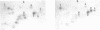Abstract
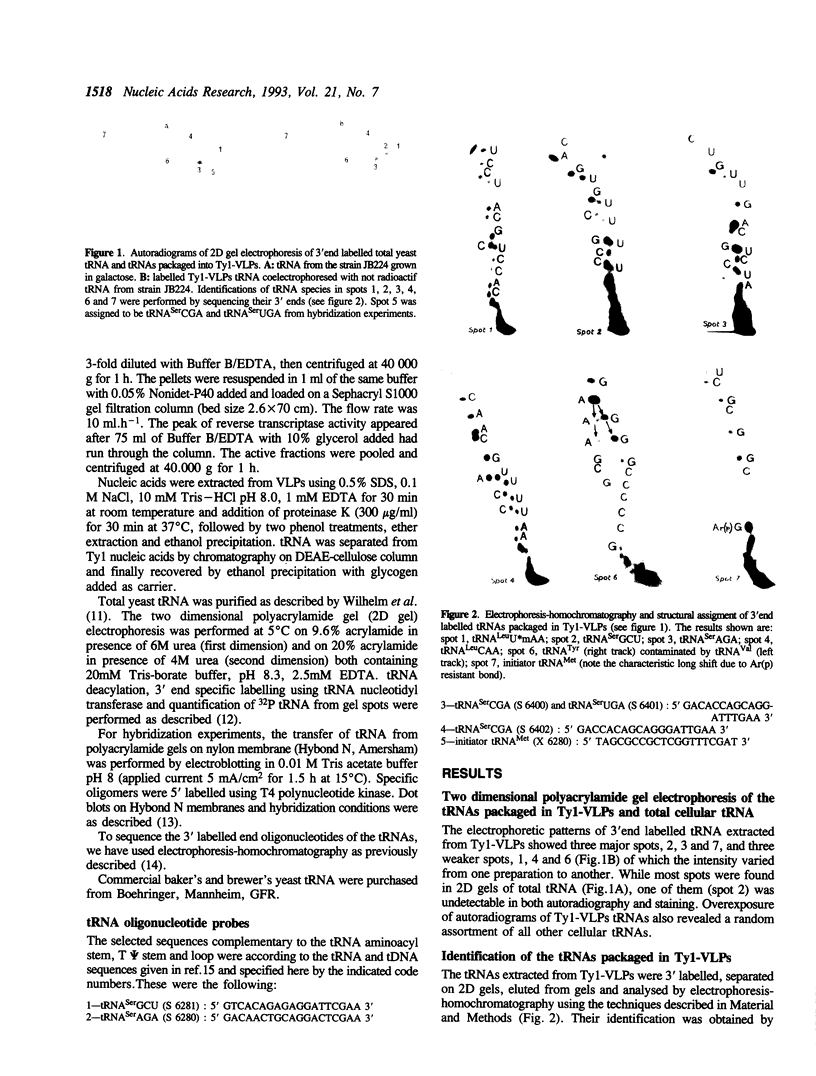
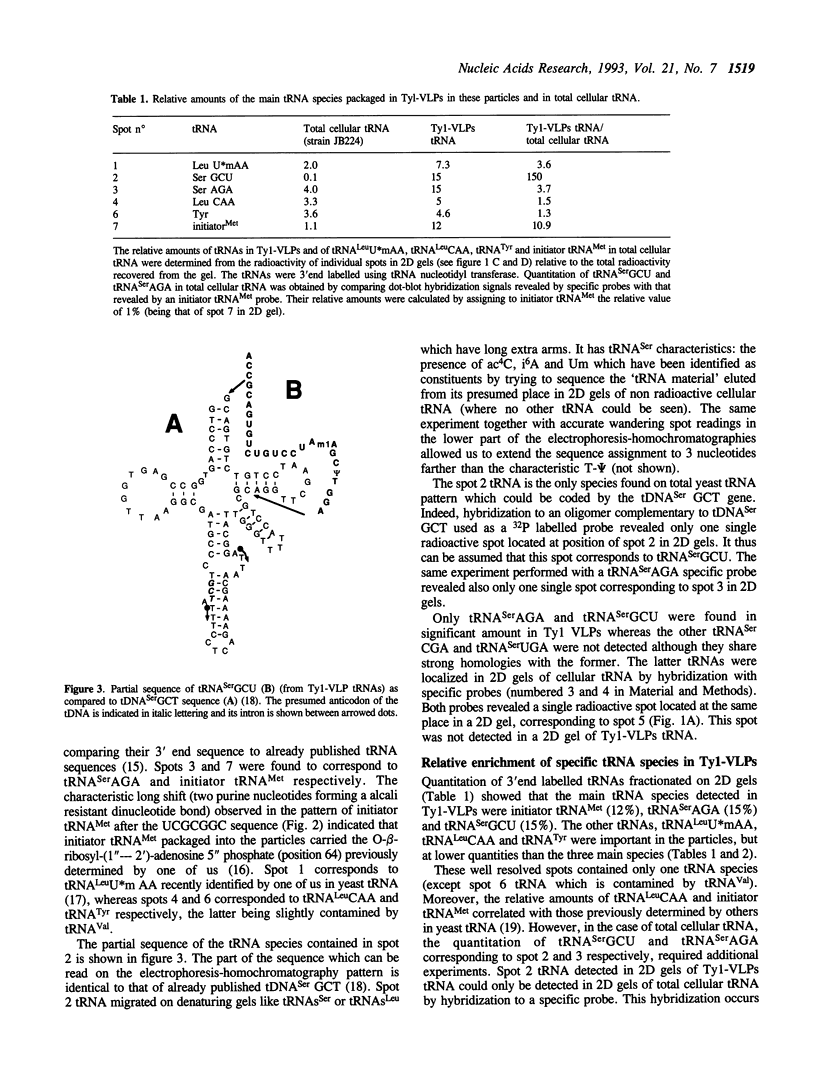
The analysis of the tRNAs associated to the virus-like particles produced by the Ty1 element revealed the specific packaging of three major tRNA species, in about equal amounts: the replication primer initiator tRNA(Met), the tRNA(Ser)AGA and a tRNA undetected until now as an expressed species in yeast. The latter tRNA is coded by the already described tDNA(Ser)GCT. This tRNA is enriched more than 150 fold in the particles as compared to its content in total cellular tRNA where it represents less than 0.1% (initiator tRNA(Met) and tRNA(Ser)AGA being 11 and 4 fold enriched respectively). This tRNA is the only species coded by the tDNA(Ser)GCT gene which is found in three copies per genome since no other corresponding expressed tRNA could be detected. This gene is thus very poorly expressed. The high concentration of tRNA(Ser)GCU in the particles compared to its very low cellular content led us to consider its possible implication in Ty specific processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Byström A. S., Fink G. R. A functional analysis of the repeated methionine initiator tRNA genes (IMT) in yeast. Mol Gen Genet. 1989 Apr;216(2-3):276–286. doi: 10.1007/BF00334366. [DOI] [PubMed] [Google Scholar]
- Chapman K. B., Byström A. S., Boeke J. D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A. M., Donahue T. F. The methionine initiator tRNA genes of yeast. Gene. 1986;41(2-3):343–348. doi: 10.1016/0378-1119(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Desgrès J., Keith G., Kuo K. C., Gehrke C. W. Presence of phosphorylated O-ribosyl-adenosine in T-psi-stem of yeast methionine initiator tRNA. Nucleic Acids Res. 1989 Feb 11;17(3):865–882. doi: 10.1093/nar/17.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P. Fractionnement des tRNA de levure de bière par distribution en contre-courant. Bull Soc Chim Biol (Paris) 1967;49(12):1679–1687. [PubMed] [Google Scholar]
- Eibel H., Gafner J., Stotz A., Philippsen P. Characterization of the yeast mobile element Ty1. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):609–617. doi: 10.1101/sqb.1981.045.01.079. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler J., Maréchal-Drouard L., Dirheimer G., Keith G. Use of a dot blot hybridization method for identification of pure tRNA species on different membranes. Biochim Biophys Acta. 1992 Feb 11;1129(3):273–277. doi: 10.1016/0167-4781(92)90503-r. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Kapsenberg M. L., Res P., Bos J. D., Schootemijer A., Teunissen M. B., Van Schooten W. Nickel-specific T lymphocyte clones derived from allergic nickel-contact dermatitis lesions in man: heterogeneity based on requirement of dendritic antigen-presenting cell subsets. Eur J Immunol. 1987 Jun;17(6):861–865. doi: 10.1002/eji.1830170620. [DOI] [PubMed] [Google Scholar]
- Keith G., Heyman T. Heterogeneities in vertebrate tRNAs(Trp) avian retroviruses package only as a primer the tRNA(Trp) lacking modified m2G in position 7. Nucleic Acids Res. 1990 Feb 25;18(4):703–710. doi: 10.1093/nar/18.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesewetter S., Ott G., Sprinzl M. The role of modified purine 64 in initiator/elongator discrimination of tRNA(iMet) from yeast and wheat germ. Nucleic Acids Res. 1990 Aug 25;18(16):4677–4682. doi: 10.1093/nar/18.16.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman L., Caudry S., Boulerice F., Wainberg M. A., Parniak M. A. Incorporation of tRNA into normal and mutant HIV-1. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1272–1280. doi: 10.1016/0006-291x(91)91559-u. [DOI] [PubMed] [Google Scholar]
- Müller F., Brühl K. H., Freidel K., Kowallik K. V., Ciriacy M. Processing of TY1 proteins and formation of Ty1 virus-like particles in Saccharomyces cerevisiae. Mol Gen Genet. 1987 May;207(2-3):421–429. doi: 10.1007/BF00331610. [DOI] [PubMed] [Google Scholar]
- Reinisch F., Heyman T. Polyacrylamide gel mapping of chicken tRNA: comparison of polysome-bound and whole-cell tRNA from normal and avian sarcoma virus-infected chicken embryo fibroblasts. Mol Cell Biol. 1982 Oct;2(10):1247–1257. doi: 10.1128/mcb.2.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Cowe E. Synonymous codon usage in Saccharomyces cerevisiae. Yeast. 1991 Oct;7(7):657–678. doi: 10.1002/yea.320070702. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Dank N., Nock S., Schön A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2127–2171. doi: 10.1093/nar/19.suppl.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Feldmann H. Structure of a Saccharomyces cerevisiae gene encoding minor (AGY)tRNA(Ser). Nucleic Acids Res. 1988 Apr 25;16(8):3583–3583. doi: 10.1093/nar/16.8.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]
- Wilhelm M. L., Keith G., Fix C., Wilhelm F. X. Pleiotropic effect of a point mutation in the yeast SUP4-o tRNA gene: in vivo pre-tRNA processing in S. cerevisiae. Nucleic Acids Res. 1992 Feb 25;20(4):791–796. doi: 10.1093/nar/20.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Adlouni C., Desgrès J., Dirheimer G., Keith G. Sequence of a new tRNA(Leu)(U*AA) from brewer's yeast. Biochimie. 1991 Nov;73(11):1355–1360. doi: 10.1016/0300-9084(91)90165-w. [DOI] [PubMed] [Google Scholar]