgenic outbred rabbits after CRPVE1/161-169, 303-311, 245-253, 42-50, 149-157 or HPV16E7/82-90 epitope DNA vaccination. Four HLA-A2.1 transgenic rabbits immunized with each of the five epitopes or a HPV16E7/82-90 epitope DNA vaccine were challenged with wtCRPV and coCRPV at four left and right back sites respectively. Significantly smaller papillomas were found in all five epitope DNA vaccinated rabbits when compared with those in HPV16E7/82-90 vaccinated rabbits (P<0.01 vs. control group, unpaired student t test). Significant difference was found between four epitope CRPVE1/149-157, 161-169, 303-311, CRPVE1/42-50 and CRPVE1/245-253 epitope vaccinated rabbits (P<0.05, unpaired student t test).
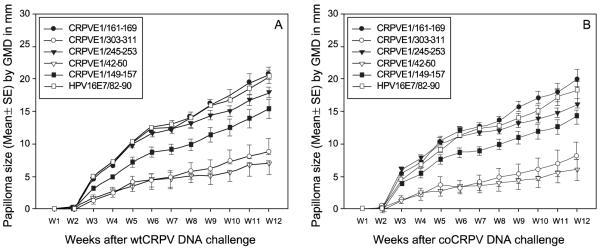
Because of the strong immunity generated by these DNA vaccinations, we wanted to test if a reduced dose of the vaccines would still be effective. Four HLA-A2.1 transgenic rabbits were immunized with half doses (12 shots) of the five epitope DNA vaccines (CRPVE1/42-50,149-157,161-169, 245-253 and 303-311) or HPV16E7/82-90 epitope DNA vaccine respectively. Two out of four rabbits immunized with either CRPVE1/149-157 or CRPVE1/303-311 epitope DNA were protected from both wtCRPV and coCRPV DNA infection (Table 2). One out of four rabbits immunized with CRPVE1/42-50 was protected while no protection was found in the CRPVE1/245-253, CRPVE1/161-169 or the HPV16E7/82-90 epitope DNA vaccine control groups (Figure 4A and B, Table 2, P>0.05, Fisher’s exact test). These results show that the threshold of DNA vaccines needed to generate effective protective immunity is dose-dependent.
Table 2.
Protective immunity induced by half-dose (12 shots) epitope DNA vaccination in outbred HLA-A2.1 transgenic rabbits.
| Vaccine (rabbit numbers) |
Papilloma sites | Protection sites | Protection rate |
|---|---|---|---|
| CRPVE1/161-169 (N=4) |
16 | 0 | 0/16 (0%) |
| CRPVE1/303-311 (N=4) |
5 | 11 | 11/16 (69%)a |
| CRPVE1/245-253 (N=4) |
16 | 0 | 0/16 (0%) |
| CRPVE1/42-50 (N=4) |
12 | 4 | 4/16 (25%)b |
| CRPVE1/149-157 (N=4) |
8 | 8 | 8/16 (50%)a |
| HPV16E7/82-90 (N=4) |
16 | 0 | 0/16 (0%) |
P=0.01
P=0.02 vs. HPV16E7/82-90 group, Fisher’s exact test
Figure 4.
Papilloma outgrowth in HLA-A2.1 transgenic rabbits after half-dose of CRPVE1/161-169, 245-253, 42-50, 303-311, 149-157 or HPV16E7/82-90 epitope DNA vaccination. Four HLA-A2.1 transgenic rabbits immunized with half-dose (6 shots/each ear instead of 12 shots for normal immunization)each of the five epitope or a HPV16E7/82-90 epitope DNA vaccine were challenged with wtCRPV and coCRPV at four left and right back sites respectively. Significantly smaller papillomas were found in CRPVE1/303-311 , 149-157 and 42-50 epitope DNA vaccinated rabbits when compared with those in other three epitope DNA and HPV16E7/82-90 vaccinated rabbits challenged with both wtCRPV DNA (A) and coCRPV DNA (B) (P<0.01 vs. control group, unpaired student t test). No significant difference was found between these two CRPVE1/245-253, CRPVE1/161-169 and HPV16E7/82-90 epitope vaccinated rabbits (P>0.05, unpaired student t test).
Cross reaction was found in normal ( non HLA A 2.1) EIII/JC inbred rabbits vaccinated with 42-50 epitope DNA vaccines
In a previous study, we noticed a possible cross-reactivity of CRPVE1/303-311 but not CRPVE1/161-169 epitope vaccine when presented by EIII/JC inbred HLA-A2.1 transgenic rabbits [20]. To identify whether any of the three remaining epitopes would show similar cross-reactivity, 4 groups of normal EIII/JC inbred rabbits were immunized with CRPVE1/42-50, 149-157, 245-253 or HPV16E7/82-90 as described in Table 3. One week after the final immunization, the rabbits were challenged with wtCRPV and coCRPV at the left and right back sites respectively. The papilloma outgrowth was monitored weekly at 3 weeks following DNA challenge. More challenge sites were protected against viral DNA challenge in CRPVE1/42-50 immunized groups but the difference was not significant (Table 3, P>0.05, Fisher’s exact test). However, both wt and coCRPV DNA-induced papilloma size was significantly smaller when compared with those in the other groups (Figure 5A and B, P<0.05, unpaired student t test). Slightly reduced size was also noticed in CRPVE1/149-157 immunized rabbits before week 10 in both wt and coCRPV DNA induced papillomas and no significant difference was found between CRPVE1/149-157 vs. control group after this time point (Figure 5A, B, P>0.05, unpaired student t test). The papilloma size in CRPVE1/245-253 and 161-169 immunized rabbits was comparable to that in the HPV16E7/82-90 epitope DNA immunized rabbits (Figure 5A, B, P>0.05, unpaired student t test). Therefore, a weak cross-reactivity was found in normal EIII/JC inbred rabbits to the CRPVE1/42-50 and 303-311 epitopes but not to CRPVE1/149-157, 161-169 and 245-253 epitopes.
Table 3.
Protective immunity induced by epitope DNA vaccination in normal (non-transgenic) EIII/JC inbred rabbits.
| Vaccine (rabbit numbers) |
Papilloma sites | Protection sites | Protection rate |
|---|---|---|---|
| CRPVE1/245-253 (N=6) |
24 | 0 | 0/24 (0%) |
| CRPVE1/42-50 (N=6) |
20 | 4 | 4/24 (17%)a |
| CRPVE1/149-157 (N=6) |
24 | 0 | 0/24 (0%) |
| HPV16E7/82-90 (N=4) |
16 | 0 | 0/16 (0%) |
P>0.05 vs. HPV16E7/82-90 group Fisher’s exact test
Figure 5.
Papilloma outgrowth in normal EIII/JC inbred rabbits after CRPVE1/161-169, 303-311, 245-253, 42-50, 149-157 or HPV16E7/82-90 epitope DNA vaccination. Four normal EIII/JC inbred rabbits immunized with each of the five epitope or an HPV16E7/82-90 epitope DNA vaccine were challenged with wtCRPV and coCRPV at four left and right back sites respectively. Significantly smaller papillomas were found in CRPVE1/42-50 and 303-311 epitope DNA vaccinated rabbits when compared with those in other three epitope DNA and HPV16E7/82-90 vaccinated rabbits challenged with both wtCRPV DNA (A) and coCRPV DNA (B) (P<0.01 vs. control group, unpaired student t test). No significant difference was found between these two CRPVE1/149-157, 245-253, 161-169 and HPV16E7/82-90 epitope vaccinated rabbits (P>0.05, unpaired student t test).
Therapeutic immunity was induced by CRPVE1/149-157 DNA vaccination
As CRPVE1/149-157 stimulated comparable protective immunity in HLA-A2.1 transgenic rabbits to that of CRPVE1/303-311, we next tested if this DNA vaccine would have a similar therapeutic effect. Experiments were done according to the availability of the animals. Two outbred and two EIII/JC inbred HLA-A2.1 transgenic rabbits were challenged with wtCRPV DNA and less vigorous mutant CRPV with E8ATG knock out (CRPVE8ATGko) (Table 4) [24]at four left and four right back sites. The rationale for including the CRPVE8ATGko mutant [24]in this study was that this latter genome produced slow-growing, small papillomas that could represent a smaller tumor burden for the therapeutic vaccine because skin tumors are very difficult to resolve. One month following DNA infection, the rabbits were immunized with CRPVE1/149-157 or HPV16E7/82-90 DNA vaccines and boosted twice at 3-week intervals. The animals were monitored weekly and papilloma sizes were recorded.
Table 4.
Therapeutic immunization after CRPVE8ATGko mutant infection in HLA-A2.1 transgenic rabbits with both EIII/JC inbred and outbred background.
| Rabbit ID | Genetic background | Vaccine | Tumor size of pooled sites (Mean GMD+SE) |
|
|---|---|---|---|---|
| Before therapy | After therapy | |||
| R1327 (C1) | Inbred | HPV16E7/82-90 | 3.17±0.38 | 3.29±0.73a |
| R1367 (C2) | ||||
| R1517 (C3) | Outbred | |||
| R1518 (C4) | ||||
| R1364 (T1) | Inbred | CRPVE1/149-57 | 3.26±0.28 | 1.51±0.71b |
| R1365 (T2) | ||||
| R1515 (T3) | Outbred | |||
| R1516 (T4) | ||||
P>0.05,
P<0.05, unpaired student t test vs. before therapy
No significant difference in wild type CRPV induced papillomas (pooled papillomas from all sixteen challenged sites) was found between CRPVE1/149-157 and HPV16E7/82-90 vaccinated outbred rabbits (Figure 6A, P>0.05, unpaired student t test). However, significantly smaller papillomas induced by CRPVE8ATGko mutant DNA were found in EIII/JC inbred HLA-A2.1 transgenic rabbits after the therapeutic treatments with CPRVE1/149-157 eptiope DNA vaccine (Figure 6B, P<0.05, unpaired student t test). In contrast, no outbred HLA-A2.1 transgenic rabbits were free of CRPVE8ATGko mutant induced papillomas in the HPV16E7/82-90 vaccinated group (Figure 6B, Table 4, P<0.05, unpaired student t test). Taken together, significantly smaller papillomas were found in the HLA-A2.1 transgenic rabbits after vaccination with CRPVE1/149-157 (Table 4, P<0.05, unpaired student t test) while no significant change in papilloma size was found in these rabbits after vaccination with HPV16E7/82-90 epitope DNA vaccine (Table 4, P>0.05, unpaired student t test). Therefore, CRPVE1/149-157 epitope DNA vaccine stimulated a strong and specific therapeutic immunity in HLA-A2.1 transgenic rabbits.
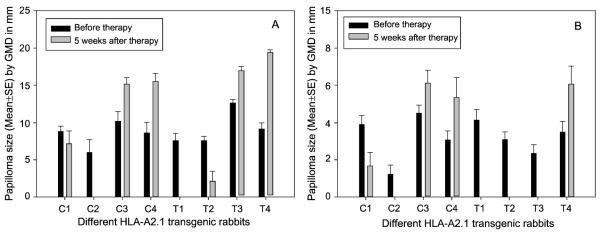
Figure 6.
Papilloma outgrowth in HLA-A2.1 transgenic rabbits after CRPVE1/149-157 epitope DNA therapeutic vaccination. Four outbred and four EIII/JC inbred transgenic rabbits were challenged with wild type CRPV (A) and CRPV E8ATGko mutant (B) at four left and right back sites respectively. Four weeks after DNA challenge, two outbred (C1-C2) and EIII/JC (C3-C4) inbred HLA-A2.1 rabbits were vaccinated with HPV16E7/82-90 epitope DNA vaccines. Two outbred (T1-T2) and two EIII/JC inbred (T3-T4) HLA-A2.1 transgenic rabbits were immunized with CRPVE1/149-157 epitope DNA vaccine at week for three times at a three week interval. Papilloma outgrowth was monitored weekly for twelve weeks. Both CRPVE1/149-157 treated EIII/JC inbred rabbits (four papillomas /per animal, eight tumor sites in total) showed significant reduction or regression of wild type CRPV induced papillomas while papillomas on one of the control rabbits regressed (A). No significant difference was found between these two CRPVE1/149-157 and HPV16E7/82-90 epitope vaccinated outbred rabbits upon challenge with wild type CRPV DNA (Eight tumor sites/ group, P>0.05, unpaired student t test).Two CRPVE1/149-157 epitope DNA vaccinated EIII/JC rabbits were free of CRPVE8ATGko mutant induced papillomas at week 5 after treatment. Taken together, when compared with those in HPV16E7/82-90 vaccinated rabbits challenged with E8ATGko mutant DNA, significantly smaller papillomas were found after CRPVE1/149-157 epitope DNA vaccination (B) (sixteen tumor sites/ group, P<0.05 vs. before therapy, unpaired student t test).
Discussion
In this report, we carried out studies using both HLA-A2.1 transgenic mouse and rabbit models to screen and characterize three remaining HLA-A2.1 restricted epitopes from our previous work(CRPVE1/ 245-253, 42-50 and 149-157) predicted by online MHCI epitope prediction programs for immunogenicity and protective immunity [19]. Our initial hypothesis was that the epitopes would behave consistently between these two transgenic animal models. Among the five tested epitopes in present and previous studies, CRPVE1/161-169, 303-311 and 149-157 showed consistency between these two models whereas the other two (CRPVE1/245-253 and 42-50) did not. In the latter case, immunogenicity was not shown in transgenic mice whereas the epitopes were able to induce strong protective immunity in HLA-A2.1 transgenic rabbits. Therefore, the HLA-A2.1 transgenic rabbit model shows potential for the screening of epitopes that might be missed by evaluation in the HLA-A2.1 transgenic mouse model (summarized in Table 5).
Table 5.
The relative rank of the five tested HLA-A2.1 restricted epitopes from CRPV E1 is summarized from HLA-A2.1 transgenic mouse and rabbit system.
| CRPV E1 Epitope |
Mouse rank | Rabbit rank | Overall rank |
||||||
|---|---|---|---|---|---|---|---|---|---|
| In vitro | In vivo | ||||||||
| Tetramer binding |
IFNγ assay |
Tetramer and 51Cr |
In vivo protection |
Mouse | Rabbit | Rabbit | |||
| Peptide vaccine |
DNA vaccine |
half dose | full dose | ||||||
| 42-50 | NR | 2 | NR | NR | 3 | 2 | 4 | NR | 3 |
| 149-157 | NR | 1 | 3 | NR | 2 | 1 | 3 | NR | 2 |
| 161-169 | 2 | NT | 2 | 2 | 4 | 2 | 2 | 2 | 4 |
| 245-253 | NR | NR | NR | NR | 4 | 2 | NR | NR | 4 |
| 303-311 | 1 | NT | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
NR—Non-responder; NT-Not Tested
The HLA-A2.1 transgenic mouse model has the advantage of reduced cost and a large panel of reagents (such as antibodies for the cytokine release assay and reagents for in vitro CTL stimulations) to test the immunogenicity of potential epitopes [29]. However, the mouse model system does not provide an effective in vivo infection model for several human pathogens such as viruses [HTLV-1/2, EBV-like virus, ocular HSV] and other diseases such as tuberculosis and syphilis [9]. In contrast, rabbits can be used as a surrogate infection model for human papillomaviruses as well as the above pathogens [8, 9, 30]. Our recent work demonstrated that the CRPV genome has a high capacity for modification without compromising its ability to induce papillomas in animals [18]. By generating hybrid and mutant genomes that are functional in the rabbits, we can test specific immunity of predicted epitopes for additional pathogens [10]. This model also has the potential to test tumor associated antigens that can be embedded into the CRPV genome. We have demonstrated here that our transgenic rabbit model system shows general agreement with the mouse model. However, the transgenic rabbit model provides increased in vivo sensitivity thus making it possible to measure the responses to epitopes that were not detected in the mouse system.
Peptide immunization has been shown to be an effective method for screening CTL epitopes in the mouse model [22]. One study compared different immunization methods and showed that DNA vaccine delivered by gene-gun actually stimulated the strongest immune response in mice [28]. Consistent with that finding, we demonstrated that CRPVE1/149-157 failed to stimulate strong specific CTLs in HLA-A2.1 transgenic mice when administered by peptide immunization but was able to elicit an immune response following DNA vaccination. Our recent study demonstrated that peptide immunization by mucosal routes (intranasal and ocular) was able to prime strong immunity in our HLA-A2.1 transgenic rabbits [31]. Therefore, the immunogenicity of a certain epitope relies not only on its composition but also on the delivery method [32]. Using the gene-gun delivery system for DNA vaccination in rabbits, we have achieved consistent protective immunity from experiment to experiment [20, 24, 33, 34]. In this study, it is also interesting to note that dose of immunization played an important role in outcome. In our previous study, we demonstrated that one booster immunization with a full dose (24 shots) was necessary to generate strong immune response in animals [19]. In the current study, we also tested whether two half dose (12 shots) immunizations could provide the same level of protection. Two of the five epitopes (CRPVE1/303-311 and 149-157) that provided complete protection by full dose immunizations now generated only sufficient immunity to protect half of the challenge sites. One (CRPVE1/42-50) of the remaining three epitopes generated protective immunity to only one fourth of the challenge sites and no protection whatsoever was found in rabbits immunized with half dose CRPVE1/161-169 and 245-253. Therefore, different epitopes had different thresholds of protection based on their immunogenicity. Taken together, our results indicate that several factors need to be taken into consideration when determining the immunogenicity of a given epitope.
Despite limited reagents to optimize in vitro stimulation and assays of CTLs generated in DNA vaccinated rabbits, we were able to demonstrate that one (CRPVE1/161-169) out of the five epitopes was capable of generating tetramer positive CTLs that killed specific target cells. CTLs from CRPVE1/303-311 immunized rabbits did not show good tetramer binding but were able to kill specific targets. The other three epitopes failed to stimulate specific CD8 T cells in vitro although they induced strong protective immunity in vivo. Recent studies have suggested that, for CD8+T cells, qualitative parameters such as the ability to proliferate upon antigen encounter, whether the cells are poly-functional, and how sensitive they are to the antigen played a more important role than did quantitative parameters in eliciting strong immunity [35]. Large quantities of CTLs could contribute to replicative senescence and even irreversible exhaustion which would then fail to provide any protection to the host [35]. Regardless of the population of in vitro stimulated CTLs, CRPVE1/303-311 and 149-157 epitope DNA vaccines not only provided complete and specific protection but also a strong therapeutic effect in HLA-A2.1 transgenic rabbits. These findings suggest that even small and sometimes undetectable levels of highly functional CTLs can provide effective protection in vivo and further suggest that the results from an in vitro stimulation assay may not necessarily reflect what happens in vivo.
To compare the performance of these five epitopes based on in vitro and in vivo data in HLA-A2.1 transgenic mouse and rabbit model systems, we ranked epitopes from 1 (the strongest responders) to 4 (weakest responders). Non-responders and not tested samples are marked as NR and NT respectively (Table 5). HLA-A2.1 transgenic mouse in vitro and rabbit in vivo data showed agreement on certain strong epitopes such as CRPVE1/303-311 but not on relatively weak epitopes such as CRPVE1/245-253. Despite their inability to stimulate CTLs in HLA-A2.1 transgenic mice, these weak epitopes were still able to promote strong protective immunity in HLA-A2.1 transgenic rabbits. The difference in the MHCI constitution of HHD mice and HLA-A2.1 transgenic rabbits might play an important role in the difference in immunogenicity displayed by the same epitope in these animals. In HHD mice, a chimeric MHCI is formed of HLA-A2.1 alpha1 and alpha 2 domains combined with H-2Db alpha 3 domain covalently attached to human beta-2 microglobulin [36]. In the transgenic rabbits, a chimeric MHCI is formed of the whole human HLA-A2.1 heavy chain in combination with rabbit beta-2 microglobulin. The compatibility of the peptide/MHCI complex from these two transgenic animals might have an impact on the display of immunogenicity by an HLA-A2.1 restricted epitope. In addition, rabbits show higher genetic homology to humans, therefore this outbred HLA-A2.1 transgenic model is more relevant to the human situation. Taken together, the HLA-A2.1 transgenic rabbits can provide a valuable additional model system to test the immunogenicity of new epitopes in vivo for protective and therapeutic immunity.
Skin papillomas are usually difficult to resolve and no effective therapeutic vaccine is available for clinical use to date [15, 37-39]. It is intriguing that epitopes CRPVE1/303-311 and 149-157 showed potential therapeutic effects in HLA-A2.1 transgenic rabbits. A stronger therapeutic effect was also found against CRPVE8ATGko challenge which generated smaller papillomas when compared to those generated by wild type CRPV especially in HLA-A2.1 transgenic rabbits with EIII/JC inbred background. This finding was true for both epitope DNA vaccinations and is consistent with our previous reports [19]. Our previous studies have demonstrated that EIII/JC inbred rabbits have distinct MHCII constitution and showed higher regression rates after CRPV infection when compared with outbred rabbits [23, 40]. CRPVE1/303-311 showed a cross reaction to normal EIII/JC inbred rabbits and therefore HLA-A2.1 transgenic rabbits with EIII/JC inbred background would display added immunity in CRPVE1/303-311 DNA immunized animals [20]. This is not true for epitope CRPVE1/149-157 that did not show cross reaction in normal EIII/JC inbred rabbits. This finding suggests specific immunity by these epitope DNA vaccinations contributed to the regression of CRPVE8ATGko mutant induced papillomas.
To date, papillomavirus E1 has not been considered a good therapeutic candidate for cancer patients because its level is undetectable in cancer samples where papillomavirus DNA is normally integrated into the host genome [14]. However, E1 immunization has been reported to induce strongly protective immunity and/or papilloma regression [41-44]. E1 has also been tested for therapeutic purposes in previous studies [33, 45-47]. Our study further confirmed that a therapeutic effect could be achieved by E1 targeted immunization for early stage papillomas in rabbits. Previous studies also demonstrated the advantage of immunizing rabbits with multiple early genes [41, 43, 47]. Therefore, vaccines containing multivalent epitopes from different early and late genes may be a desirable extension of current therapeutic vaccines.
In summary, we have presented here a unique HLA-A2.1 transgenic rabbit model system to test the immunogenicity of HLA-A2.1 restricted epitopes in vitro and in vivo. Although moderate consistency of epitope responsiveness between these two models was noted, the HLA-A2.1 transgenic rabbit model is more sensitive in its ability to identify specific targets for protective and therapeutic purposes when compared with the HLA-A2.1 transgenic mouse model in this study. We anticipate that the HLA-A2.1 transgenic rabbit model will be valuable for the development of therapeutic vaccines for HPV and other rabbit susceptible human pathogens [37, 38].
Acknowledgements
We thank Jeremy Haley for excellent help with the animals. This work was supported by the National Cancer Institute grant R01 CA47622 from the National Institutes of Health and the Jake Gittlen Memorial Golf Tournament.
References
- 1.Epstein H, Hardy R, May JS, Johnson MH, Holmes N. Expression and function of HLA-A2.1 in transgenic mice. Eur J Immunol. 1989;19:1575–1583. doi: 10.1002/eji.1830190909. [DOI] [PubMed] [Google Scholar]
- 2.Le AX, Bernhard EJ, Holterman MJ, Strub S, Parham P, et al. Cytotoxic T cell responses in HLA-A2.1 transgenic mice. Recognition of HLA alloantigens and utilization of HLA-A2.1 as a restriction element. J Immunol. 1989;142:1366–1371. [PubMed] [Google Scholar]
- 3.Himoudi N, Abraham JD, Fournillier A, Lone YC, Joubert A, et al. Comparative vaccine studies in HLA-A2.1-transgenic mice reveal a clustered organization of epitopes presented in hepatitis C virus natural infection. J Virol. 2002;76:12735–12746. doi: 10.1128/JVI.76.24.12735-12746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami Y, Zakut R, Topalian SL, Stötter H, Rosenberg SA. Shared human melanoma antigens: Recognition by tumor-infiltrating lymphocytes in HLA-A2.1-transfected melanomas. J Immunol. 1992;148:638–643. [PubMed] [Google Scholar]
- 5.Meng WS, Butterfield LH, Ribas A, Heller JB, Dissette VB, et al. Fine specificity analysis of an HLA-A2.1-restricted immunodominant T cell epitope derived from human alpha-fetoprotein. Mol Immunol. 2000;37:943–950. doi: 10.1016/s0161-5890(01)00017-7. [DOI] [PubMed] [Google Scholar]
- 6.Ressing ME, Sette A, Brandt RM, Ruppert J, Wentworth PA, et al. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 7.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, et al. A Novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010;184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao TM, Hague B, Caudell DL, Simpson RM, Kindt TJ. Quantification of HTLV-I proviral load in experimentally infected rabbits. Retrovirology. 2005;2:34. doi: 10.1186/1742-4690-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng CK, Hughes MA, Hsu PL, Mahoney S, Duvic M, et al. Syphilis superinfection activates expression of human immunodeficiency virus 1 in latently infected rabbits. Am J Pathol. 1991;138:1149–1164. [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, et al. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol. 2006;177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Peng X, Budgeon LR, Cladel NM, Balogh KK, et al. Establishment of a cottontail rabbit papillomavirus/HLA-A2.1 transgenic rabbit model. J Virol. 2007;81:7171–7177. doi: 10.1128/JVI.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 13.Campo MS. Animal models of papillomavirus pathogenesis. Virus Res. 2002;89:249–261. doi: 10.1016/s0168-1702(02)00193-4. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls PK, Stanley MA. The immunology of animal papillomaviruses. Vet Immunol Immunopathol. 2000;73:101–127. doi: 10.1016/s0165-2427(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 15.Brandsma JL. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. Methods Mol Med. 2005;119:217–235. doi: 10.1385/1-59259-982-6:217. [DOI] [PubMed] [Google Scholar]
- 16.Breitburd F, Salmon J, Orth G. The rabbit viral skin papillomas and carcinomas: a model for the immunogenetics of HPV-associated carcinogenesis. Clin Dermatol. 1997;15:237–247. doi: 10.1016/s0738-081x(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 17.Christensen ND. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. Antivir Chem Chemother. 2005;16:355–362. doi: 10.1177/095632020501600602. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Cladel NM, Balogh K, Budgeon L, Christensen ND. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2007;358:384–390. doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Cladel N, Peng X, Balogh K, Christensen ND. Protective immunity with an E1 multivalent epitope DNA vaccine against cottontail rabbit papillomavirus (CRPV) infection in an HLA-A2.1 transgenic rabbit model. Vaccine. 2008;26:809–816. doi: 10.1016/j.vaccine.2007.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Schell TD, Peng X, Cladel NM, Balogh K, et al. Strong and Specific Protective and Therapeutic Immunity Induced by Single HLA-A2.1 Restricted Epitope DNA Vaccine in Rabbits. Procedia in Vaccinology. 2009;1:4–14. [Google Scholar]
- 21.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, et al. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schell TD, Lippolis JD, Tevethia SS. Cytotoxic T lymphocytes from HLA-A2.1 transgenic mice define a potential human epitope from simian virus 40 large T antigen. Cancer Res. 2001;61:873–879. [PubMed] [Google Scholar]
- 23.Hu J, Cladel NM, Pickel MD, Christensen ND. Amino Acid residues in the carboxy-terminal region of cottontail rabbit papillomavirus E6 influence spontaneous regression of cutaneous papillomas. J Virol. 2002;76:11801–11808. doi: 10.1128/JVI.76.23.11801-11808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Han R, Cladel NM, Pickel MD, Christensen ND. Intracutaneous DNA vaccination with the E8 gene of cottontail rabbit papillomavirus induces protective immunity against virus challenge in rabbits. J Virol. 2002;76:6453–6459. doi: 10.1128/JVI.76.13.6453-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holden HT, Oldham RK, Ortaldo JR, Herberman RB. Standardization of the chromium-51 release, cell-mediated cytotoxicity assay: Cryopreservation of mouse effector and target cells. J Natl Cancer Inst. 1977;58:611–622. doi: 10.1093/jnci/58.3.611. [DOI] [PubMed] [Google Scholar]
- 26.Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J Virol Methods. 2008;148:34–39. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimble C, Lin CT, Hung CF, Pai S, Juang J, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 29.Engelhard VH, Lacy E, Ridge JP. Influenza A-specific, HLA-A2.1-restricted cytotoxic T lymphocytes from HLA-A2.1 transgenic mice recognize fragments of the M1 protein. J Immunol. 1991;146:1226–1232. [PubMed] [Google Scholar]
- 30.Koirala TR, Hayashi K, Jin Z, Onoda S, Tanaka T. Induction and prevention of virus-associated malignant lymphoma by serial transmission of EBV-related virus from cynomolgus by blood transfusion in rabbits. Acta Med Okayama. 2004;58:67–74. doi: 10.18926/AMO/32097. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Cladel N, Balogh K, Christensen N. Mucosally delivered peptides prime strong immunity in HLA-A2.1 transgenic rabbits. Vaccine. 2010;28:3706–3713. doi: 10.1016/j.vaccine.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XS, Abdul-Jabbar I, Qi YM, Frazer IH, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252:39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 33.Han R, Cladel NM, Reed CA, Peng X, Budgeon LR, et al. DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits. J Virol. 2000;74:9712–9716. doi: 10.1128/jvi.74.20.9712-9716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J, Cladel NM, Budgeon LR, Reed CA, Pickel MD, et al. Protective cell-mediated immunity by DNA vaccination against Papillomavirus L1 capsid protein in the Cottontail Rabbit Papillomavirus model. Viral Immunol. 2006;19:492–507. doi: 10.1089/vim.2006.19.492. [DOI] [PubMed] [Google Scholar]
- 35.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 36.Firat H, Garcia-Pons F, Tourdot S, Pascolo S, Scardino A, et al. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur J Immunol. 1999;29:3112–3121. doi: 10.1002/(SICI)1521-4141(199910)29:10<3112::AID-IMMU3112>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albers AE, Kaufmann AM. Therapeutic human papillomavirus vaccination. Public Health Genomics. 2009;12:331–342. doi: 10.1159/000214923. [DOI] [PubMed] [Google Scholar]
- 39.Campo MS, Roden RB. Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol. 2010;84:1214–1220. doi: 10.1128/JVI.01927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu J, Cladel NM, Christensen ND. Increased immunity to cottontail rabbit papillomavirus infection in EIII/JC inbred rabbits after vaccination with a mutant E6 that correlates with spontaneous regression. Viral Immunol. 2007;20:320–325. doi: 10.1089/vim.2006.0104. [DOI] [PubMed] [Google Scholar]
- 41.Han R, Cladel NM, Reed CA, Peng X, Christensen ND. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J Virol. 1999;73:7039–7043. doi: 10.1128/jvi.73.8.7039-7043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selvakumar R, Borenstein LA, Lin YL, Ahmed R, Wettstein FO. Immunization with nonstructural proteins E1 and E2 of cottontail rabbit papillomavirus stimulates regression of virus-induced papillomas. J Virol. 1995;69:602–605. doi: 10.1128/jvi.69.1.602-605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leachman SA, Shylankevich M, Slade MD, Levine D, Sundaram RK, et al. Ubiquitin-fused and/or multiple early genes from cottontail rabbit papillomavirus as DNA vaccines. J Virol. 2002;76:7616–7624. doi: 10.1128/JVI.76.15.7616-7624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston KB, Monteiro JM, Schultz LD, Chen L, Wang F, et al. Protection of beagle dogs from mucosal challenge with canine oral papillomavirus by immunization with recombinant adenoviruses expressing codon-optimized early genes. Virology. 2005;336:208–218. doi: 10.1016/j.virol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Moore RA, Walcott S, White KL, Anderson DM, Jain S, et al. Therapeutic immunisation with COPV early genes by epithelial DNA delivery. Virology. 2003;314:630–635. doi: 10.1016/s0042-6822(03)00465-3. [DOI] [PubMed] [Google Scholar]
- 46.Brandsma JL, Shylankevich M, Su Y, Roberts A, Rose JK, et al. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J Virol. 2007;81:5749–5758. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brandsma JL, Shlyankevich M, Zelterman D, Su Y. Therapeutic vaccination of rabbits with a ubiquitin-fused papillomavirus E1, E2, E6 and E7 DNA vaccine. Vaccine. 2007;25:6158–6163. doi: 10.1016/j.vaccine.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christensen ND. Emerging human papillomavirus vaccines. Expert Opin Emerg Drugs. 2005;10:5–19. doi: 10.1517/14728214.10.1.5. [DOI] [PubMed] [Google Scholar]