Abstract
The transverse tubular system (t-system) is a major site for signaling in mammalian ventricular cardiomyocytes including electrical signaling and excitation-contraction coupling. It consists of membrane invaginations, which are decorated with various proteins including mechanosensitive ion channels. Here, we investigated mechanical modulation of the t-system. By applying fluorescent markers, three-dimensional scanning confocal microscopy, and methods of digital image analysis, we studied isolated ventricular cardiomyocytes under different strains. We demonstrate that strain at the cellular level is transmitted to the t-system, reducing the length and volume of tubules and altering their cross-sectional shape. Our data suggest that a cellular strain of as little as 5% affects the shape of transverse tubules, which has important implications for the function of mechanosensitive ion channels found in them. Furthermore, our study supports a prior hypothesis that strain can cause fluid exchange between the t-system and extracellular space.
Mammalian ventricular myocytes exhibit a transverse tubular system (t-system), which consists of membrane invaginations (1). Geometry and morphology of the t-system were found to be dependent on species and cell type (2). The t-system is an important site for excitation-contraction coupling and essential for rapid electrical signaling from the outer sarcolemma into the cell interior. Recent interest in the t-system has been renewed by studies demonstrating that transverse tubules (t-tubules) are less dense and their arrangement is disorganized in diseased ventricular cardiomyocytes (3,4).
It has been suggested that t-tubular loss reduces the efficiency of cardiac excitation-contraction coupling (5). It has also been suggested that mechanical deformation of the t-system can contribute to fluid exchange between it and the interstitial space (2,6). Such a pumping mechanism would support transport of nutrients, metabolites, and ions into the myocyte. Any t-system deformation may contribute to mechanical modulation of ion channels. Mechanosensitive ion channels found in the t-system include stretch-activated transient receptor potential cation channels (TRPC6) and stretch-modulated inward rectifier potassium channels (Kir2.3) (7).
The aim of this study was to characterize the transfer of strain at cellular level to the t-system. The study is based on our previous work, which applied three-dimensional scanning confocal microscopy on living isolated cardiomyocytes to characterize geometrical features of the t-system (2). We found that the rabbit t-system rarely exhibits longitudinal tubules. We demonstrated flattening of t-tubule cross sections and alignment of their short axis with the long axis of myocytes. We suggested that the flattening is related to the myocytes being at a slack length and is altered when they shorten or lengthen.
Using this experimental and analytical approach, we studied mechanical deformation of the t-system of myocytes. Strain was applied statically by longitudinal stretching of the myocytes. We hypothesized that 1), cellular strain is transmitted to the t-system; and 2), mechanical deformation of myocytes contributes to fluid transport between the t-system cavities and extracellular space. We tested these hypotheses by imaging and comparison of geometrical features of t-tubules in quiescent myocytes at rest and during static strain.
The protocol used for isolating rabbit myocytes is described in the Supporting Material. The myocytes were transferred to an imaging chamber, suffused with a membrane-impermeable dextran conjugated to fluorescent dye (Alexa 488; Invitrogen, Carlsbad, CA), and imaged using a LSM 5 Duo confocal microscope (Carl Zeiss, Jena, Germany). The setup for imaging and straining of myocytes is shown in Fig. S1 in the Supporting Material. Exemplary images obtained from a myocyte before and during strain are shown in Fig. 1.
Figure 1.
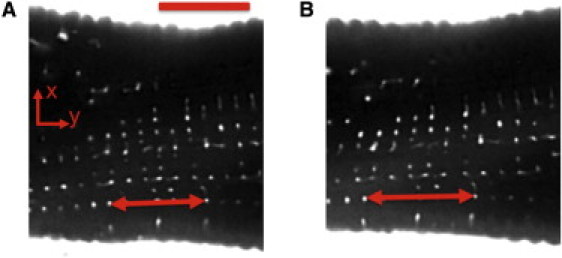
Image of myocyte segment before (A) and during (B) 15% static strain. Extracellular space and t-system exhibit fluorescent signal. Two corresponding t-tubules are marked in each image (arrows). Longitudinal t-tubular spacing Δ was (A) 1.80 and (B) 2.06 μm. Scale bar: 10 μm.
The image stacks were deconvolved and corrected for background signals and depth-dependent attenuation (8). Longitudinal spacing of t-tubules, Δ, was determined by maxima in Fourier spectra of the three-dimensional images. Strain was defined as ΔStrained/ΔUnstrained with ΔStrained and ΔUnstrained describing the spacing after and before strain, respectively. Fractional volume of the t-system was calculated based on fluorescence ratios (9).
T-tubules were automatically segmented with the region-growing method (10). Characterization of t-tubules by principal component analysis was based on the image moments of spherical regions (10). The centers of these regions were regularly spaced (∼0.2 μm) along the t-tubule longitudinal axis. Centroids of these regions were determined by first-order image moments given by
with the three-dimensional image I and the set of voxel indexes in the spherical region S. A matrix of second-order central image moments M2 was set up as
with the moments
Eigenvalues, λ1, λ2, and λ3, and eigenvectors, e1, e2, and e3, of M2 were calculated by singular value decomposition. Several measures served for characterization of t-tubule cross-sections: ellipticity and orientation. Ellipticity ɛ of tubules was defined as
With this measure, a decrease of ellipticity corresponds to more circular cross-sections. The orientation α of the minor eigenvector e3, i.e., the minor axis of the t-tubule cross-section, versus the myocyte long axis m1, was calculated by
Only t-tubules of simple topology were used for further analysis. Mouth and end regions of the t-tubules were excluded from analysis to avoid problems with detection of these regions and image blurring.
Analysis of a t-tubule (Fig. 2) revealed that it had a length of 2.3 μm. The cross-sectional area shows a maximum at its mouth and a constriction at 0.8 μm (Fig. 2 B). Ellipticity ranged between 0.07 and 0.23, indicating a slight flattening of the cross sections (Fig. 2 C). Orientation ranged between 1 and 32° (Fig. 2 D).
Figure 2.
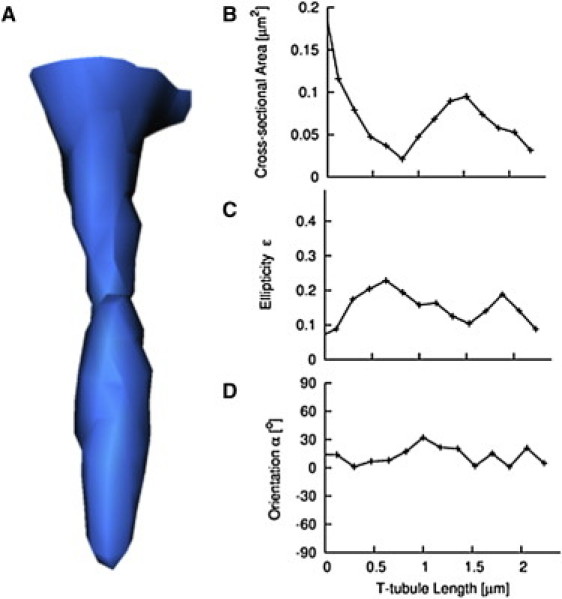
Reconstruction and analysis of t-tubule from rabbit myocyte. (A) Three-dimensional reconstruction of t-tubule at rest with simple topology. (B) Cross-sectional area, (C) ellipticity ɛ, and (D) orientation α were determined along the length of the t-tubule. An orientation α equal to zero denotes that the minor axis of the t-tubule cross-section is parallel to the myocyte long axis.
A statistical analysis of 28 cells from 14 animals is presented in Fig. 3 and Table 1. T-tubules (n =1048) and their cross sections (n = 10,328) were grouped according to the strain of cells, i.e., control (before strain), 5% strain (2.5–7.5%), and 15% strain (12.5–17.5%). At 15% strain, mean t-tubule length and volume decreased by 10.3% and 12.7%, respectively. The fractional volume of the t-system decreased by 16.5%. The mean orientation angle of the minor eigenvector increased with increasing strain.
Figure 3.
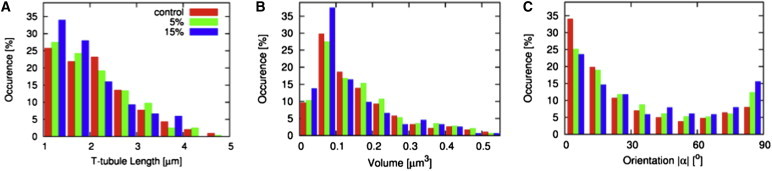
Statistical analysis. Histograms of (A) t-tubule length, (B) volume, and (C) orientation of cross sections are presented for control cells and cells at 5% and 15% strain. Strain was associated with an increase of short t-tubules and small volume. Cross sections of t-tubules of strained cells tended toward having minor axes perpendicular to the myocyte long axis.
Table 1.
Statistical analysis of t-tubules presented as mean ± standard deviation
| Feature | Controls | 5% | 15% |
|---|---|---|---|
| Length [μm] | 2.12 ± 0.84 | 2.07 ± 0.84 | 1.90 ± 0.75∗ |
| Volume [μm3] | 0.16 ± 0.12 | 0.17 ± 0.12 | 0.14 ± 0.11∗ |
| Fractional volume [%] | 4.82 ± 1.33 | 4.41 ± 1.00 | 4.01± 0.87∗ |
| Ellipticity ɛ | 0.20 ± 0.11 | 0.20 ± 0.11 | 0.19 ± 0.10∗ |
| Orientation |α| [°] | 28.7 ± 27.4 | 34.2 ± 28.6∗ | 38.7 ± 30.0∗ |
p < 0.05 versus control.
Our study demonstrates that cellular strain alters the shape and volume of the t-system in myocytes. The measured decrease of volume is associated with a decrease of t-tubule length. This finding contrasts with results from a previous study on the t-system from toad skeletal muscle fibers. Based on intensity analysis of two-dimensional confocal micrographs, it has been observed that sarcomere length, which varied in the range from 1.93 to 3.30 μm, did not affect the steady-state fractional volume (11).
Our data suggest that geometrical changes of the t-system begin at small cellular strains (5%). Geometrical changes of t-tubules are associated with changes of the stress distribution on the sarcolemma, which has been suggested as a mechanism for gating of stretch-activated ion channels (7). Further implications of our finding are related to a possible mechanism for pumping fluid into and out of the t-system. Our data on volume changes indicate that high strain (15%) along the cell long axis causes flux of fluid out of the t-system into the interstitial space.
Limitations of our imaging approach are discussed in our previous publication (2) and the Supporting Material. This study focused on the rabbit t-system. Comparative studies are needed in other species to generalize our findings.
Acknowledgments
We thank Dr. Kenneth Spitzer for useful discussions and his help in the presented studies.
This study was supported by the Richard A. and Nora Eccles Fund for Cardiovascular Research and awards from the Nora Eccles Treadwell Foundation.
Supporting Material
References and Footnotes
- 1.Fawcett D.W., McNutt N.S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J. Cell Biol. 1969;42:1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savio-Galimberti E., Frank J., Sachse F.B. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys. J. 2008;95:2053–2062. doi: 10.1529/biophysj.108.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balijepalli R.C., Lokuta A.J., Kamp T.J. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc. Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 4.Wei S., Guo A., Song L.S. T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louch W.E., Bito V., Sipido K.R. Reduced synchrony of Ca2+ release with loss of T-tubules—a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc. Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Kohl P., Cooper P.J., Holloway H. Effects of acute ventricular volume manipulation on in situ cardiomyocyte cell membrane configuration. Prog. Biophys. Mol. Biol. 2003;82:221–227. doi: 10.1016/s0079-6107(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 7.Dyachenko V., Husse B., Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium. 2009;45:38–54. doi: 10.1016/j.ceca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Savio E., Goldhaber J.I., Sachse F.B. A framework for analyzing confocal images of transversal tubules in cardiomyocytes. Lect. Notes Comput. Sci. 2007;4466:110–119. [Google Scholar]
- 9.Soeller C., Cannell M.B. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ. Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez R.C., Woods R.E. Addison-Wesley; Reading, MA: 1992. Digital Image Processing. [Google Scholar]
- 11.Launikonis B.S., Stephenson D.G. Tubular system volume changes in twitch fibers from toad and rat skeletal muscle assessed by confocal microscopy. J. Physiol. 2002;538:607–618. doi: 10.1113/jphysiol.2001.012920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


