Abstract
Cyclic AMP signalling promotes VSMC quiescence in healthy vessels and during vascular healing following injury. Cyclic AMP inhibits VSMC proliferation via mechanisms that are not fully understood. We investigated the role of PKA and Epac signalling on cAMP-induced inhibition of VSMC proliferation. cAMP-mediated growth arrest was PKA-dependent. However, selective PKA activation with 6-Benzoyl-cAMP did not inhibit VSMC proliferation, indicating a requirement for additional pathways. Epac activation using the selective cAMP analogue 8-CPT-2′-O-Me-cAMP, did not affect levels of hyperphosphorylated Retinoblastoma (Rb) protein, a marker of G1-S phase transition, or BrdU incorporation, despite activation of the Epac-effector Rap1. However, 6-Benzoyl-cAMP and 8-CPT-2′-O-Me-cAMP acted synergistically to inhibit Rb-hyperphosphorylation and BrdU incorporation, indicating that both pathways are required for growth inhibition. Consistent with this, constitutively active Epac increased Rap1 activity and synergised with 6-Benzoyl-cAMP to inhibit VSMC proliferation. PKA and Epac synergised to inhibit phosphorylation of ERK and JNK. Induction of stellate morphology, previously associated with cAMP-mediated growth arrest, was also dependent on activation of both PKA and Epac. Rap1 inhibition with Rap1GAP or siRNA silencing did not negate forskolin-induced inhibition of Rb-hyperphosphorylation, BrdU incorporation or stellate morphology. This data demonstrates for the first time that Epac synergises with PKA via a Rap1-independent mechanism to mediate cAMP-induced growth arrest in VSMC. This work highlights the role of Epac as a major player in cAMP-dependent growth arrest in VSMC.
Keywords: Epac, PKA, Rap1, cAMP, Proliferation, Vascular smooth muscle cell
Research Highlights
► PKA is required but not sufficient for the anti-mitogenic effects of cAMP in VSMC. ► cAMP-induced smooth muscle cell growth arrest is Epac-dependent but Rap1-independent. ► PKA and Epac synergise to inhibit smooth muscle cell proliferation. ► cAMP inhibits ERK and JNK phosphorylation in a PKA- and Epac-dependent manner. ► cAMP-induced stellate morphology of SMC requires PKA and Epac.
1. Introduction
Vascular smooth muscle cell (VSMC) proliferation contributes towards intima formation during numerous vasculo-proliferative diseases, including atherosclerosis, transplant vasculopathy and pulmonary hypertension. VSMC proliferation also contributes towards neointima formation after balloon angioplasty, with and without stenting, and after bypass vein grafting, thus limiting the success of clinical interventions designed to treat atherosclerosis. A complete understanding of the mechanisms regulating VSMC proliferation during these processes is therefore essential.
In healthy vessels, VSMC exhibit very low rates of proliferation [1]. However, VSMC proliferation rates are dramatically elevated in response to vessel injury. Mechanisms responsible for this include release of growth factors and remodelling of the vascular extracellular matrix [2,3] that activate cell signalling pathways required for cell-cycle progression.
VSMC proliferation is also subject to negative regulation [4]. cAMP, a major second messenger produced by adenylate-cyclase has a well documented role as an inhibitor of VSMC proliferation. cAMP synthesis in response to endothelial-derived prostacyclin is an important mechanism promoting VSMC quiescence and vascular healing following injury. For example, elevated cAMP levels have been shown to inhibit VSMC proliferation in vitro and after vascular injury-induced proliferation in vivo, ultimately leading to a reduction in restenosis [4,5]. The mechanisms underlying these growth-inhibitory properties are only partially understood. Elevated cAMP levels inhibit expression of the G1-S phase cell-cycle regulators, Cyclin D and Skp2 [4,6], which accounts at least in part for cAMP-mediated G1-arrest and reduction in neointima development [7–9]. However, upstream signalling pathways responsible for these important biological effects of cAMP remain unclear.
At one time, the biological effects of cAMP were thought to be mediated exclusively by Protein Kinase A (PKA). Consistent with this, PKA inhibitors have been shown to reverse the effects of cAMP-elevating agents on VSMC proliferation [5]. However, we recently reported that cAMP-mediated inhibition of Skp2 expression, a key regulator of G1-S phase progression, could only be partially reversed by a PKA inhibitor [4]. Other studies demonstrated PKA-independent effects of cAMP analogues on cell-cycle regulatory proteins in other cell types [10,11]. These observations indicate the involvement of additional PKA-independent cAMP-sensitive pathways involved in cell-cycle regulation. The recent identification of a new family of cAMP-sensitive proteins, the Epac family (Epac 1 and 2) established the existence of a distinct PKA-independent signalling pathway [12–14]. Epac 1 and 2 both contain N-terminal cAMP-binding domains and C-terminal guanine-nucleotide exchange factor (GEF) domains allowing them to function as cAMP-binding proteins with intrinsic GEF activity that can couple cAMP levels to the activation of members of the Ras-like family of GTPases such as Rap1 [12,15]. Numerous highly cell-type specific functions for Epac proteins have been proposed, including integrin-mediated cell adhesion, cell–cell junctions, migration and apoptosis [16–18] as well as having both pro- and anti-proliferative properties [19,20], depending on the cell type under investigation. However, the role of the Epac signalling pathway in cAMP-mediated inhibition of VSMC proliferation is not known.
Here we show for the first time that Epac1 acts synergistically with PKA to mediate cAMP-dependent cell-cycle arrest and associated induction of stellate morphology in vascular smooth muscle cells. We also demonstrate that Epac mediates its effects on cell-cycle arrest in a Rap1-independent manner.
2. Materials and methods
Male Sprague–Dawley (SD) rats were obtained from Charles River. Culture media and additives were obtained from Lonza. Antibodies used were Skp2 (#32-3400; Zymed Inc.), Rap1, GAPDH (#07-916, #MAB374; Millipore), Cyclin D1 (#CC12; Calbiochem), Epac (#sc-28366; Santa Cruz), VASP (#ALX-210-880; Alexis), hyperphosphorylated-Rb, phospho-ERK, ERK, phospho-JNK, JNK (#9308, #9101, #9102, #9251, #9258; Cell Signalling), V5-tag (#R960-25; Invitrogen), paxillin (#610051; BD Biosciences), BrdU (#B2531; Sigma) and Rap1GAP (#ab32373; Abcam). Db-cAMP and forskolin were obtained from Sigma. N6-Benzoyl-cAMP and 8-CPT-2′-O-Me-cAMP were from Calbiochem. Sp-8-CPT-2′-O-Me-cAMPS was obtained from Biolog.
2.1. RT PCR
Total RNA was extracted using Qiagen RNease kit (Qiagen), quantitated using a ND1000 nanodrop spectrophotometer and between 100 and 400 ng reverse transcribed using a QuantiTect reverse transcription kit (Qiagen). Quantitative RT-PCR was performed using Qiagen QuantiTect SYBR green on a Roche Lightcycler using the following primers: Epac1 forward 5′-CGACACCACAGGTTGGAGAATG-3′, Epac1 reverse 5′-AAGCTGCCATCACTTCCCTCAC-3′, Epac2 forward 5′-TGTGCACGAGCTGGAGCTAATC-3′, Epac2 reverse 5′-TGTACGCCCTGTGATTTCTGGA-3′, Rap1A forward 5′-AGATTGCCAACAGTGTATGCTGGA-3′, Rap1A reverse 5′-ATTGCCAACCAAAATCATTGGAAC-3′, Rap1B forward 5′-GATACTGCAGGAACGGAGCAGTTT-3′, Rap1B reverse 5′-GTCTTGCCAGGTTCTGACCTTGTT-3′, Copy number was calculated by extrapolation from a standard curve.
2.2. Smooth muscle cell culture and bromo-deoxyuridine labelling
Isolated VSMC were prepared using a modification of the explant technique described previously [1]. Unless otherwise stated, all cells were cultured in the presence of 10% foetal calf serum and used between passage 3 and 8. Where indicated, cells were rendered quiescent by serum deprivation for 72 h. VSMC proliferation was quantified by labelling cells with 10 μM BrdU for 6 h, unless otherwise stated. Cells were fixed and analysed for BrdU incorporation by immuno-histochemistry with diaminobenzidine staining and counted using NIH ImageJ software.
2.3. Western blotting
Total cell lysates were prepared using SDS-lysis buffer (50 mM Tris–HCl, pH 6.8, 10% glycerol, 1% SDS). Protein content was determined (Micro BCA assay kit, Pierce), and equal amounts of reduced protein (20–70 μg) were separated by PAGE, transferred to PVDF membrane, blocked with TBS containing 0.2% Tween and 6% milk powder before incubation in primary antibody. Specific proteins were detected using HRP-conjugated secondary antibodies (Dako, Ely, UK) and enhanced chemoluminescence. Westerns detecting exogenously expressed proteins were exposed to correctly detect exogenous proteins and thus endogenous expression may not be visible.
2.4. Recombinant adenoviruses and infection of VSMC
Recombinant adenovirus encoding Rap1GAP was a kind gift from Patrick J. Casey (Duke University Medical Center, North Carolina). Adenovirus expressing PKA inhibitor was a kind gift from Prof. David Murphy (University of Bristol, U.K.) [21]. Adenovirus encoding constitutively active mutant of Epac was constructed by PCR to amplify the C-terminal GEF domain (aa 322–881) without the N-terminal DEP and cAMP-binding domains. This cDNA was sub-cloned with a 5′ V5 tag into the pDC515 adenovirus shuttle vector (Microbix). Replication-deficient adenoviruses were generated by recombination of co-transfected shuttle and genomic plasmids in HEK293 cells. Virus stocks were plaque-purified, amplified, CsCl-banded, and titrated by plaque assay. Asynchronous rat VSMC were infected with adenovirus at 108 pfu/ml for 16 h, unless otherwise stated.
2.5. Rap1 activity assays
Prokaryotic expression vector for GST-tagged Ral-GDS was a kind gift from Prof. Peter Cullen (University of Bristol, U.K.). GST-Ral-GDS protein was purified from BL21 E. coli on glutathione-sepharose using standard protocols. VSMC were stimulated as indicated and lysed in NP-40 lysis buffer (50 mM Tris pH 7.4, 200 mM NaCl, 5 mM MgCl2, 1% NP-40 alternative, 10% glycerol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF). Insoluble material was pelleted by centrifugation at 14,000 rpm and active GTP-bound Rap1 affinity purified on the GST-Ral-GDS resin at 4 °C for 45 min with constant mixing. Resin was washed 6 times in NP-40 lysis buffer and bound Rap1 eluted in SDS-lysis buffer. Total Rap1 in cell lysates and purified active Rap1 were quantified by western blotting.
2.6. PKA activity assays
PKA activity was assessed using the fluorescent PepTag assay system (Promega) following the manufacturers protocol, with the following changes: cells were lysed in buffer containing 25 mM Tris pH 7.5, 0.5% NP-40 alternative, 1 mM NaF, 1 mM Na-vanadate, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin. Lysates were centrifuged without homogenization. PKA activator 5× solution was not added to sample reaction tubes. PKA phosphorylated peptide gained a net negative charge and was separated from un-phosphorylated peptide by electrophoresis on 0.8% agarose gels. Bands were visualised using a UV transilluminator.
2.7. Phalloidin and paxillin immunofluoresence staining and quantification of focal adhesion area
F-actin was stained with fluorescein-phalloidin (Invitrogen) following the manufacturers protocol. Briefly, cells were fixed with 4% PFA/PBS, permeabilised with 0.1% Triton/PBS, blocked with 1% BSA/PBS and labelled with phalloidin. For paxillin staining, cells were fixed in 4% PFA/PBS, permeabilised with 0.1% Triton/PBS, blocked with 20% goat serum, labelled with anti-paxillin and visualised with Alexa 488-conjugated anti-mouse IgG. Coverslips were mounted on slides in DAPI Vectashield mounting medium (Vector). Mean focal adhesion areas (typically 90 focal adhesions per condition in each experiment) in individual cells were manually measured using NIH ImageJ software.
2.8. SiRNA-mediated Rap1 silencing
VSMC were transfected in 100 μl with 600 nM of control siRNA (All Stars negative) or a combination (total of 600 nM) of two siRNAs targeting Rap1A (Sense 5′-CUGGGAUAACUGAUUUCUATT-3′ and 5′-UAACGCGGGUUGUCAAUAUUUATT-3′) and two siRNAs targeting Rap1B (Sense 5′-GCAGAUUCUUCGGGUUAAATT-3′ and 5′-AGAUAAAUGUUAACGAGAUTT-3′) using the basic smooth muscle cell electroporation kit (Amaxa). SiRNA-mediated gene silencing was quantified 24 h post transfection.
2.9. Statistical analysis
After calculating means and standard errors of the means, analysis was performed using a two-tailed paired t-test or ANOVA (Student–Newman–Keuls) as indicated. Significant differences were taken when p < 0.05.
3. Results
3.1. Components of the Epac–Rap1 pathway are expressed and functional in VSMC
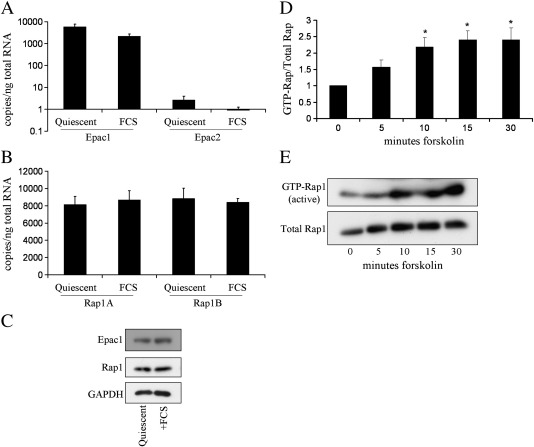
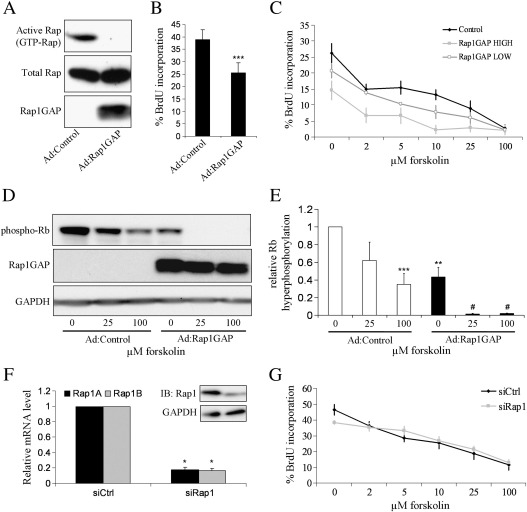
We initially sought to determine which components of the Epac–Rap pathway were expressed by rat aortic vascular smooth muscle cells. Quantitative reverse transcription PCR for Epac1 and Epac2 mRNA expression demonstrated Epac1 to be the predominant Epac expressed, being 200 fold higher than Epac2 mRNA levels (Fig. 1A). Epac1 protein expression was similar in both quiescent and mitogen stimulated cells (Fig. 1C). We next sought to determine which Rap isoforms were expressed in VSMC. Since Epac exhibits relatively poor GEF activity towards Rap2 compared to Rap1 [22], we focused on the Rap1 isoforms Rap1A and Rap1B, the major GTPases reported to be activated by Epac. Quantitative RT-PCR detected similar levels of both Rap1A and Rap1B isoforms in both quiescent and mitogen stimulated VSMC (Fig. 1B). A pan-Rap1 antibody also detected similar levels of 21 kDa Rap1 protein in quiescent and mitogen stimulated cells (Fig. 1C). This data demonstrates that rat VSMC express Epac1 and both isoforms of Rap1. Importantly, stimulation with the adenylate-cyclase activator forskolin resulted in a significant increase in Rap1 activity (Fig. 1D), indicating that the Epac–Rap1 pathway is functional in VSMC.
Fig. 1.
The Epac–Rap pathway is expressed and functional in rat VSMC. VSMC were rendered quiescent by serum deprivation and stimulated with FCS as indicated: A and B, Epac1, Epac2, Rap1A and Rap1B mRNA copy number was calculated by quantitative RT-PCR, n = 5; C, Epac1 and Rap1 protein expression was verified by western blotting. D, VSMC were stimulated with 100 μM forskolin for indicated times, and assayed for Rap1 activity by Ral-GDS pulldown, n = 6. E, A representative western blot is shown. * indicates p < 0.01 versus 0 min.
3.2. Selective activation of PKA and Epac signalling using selective cAMP analogues
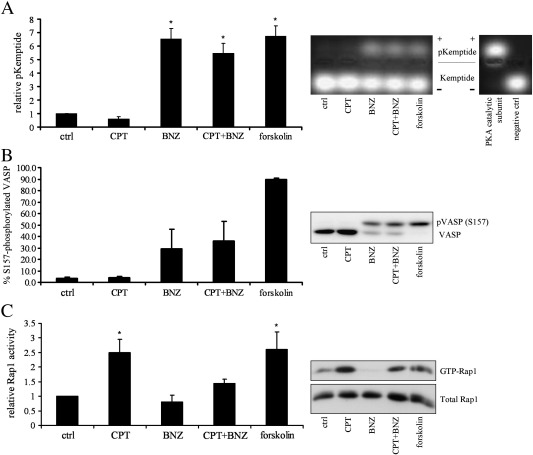
To test the relative contribution of Epac and PKA signalling in the regulation of VSMC proliferation, we used the PKA-selective cAMP analogue N6-Benzoyl-cAMP (BNZ) and the Epac-selective cAMP analogue 8-CPT-2′-O-Me-cAMP (CPT) [23,24]. Stimulation of VSMC with BNZ but not CPT resulted in a significant activation of PKA activity (6.5 ± 0.75 fold, n = 3, p < 0.001), measured by kemptide phosphorylation assay (Fig. 2A). Consistent with this, VSMC stimulation with CPT did not increase phosphorylation of the PKA substrate VASP (4 ± 1.3% compared to 3.2 ± 1.3% phosphorylated in control) (Fig. 2B) but did significantly activate the Epac target Rap1 (Fig. 2C, 2.5 ± 0.4 fold compared to control, n = 6, p < 0.05). BNZ stimulation resulted in a 29.3 ± 16.8% phosphorylation of PKA substrate VASP (Fig. 2B), without elevating Rap1 activity (Fig. 2C). Co-stimulation with BNZ plus CPT did not result in additional kemptide or VASP phosphorylation but did result in a reduced activation of Rap1 that was no longer significantly different from controls (Fig. 2). Elevation of endogenous cAMP levels with forskolin resulted in 89.8 ± 1.3% phosphorylation of VASP and a 2.6 ± 0.6 fold activation of Rap1, indicating an activation of both pathways. This data demonstrated that PKA and Epac signalling pathways can be selectively activated in VSMC by BNZ and CPT respectively.
Fig. 2.
Selective activation of PKA and Epac signalling with N6-Benzoyl-cAMP and 8-CPT-2′-O-Me-cAMP, respectively. VSMC were serum deprived for 24 h and stimulated with 200 μM BNZ, CPT, BNZ plus CPT or 100 μM forskolin for 30 min as indicated. A, Cell lysates were analysed for PKA activity by kemptide phosphorylation assay. Upper bands represent PKA phosphorylated kemptide while lower bands are un-phosphorylated kemptide. Recombinant PKA catalytic subunit and lysis buffer were included in the assay as positive and negative controls respectively. B, Cell lysates were analysed for phospho-VASP (slower migrating band) by western blotting with a total VASP antibody, n = 3. C, Rap1 activity was quantified by Ral-GDS pulldown, n = 6. * indicates p < 0.05 versus control.
3.3. PKA and Epac synergise to inhibit VSMC proliferation
To investigate whether activation of PKA alone is sufficient for VSMC growth arrest, we stimulated VSMC with the PKA-selective analogue BNZ. Stimulation with BNZ had no effect on BrdU incorporation, Rb-hyperphosphorylation or the expression levels of Cyclin D or Skp2 (Fig. 3) despite robust PKA activation (Figs. 2A, B and 4C). However, consistent with previous findings, stimulation with the non-selective cAMP analogue dibutyryl-cAMP (db-cAMP) significantly inhibited BrdU incorporation (to 59.3 ± 7.8% of control, n = 6, p < 0.01) (Fig. 3A) and Rb-hyperphosphorylation (to 29.3 ± 6.5% of control, n = 3, p < 0.05) (Fig. 3B). Db-cAMP also inhibited expression of G1-phase cell-cycle proteins Cyclin D and Skp2 (Fig. 3C). Taken together, these observations indicate that PKA activity is required but alone insufficient for cAMP-induced growth inhibition in VSMC. Therefore we investigated the role of the Epac signalling pathway.
Fig. 3.
Epac and PKA synergise to inhibit VSMC cell-cycle progression. Asynchronous VSMC were stimulated with 200 μM BNZ, 200 μM CPT, 200 μM BNZ plus 200 μM CPT, or 200 μM db-cAMP: A, for 24 h with the last 6 h in the presence of 10 μM BrdU and analysed for BrdU incorporation, n = 6; B and C, for 18 h and cell lysates analysed by western blotting as indicated, n = 3. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001 versus control.
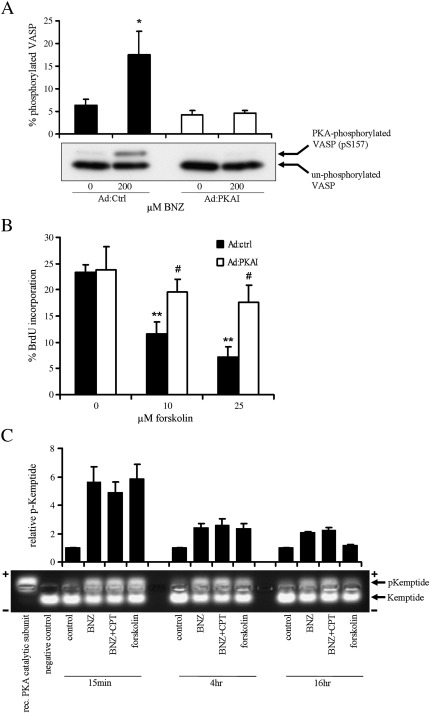
Fig. 4.
PKA is required but not sufficient to inhibit VSMC cell-cycle progression. VSMC were infected with either Ad:Control or Ad:PKAI at 2 × 108 pfu/ml. A, 40 h later, cells were rendered quiescent by serum deprivation for a further 6 h followed by stimulation with 200 μM BNZ for 15 min. Total cell lysates were analysed for non-phosphorylated (lower band) and phosphorylated (upper band) VASP by western blotting, n = 3. B, Asynchronous VSMC were stimulated with forskolin for 24 h with the last 6 h in the presence of 10 μM BrdU. BrdU incorporation was quantified by immuno-staining for BrdU and counting of positively stained nuclei, n = 7. C, Asynchronous VSMC were stimulated for the indicated times with 200 μM BNZ, 200uM CPT, 200 μM BNZ plus 200 μM CPT, or 100 μM forskolin, and analysed for PKA activity by kemptide phosphorylation assay. Upper bands represent PKA phosphorylated kemptide while lower bands are un-phosphorylated kemptide. Recombinant PKA catalytic subunit and lysis buffer were included in the assay as positive and negative controls respectively. * indicates p < 0.05, ** indicates p < 0.01 versus Ad:Control alone. # indicates p < 0.05 versus corresponding dose in Ad:Control.
Stimulation with the Epac-selective cAMP-analogue CPT alone had no effect on BrdU incorporation, Rb-hyperphosphorylation or expression of Cyclin D or Skp2 (Fig. 3) despite robust activation of Rap1 (Fig. 2C). This indicates that Epac activation in isolation is also insufficient to inhibit VSMC proliferation. Given that elevation of endogenous cAMP with forskolin or treatment with the non-selective cAMP analogue db-cAMP induces VSMC growth arrest, we tested the hypothesis that coordinated activation of both PKA and Epac signalling is required. Consistent with this, stimulation with both BNZ and CPT together significantly inhibited BrdU incorporation (to 48.3 ± 15.1% of control, n = 6, p < 0.001) and Rb-hyperphosphorylation (to 20.3 ± 14.8% of control, n = 3, p < 0.05) (Fig. 3). Furthermore, BNZ plus CPT stimulation potently inhibited expression of Cyclin D and Skp2 to levels similar to that in db-cAMP treated cells (Fig. 3C). This indicates that while activation of PKA or Epac alone is insufficient, the simultaneous activation of both of these cAMP-dependent pathways results in growth arrest in VSMC.
Given that some reports have demonstrated Epac-independent effects of CPT metabolites [25,26], we used the non-hydrolysable derivative of CPT, Sp-8-CPT-2′-O-Me-cAMPS (CPTSp) to confirm that the effects observed were indeed operating through activation of Epac. Consistent with our earlier observations (Fig. 3), stimulation of VSMC with BNZ or CPTSp alone had no effect on BrdU incorporation, while simultaneous stimulation with both BNZ and CPTSp significantly inhibited BrdU incorporation to 77.1 ± 7.3% of control (n = 6, p < 0.05) (Supplemental Fig. 1). The magnitude of growth inhibition induced by CPTSp plus BNZ was less that that induced by CPT plus BNZ (Fig. 3B). This probably reflects the lower potency of CPTSp for Epac compared to CPT [27].
Supplemental Fig. 1.
Epac and PKA synergise to inhibit VSMC proliferation. A, Asynchronous VSMC were stimulated with 200 μM BNZ, with or without 200 μM CPTSp stimulation, for 24 h with the last 6 h in the presence of 10 μM BrdU and analysed for BrdU incorporation, n = 6. * indicates p < 0.05 versus control.
To confirm the role of PKA in cAMP-mediated growth arrest, we used BNZ and PKA inhibitor expressing adenovirus (Ad:PKAI). Infection with Ad:PKAI but not Ad:Control completely inhibited BNZ-stimulated phosphorylation of VASP, demonstrating potent PKA inhibition in Ad:PKAI infected cells (Fig. 4A). Forskolin stimulation of Ad:Control infected cells resulted in dose-dependent and significant inhibition of BrdU incorporation that was prevented by infection with Ad:PKAI (Fig. 4B). BrdU incorporation in forskolin stimulated Ad:PKAI infected cells was significantly higher (n = 7, p < 0.05) than in Ad:Control infected cells at both doses of forskolin tested, demonstrating a requirement for PKA activity in cAMP-induced growth arrest of VSMC.
Since prolonged PKA activity is necessary for cAMP-mediated VSMC growth arrest [28], we investigated the effect of Epac stimulation on the level and duration of BNZ-induced PKA activity. BNZ stimulation alone caused an induction of PKA activity similar to that caused by forskolin and lasting at least 16 h (Fig. 4C). Activation of Epac with CPT did not enhance PKA activity further. This indicates that the synergistic actions of PKA and Epac occur downstream of PKA activity, and emphasises that prolonged PKA activation alone is insufficient for cAMP-mediated growth arrest of VSMC, implying a requirement for an additional cAMP-sensitive pathway, such as Epac.
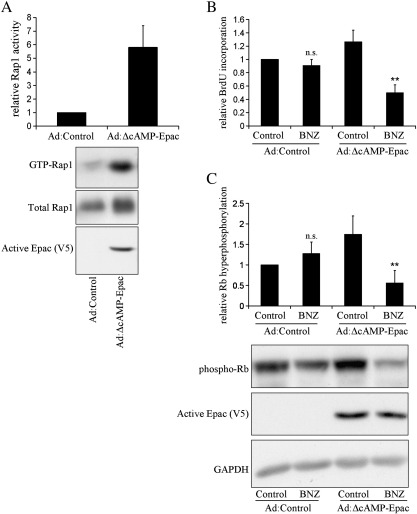
To test if Epac activation synergises with PKA to inhibit VSMC proliferation we used adenovirus-mediated expression of constitutively active Epac (Ad:ΔcAMP-Epac) lacking the N-terminal DEP and cAMP-binding domains. Ad:ΔcAMP-Epac infection resulted in expression of a ~ 60 kDa ∆cAMP-Epac protein and a significant 5.8 ± 1.6 fold increase in Rap1 activity (Fig. 5A), confirming constitutively active GEF activity. Expression of ΔcAMP-Epac alone did not significantly alter BrdU incorporation or Rb-hyperphosphorylation (Fig. 5), consistent with our results obtained with CPT. BNZ stimulation of Ad:Control infected cells had no effect on BrdU incorporation or Rb-hyperphosphorylation (Fig. 5), consistent with our earlier observation (Fig. 3). However, BNZ treatment in Ad:ΔcAMP-Epac infected cells induced a significant inhibition of BrdU incorporation (to 39.5 ± 7.7% of Ad:ΔcAMP-Epac alone, n = 4, p < 0.01) and Rb-hyperphosphorylation (to 28.1 ± 10.5% of Ad:ΔcAMP-Epac alone, n = 6, p < 0.01), further supporting the hypothesis that PKA and Epac signalling synergises to induce VSMC growth arrest.
Fig. 5.
Constitutively active Epac synergises with PKA to inhibit VSMC cell-cycle progression. Asynchronous VSMC were infected with an adenovirus encoding ΔcAMP-Epac as indicated. A, Rap1 activity in expressing and non-expressing cells was detected by Ral-GDS pulldown, n = 4. Cells were stimulated with 200 μM BNZ: B, for 24 h with the last 6 h in the presence of 10 μM BrdU and analysed for BrdU incorporation, n = 4; C, for 18 h and cell lysates were analysed by western blotting for Rb-hyperphosphorylation, n = 6. ** indicates p < 0.01 versus Ad:ΔcAMP-Epac.
3.4. cAMP-dependent growth arrest is Rap1-independent
Since Rap1, the best characterised Epac target protein, was activated by elevated cAMP levels or expression of constitutively active Epac, we tested the hypothesis that Epac-dependent activation of Rap1 was involved in cAMP-induced growth arrest and induction of stellate morphology in VSMC. We used adenovirus-mediated expression of Rap1GAP (Ad:Rap1GAP), which specifically inactivates Rap1 by increasing GTP hydrolysis [29] and completely inhibited Rap1 activity in VSMC (Fig. 6A). Ad:Rap1GAP infection resulted in a significant inhibition of basal BrdU incorporation rates (from 38.8 ± 4.0% in Ad:Control cells to 25.6 ± 4.0% in Rap1GAP infected cells, n = 9, p = 0.0004) (Fig. 6B) and Rb-hyperphosphorylation (to 43.5 ± 10.8% of Ad:Control, n = 5, p < 0.01) (Fig. 6E), indicative of a pro-proliferative role for Rap1 in VSMC. Consistent with our earlier observations, forskolin stimulation of Ad:Control infected cells resulted in a significant and dose-dependent inhibition of BrdU incorporation (p < 0.01 for all forskolin doses tested) (Fig. 6C) and Rb-hyperphosphorylation (65.0 ± 12.1% inhibition for 100 μM forskolin, n = 5, p < 0.001) (Fig. 6E). Importantly, inhibition of Rap1 activity with Ad:Rap1GAP did not prevent forskolin-induced arrest. Forskolin stimulation still resulted in a significant inhibition of BrdU incorporation (p < 0.05 for all forskolin doses tested at both Ad:Rap1GAP doses) the magnitude of which was not significantly different from that of Ad:Control infected cells at any dose of forskolin tested. Furthermore, forskolin stimulation resulted in a significant inhibition of Rb-hyperphosphorylation in Ad:Rap1GAP cells (94.9 ± 2.4% and 94.6 ± 2.2% inhibition compared to Ad:Rap1GAP alone for 25 and 100 μM forskolin respectively, n = 5, p < 0.05) that was not smaller in magnitude than that in Ad:Control infected cells. In fact, Rap1 inhibition resulted in a significantly larger inhibition of Rb-hyperphosphorylation in response to 25 μM forskolin compared to Ad:Control cells (94.9 ± 2.4% versus 37.8 ± 20.6%, n = 5, p < 0.05), consistent with a pro-proliferative, rather than an anti-proliferative, role for Rap1 in VSMC. Rap1 inhibition with Ad:Rap1GAP also had no effect on the magnitude of growth inhibition induced by a combination of BNZ plus CPT (59.1 ± 9.5% inhibition and 59.3 ± 19.2% inhibition in Ad:Control and Ad:Rap1GAP infected cells respectively).
Fig. 6.
Inhibition of Rap1 does not negate the effects of cAMP on cell-cycle progression. Where indicated, asynchronous VSMC were infected with an adenovirus encoding Rap1GAP at 108 pfu/ml (high) or 2 × 107 pfu/ml (low) (A–E), or transfected with siRNA specific for Rap1 as described (F, G). High dose of Ad:Rap1GAP was used unless otherwise indicated. VSMC were stimulated with the indicated forskolin doses for 18 h. A, Rap1GAP activity was confirmed by Rap1 activity assay. B, VSMC were incubated for 6 h in the presence of 10 μM BrdU and analysed for BrdU incorporation, n = 9. C, VSMC were stimulated with the forskolin doses indicated for a further 6 h in the presence of 10 μM BrdU and analysed for BrdU incorporation, n = 3; D and E, cell lysates were analysed by western blotting as indicated, n = 5. F, 24 h post transfection, mRNA levels of Rap1A and Rap1B were quantified by RT-PCR, n = 4; F (inset), and cell lysates were analysed by western blotting with a pan-Rap1A/B antibody; G, VSMC were stimulated with the indicated forskolin doses a further 6 h in the presence of 10 μM BrdU and analysed for BrdU incorporation, n = 6. * indicates p < 0.05 versus siCtrl. ** indicates p < 0.01, *** indicates p < 0.001 versus Ad:Control. # indicates p < 0.05, ## indicates p < 0.01 versus Ad:Rap1GAP.
To further confirm the Rap1-independence of cAMP-induced growth arrest in VSMC, we used siRNA to specifically silence expression of both Rap1A and Rap1B isoforms. Co-transfection with siRNA targeting both Rap1A and Rap1B resulted in a significant inhibition of Rap1A and Rap1B mRNA expression (82.0 ± 3.3% silencing for Rap1A and 83.4 ± 3.2% silencing for Rap1B, n = 4, p < 0.05) but not GAPDH mRNA expression, compared to control siRNA transfected cells (Fig. 6F). Rap1-siRNA also significantly inhibited expression of Rap1 protein detected with a pan-Rap1A/B antibody (51.1 ± 11.4% inhibition, n = 3, p < 0.05) but did not affect GAPDH protein levels. Inhibition of Rap1 with siRNA resulted in a significant inhibition of basal proliferation rates (from 46.4 ± 3.4% to 38.5 ± 1.2%, n = 3, p < 0.05), consistent with our data obtained with Rap1GAP (Fig. 6G). However, silencing of Rap1 did not result in reversal of forskolin-induced growth arrest. Taken together, this data demonstrates that cAMP-mediated growth arrest and induction of stellate morphology occurs via a Rap1-independent mechanism in VSMC.
3.5. PKA and Epac synergise to inhibit ERK and JNK signalling
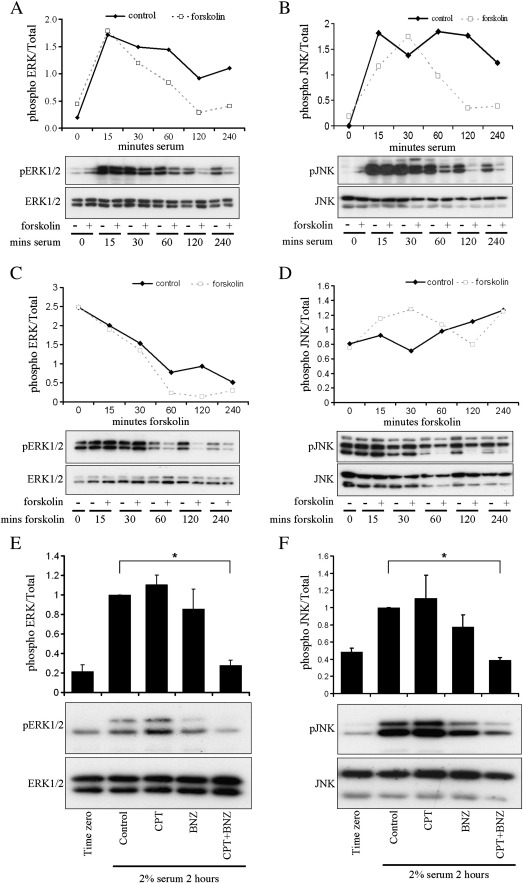
Some reports suggest that the growth-inhibitory effects of cAMP can be mediated through inhibition of ERK signalling [30–32]. In addition, the reported regulation of JNK by Epac, independent of its GEF activity [33], represents a Rap1-independent mechanism for Epac signalling. In order to elucidate the role of ERK and JNK in cAMP-mediated inhibition of VSMC proliferation, we examined their phosphorylation states in mitogen stimulated VSMC. Elevation of endogenous cAMP with forskolin, which results in activation of both PKA and Epac (Fig. 2), inhibited both ERK1/2 and JNK phosphorylation induced by acute serum stimulation (Figs. 7A and B). Inhibition of JNK phosphorylation was only evident at late (1–4 h) but not early (15–30 min) time points (Fig. 7B). Forskolin also inhibited ERK1/2 but not JNK phosphorylation in cells grown continuously in the presence of serum (Figs. 7C and D). To determine the relative contribution of PKA and Epac to this inhibition of ERK1/2 and JNK signalling, we treated serum deprived cells with BNZ, CPT or BNZ plus CPT before 2% serum stimulation (in the continued presence of the indicated cAMP analogues) for two hours. BNZ or CPT alone had no significant effect on ERK1/2 or JNK phosphorylation (Figs. 7E and F). However, stimulation with both BNZ and CPT together significantly inhibited ERK1/2 and JNK phosphorylation, indicating that PKA and Epac synergise to inhibit these kinases (Figs. 7E and F).
Fig. 7.
Epac and PKA synergise to inhibit phosphorylation of ERK1/2 and JNK. A and B, Effect of forskolin on acute serum stimulated ERK1/2 and JNK phosphorylation. VSMC were rendered quiescent by serum deprivation for 24 h and pre-treated with 25 μM forskolin as indicated for 30 min before stimulation with 10% serum in the presence of 25 μM forskolin for the timepoints (minutes) indicated. C and D, Effect of forskolin on ERK1/2 and JNK phosphorylation in cells grown continuously in the presence of serum. VSMC were stimulated with 25 μM forskolin in the presence of 10% serum for the timepoints (minutes) indicated. E and F, Effect of PKA and Epac-selective analogues on acute serum stimulated ERK1/2 and JNK phosphorylation. VSMC were rendered quiescent for 24 h and pre-treated with 200 μM of the indicated cAMP analogues before 2% serum stimulation for the indicated timepoints (minutes). Levels of phosphorylated and total ERK1/2 and JNK were quantified by western blotting. * indicates p < 0.05; ANOVA with Student–Newman–Keuls post test on logged data.
3.6. PKA and Epac synergise to induce stellate morphology and associated actin cytoskeleton and focal adhesion disruption in a Rap1-independent manner
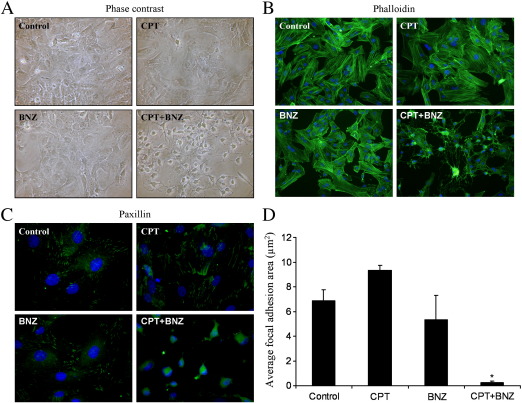
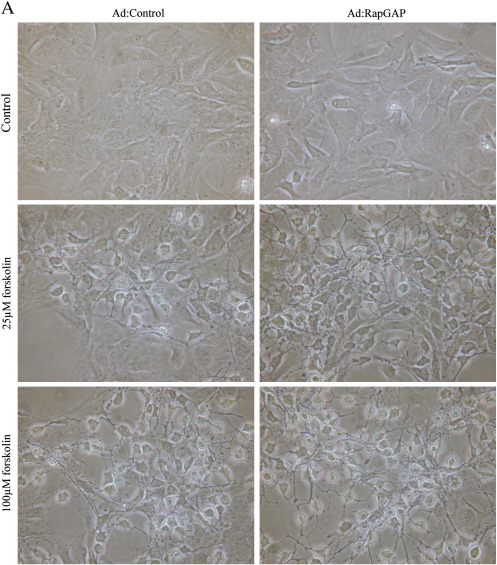
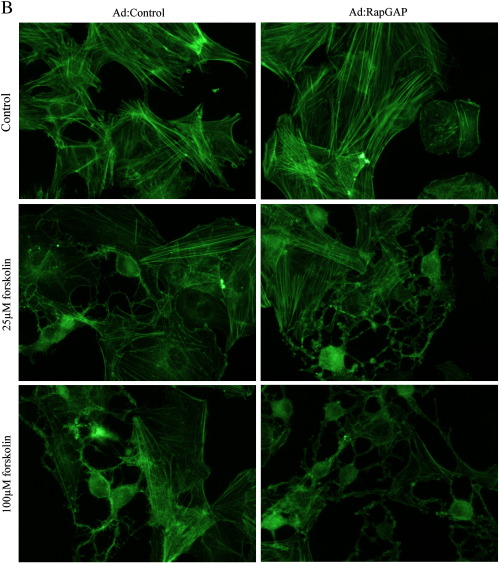
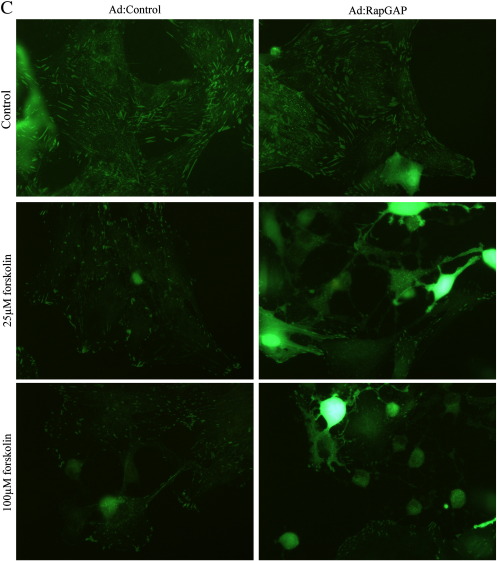
Our previous observations demonstrated that cAMP-mediated growth arrest of VSMC is associated with induction of stellate morphology, characterised by loss of actin stress fibres and focal adhesions, both of which are essential for cell-cycle progression [34,35]. We therefore tested the hypothesis that activation of PKA and Epac signalling is associated with a synergistic induction of stellate morphology and cytoskeleton disruption. Consistent with this, BNZ or CPT stimulation alone did not result in induction of stellate morphology. These cells retained prominent actin stress fibres and there was no significant effect on focal adhesion area (Fig. 8). Importantly, co-stimulation with BNZ plus CPT induced a pronounced stellate morphology (Fig. 8A) associated with loss of actin stress fibres (Fig. 8B) and a significant decrease in focal adhesion area (Fig. 8D). Furthermore, VSMC infected with Ad:Rap1GAP or Ad:Control adopted a similar stellate morphology in response to forskolin stimulation, with associated loss of actin stress fibres and focal adhesions (Supplemental Fig. 2), indicating that these effects are not mediated by Rap1. This coordinated induction of stellate morphology represents a potential downstream mechanism by which the synergy between PKA and Epac signalling (independent of Rap1) may mediate cAMP-induced VSMC growth arrest.
Fig. 8.
Epac and PKA synergise to induce stellate morphology. Asynchronous VSMC were stimulated with 200 μM BNZ, CPT or BNZ plus CPT as indicated. A, Phase contrast images showing cell morphology. B, Phalloidin staining for F-actin. C, Paxillin staining representing focal adhesions, D, the area of which was quantified using NIH ImageJ, n = 3. * indicates p < 0.05 versus control.
Supplemental Fig. 2.
Inhibition of Rap does not negate the effects of cAMP on morphology. Asynchronous VSMC were infected with an adenovirus encoding Rap1GAP and cells were stimulated with 25 and 100 μM forskolin. A, Phase contrast images showing cell morphology. B, Phalloidin staining for F-actin. C, Paxillin staining representing focal adhesions.
4. Discussion
cAMP is a major second messenger involved in the regulation of VSMC proliferation. The ability of vascular cells to synthesise cAMP is high in healthy vessels and contributes toward the maintenance of VSMC quiescence. Reduced synthesis of cAMP after vessel injury is likely to be an important mechanism in removing the “brake” on proliferation. In this study, we show for the first time that PKA and Epac act synergistically to induce growth arrest in VSMC via a Rap1-independent mechanism.
The growth-inhibitory properties of cAMP in VSMC have been recognised for many years [5]. Elevated cAMP levels arrest cells in G1 phase of the cell cycle, at least in part through inhibition of G1-regulatory proteins Cyclin D1 and Skp2 [4,6]. However, upstream signalling mechanisms underlying these anti-proliferative effects have remained elusive. Several studies demonstrated a clear role for PKA, at a time when this was the only known cAMP-sensitive protein. For example, PKA inhibition completely reversed cAMP inhibition of VSMC proliferation and injury-induced neointima formation in vivo [5] and our results are consistent with this essential role of PKA in cAMP-mediated growth arrest. Our data now demonstrates that PKA activation is essential for cAMP-mediated growth arrest but alone is unable to induce these effects, indicating a requirement for an additional cAMP-sensitive pathway. The recent identification of the Epac family of cAMP-sensitive GEFs demonstrated the existence of a distinct signalling pathway directly activated by cAMP [12]. Characterisation of Epac proteins in different cell types shows diverse and highly cell-type specific functions, reflecting the specific effects of cAMP in those cells. These include integrin-dependent adhesion, cell–cell junction formation, apoptosis, cardiac hypertrophy, cell differentiation, cytoskeleton rearrangements, gene expression and cell proliferation [18]. However, the function of Epac signalling in cAMP-mediated growth arrest of VSMC remained unknown. We show that Epac1 but not Epac2 is expressed in VSMC together with the Epac-effector proteins Rap1A and Rap1B. Furthermore, we show that Rap1 is activated in response to forskolin or by an Epac-selective cAMP analogue, demonstrating that this pathway is present and functional in VSMC. The development of cAMP analogues, selective for PKA or Epac [23], has provided invaluable tools for the analysis of PKA and Epac signalling and we confirm their specificity in VSMC. Using these analogues, we show for the first time that activation of either pathway alone had little effect on proliferation, while dual activation potently suppressed it. This indicates that Epac plays a major role, in synergy with PKA, in mediating cAMP-dependent growth inhibition of VSMC. This novel function for Epac in VSMC was confirmed using adenovirus-mediated expression of a constitutively activated truncation of Epac. Activation of Epac in this way, independent of PKA stimulation, had no effect of cell proliferation. However, co-stimulation of PKA potently suppressed proliferation and Rb-hyperphosphorylation, again demonstrating a synergistic interaction between PKA and Epac signalling in mediating VSMC growth arrest. In order to further confirm the role of Epac in cAMP-mediated growth inhibition in VSMC, we employed siRNA-mediated gene silencing (data not shown). Interestingly, Epac siRNA failed to reverse forskolin-induced growth arrest despite efficient silencing of Epac protein expression. However, great care must be taken when interpreting data from Epac silencing experiments since Epac is known to control both cAMP-dependent and cAMP-independent signalling pathways [33]. This considered, Epac silencing does not equate to inhibition of Epac activity. For example, Epac has recently been shown to control JNK signalling in a cAMP-independent manner [33] and consistent with this, we find that Epac silencing also inhibits JNK activity in VSMC (data not shown). These cAMP-independent functions of Epac complicate analysis of Epac silencing data and probably explain the inability of Epac silencing to reverse cAMP-mediated growth arrest. Future development of specific pharmacological Epac antagonists should facilitate this type of functional analysis of Epac and allow both in vitro and in vivo confirmation of the role of Epac in VSMC growth regulation.
Co-operation or synergy between PKA and Epac has been recently reported in PCCL3 thyroid cell line in which cAMP is pro-mitogenic, in contrast to VSMC [19]. Our study demonstrates for the first time that PKA and Epac also synergise to inhibit cell proliferation in a cell type where cAMP in anti-mitogenic. However, other studies have indicated both pro- [36] and anti-proliferative [20] properties of Epac signalling independent of PKA, highlighting the complex and highly cell-type specific nature of this signalling network.
There is evidence that the growth-inhibitory effect of cAMP in VSMC is dependent on the prolonged rather than transient PKA activation [28]. However, our data demonstrates that stimulation with a PKA-selective cAMP analogue fails to induce growth arrest, despite inducing robust PKA activation that persists for at least 16 h and is actually greater than that induced by forskolin at later time points. Furthermore, we show that Epac activation did not further increase PKA activity. This data indicates that synergy between PKA and Epac signalling does not occur at the level of PKA activation.
Several studies have demonstrated a role for Epac signalling in the activation of Rap1, with an additional role for PKA signalling [12,37,38]. This suggests that Rap1 may act as a common target for PKA and Epac in some cell types, raising the possibility of synergy between PKA and Epac acting at the level of Rap1 activation. However, our data demonstrates that PKA signalling did not enhance Epac-dependent Rap1 activation in VSMC but instead decreased it, indicating that PKA actually antagonised Epac-dependent Rap1 activation. Rap1 activation is therefore an unlikely target for the synergy between these two pathways. In accordance with this, specific and complete inhibition of Rap1 activity with Rap1GAP did not reverse the effects of cAMP-elevation on cell proliferation, demonstrating that cAMP-dependent growth inhibition is Rap1-independent in VSMC. Rap1-independence was further confirmed using siRNA-mediated silencing of Rap1 A/B. This clearly implicates a role for other currently unidentified Epac-effector proteins in cAMP-dependent growth arrest. Although many studies demonstrate a role for Rap1 signalling in mediating the effects of Epac activation in other cell types [18], several studies have also described Rap1-independent effects of Epac, similar to those described in this study [33,39]. Future research, beyond the scope of the current study, should focus on identifying and characterising other Epac-effector proteins to advance our understanding of the mechanisms underlying cAMP-dependent signalling. Taken together, this data indicates that the synergy between PKA and Epac signalling must occur at a level further downstream and not at the level of PKA or Epac activity itself.
The level at which PKA and Epac pathways synergise remains unclear. One plausible mechanistic model is that PKA and Epac independently target different, redundant downstream cell-cycle regulators, such as the G1-phase regulatory proteins Cyclin D and Skp2. In this model, growth inhibition would only occur when expression of both cell-cycle regulators were suppressed by the simultaneous activation of both pathways. However, expression of Cyclin D and Skp2, known targets of cAMP signalling, is not affected by PKA or Epac signalling in isolation but potently suppressed when both are activated together. This suggests that PKA and Epac synergise upstream of G1 regulatory proteins.
Our data also clearly demonstrates that cAMP-induced stellate morphology in VSMC, with the associated loss of actin stress fibres and focal adhesions, is dependent upon activation of both PKA and Epac signalling suggesting that synergy may occur at the level of cytoskeleton organisation. Both PKA and Epac have been previously implicated in cytoskeleton regulation in other cell types by targeting numerous downstream proteins involved in the regulation of actin-polymerisation, including VASP, RhoA, Rac1 and Merlin [18,40–42]. cAMP-dependent growth inhibition in VSMC is associated with this induction of stellate morphology and loss of cytoskeleton integrity suggesting a possible causative role. Numerous studies have demonstrated a critical requirement forcytoskeleton integrity and organisation for G1-S phase progression [34,43], suggesting that the cytoskeleton may act as a nexus for PKA and Epac-dependent signals, ultimately controlling VSMC proliferation.
It remains to be determined whether cytoskeleton remodelling represents the primary mechanism for cAMP-dependent growth arrest in VSMC. However, several studies have demonstrated that mitogenic signalling through the ERK1/2 pathway is dependent on cytoskeleton integrity. We investigated whether inhibition of members of the MAPK pathway could be a target for synergy between PKA and Epac signalling since they have been implicated in cAMP-mediated growth arrest in some cell types [24,44], but not others [45,46]. For example, Epac signalling has been reported to regulate JNK signalling in HEK-293T cells while elevated cAMP has also been shown to inhibit ERK activation [24,33]. Whether cAMP inhibits VSMC proliferation via ERK1/2 in VSMC remains controversial [44,45]. However, elevation of endogenous cAMP with forskolin in our experiments resulted in inhibition of both basal and serum stimulated ERK and JNK phosphorylation. CPT and BNZ stimulation synergised to inhibit ERK and JNK phosphorylation, indicating that coordinated signalling of PKA and Epac mediates inhibition of these MAPKs and may contribute to the anti-proliferative effects of cAMP in VSMC.
The requirement for two distinct signalling pathways for cAMP-induced growth arrest likely represents an important mechanism allowing VSMC to finely modulate their cellular responses to cAMP-elevating stimuli. Our data demonstrates for the first time that activation of both pathways in VSMC results in potent and synergistic growth arrest and these observations highlight Epac alongside PKA as a major player in cAMP-dependent growth arrest in VSMC in vitro. The future development of Epac-specific pharmacological antagonists and analysis of Epac-deficient mice will allow validation of the relative roles of PKA and Epac in the regulation of VSMC proliferation during the development of vascular disease in vivo.
The following are the supplementary materials related to this article.
Acknowledgements
This work was supported by British Heart Foundation grant FS/07/038 and by the NIHR Bristol BRU in Cardiovascular Medicine. We would like to thank Prof. Patrick Casey and Candice Bailey for the generous gift of the Rap1GAP adenovirus, Prof. David Murphy for the PKAI adenovirus and Prof. Peter Cullen for the GST-Ral-GDS expression vector. We thank Gill Tarlton and Sue Finerty for their expert support. We also thank Prof. Nickolai Dulin and Prof. Donald Maurice for their helpful advice and discussion.
References
- 1.Izzard T., Taylor C., Birkett S., Jackson C., Newby A. Mechanisms underlying maintenance of smooth muscle cell quiescence in rat aorta: role of the cyclin dependent kinases and their inhibitors. Cardiovasc Res. 2002;52:242–252. doi: 10.1016/s0008-6363(01)00444-8. [DOI] [PubMed] [Google Scholar]
- 2.Lewis C.D., Olson N.E., Raines E.W., Reidy M.A., Jackson C.L. Modulation of smooth muscle proliferation in rat carotid artery by platelet-derived mediators and fibroblast growth factor-2. Platelets. 2001;12:352–353. doi: 10.1080/09537100120071013. [DOI] [PubMed] [Google Scholar]
- 3.Hedin U., Roy J., Tran P.K. Control of smooth muscle cell proliferation in vascular disease. Curr Opin Cell Biol. 2004;15:559–565. doi: 10.1097/00041433-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y., Bond M., Sala-Newby G., Newby A. Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ Res. 2006;98:1141–1150. doi: 10.1161/01.RES.0000219905.16312.28. [DOI] [PubMed] [Google Scholar]
- 5.Indolfi C., Avvedimento E.V., DiLorenzo E., Esposito G., Rapacciuolo A., Giuliano P. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med. 1997;3:775–779. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- 6.LAllemain G., Lavoie J., Rivard N., Baldin V., Pouyssegur J. Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle entry in fibroblasts. Oncogene. 1997;14:1981–1990. doi: 10.1038/sj.onc.1201038. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi S., Morishita R., Matsushita H., Nakagami H., Taniyama Y., Nakamura T. Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension. 2000;35:237–243. doi: 10.1161/01.hyp.35.1.237. [DOI] [PubMed] [Google Scholar]
- 8.Bond M., Wu Y.-J., Sala-Newby G., Newby A. Biphasic effect of p21Cip1 on vascular smooth muscle cell proliferation: role of PI3-kinase signalling and Skp2-mediated degradation. Cardiovasc Res. 2005;69:198–206. doi: 10.1016/j.cardiores.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y.J., Sala-Newby G.B., Shu K.T., Yeh H.I., Nakayama K.I., Nakayama K. S-phase kinase-associated protein-2 (Skp2) promotes vascular smooth muscle cell proliferation and neointima formation in vivo. J Vasc Surg. 2009;50:1135–1142. doi: 10.1016/j.jvs.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H.T., Kay E.P. Regulatory role of cAMP on expression of Cdk4 and p27Kip1 by inhibiting phosphatidylinositol 3-kinase in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2003;44:3816–3825. doi: 10.1167/iovs.03-0147. [DOI] [PubMed] [Google Scholar]
- 11.Hecquet C., Lefevre G., Valtink M., Engelmann K., Mascarelli F. CAMP inhibits the proliferation of retinal pigmented epithelial cells through the inhibition of ERK1/2 in a PKA-independent manner. Oncogene. 2002;21:6101–6112. doi: 10.1038/sj.onc.1205765. [DOI] [PubMed] [Google Scholar]
- 12.de Rooij J., Zwartkruis F.J.T., Verheijen M.H.G., Cool R.H., Nijman S.M.B., Wittinghofer A. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 13.Bos J. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki H., Springett G.M., Mochizuki N., Toki S., Nakaya M., Matsuda M. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 15.de Rooij J., Rehmann H., van Triest M., Cool R.H., Wittinghofer A., Bos J.L. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama U., Minamisawa S., Quan H., Akaike T., Jin M.H., Otsu K. Epac1 is upregulated during neointima formation and promotes vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2008;295:H1547–H1555. doi: 10.1152/ajpheart.01317.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta M., Yarwood S.J. MAP1A light chain 2 interacts with exchange protein activated by cyclic AMP 1 (EPAC1) to enhance Rap1 GTPase activity and cell adhesion. J Biol Chem. 2005;280:8109–8116. doi: 10.1074/jbc.M413697200. [DOI] [PubMed] [Google Scholar]
- 18.Roscioni S.S., Elzinga C.R.S., Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedeberg's Arch Pharmacol. 2008;377:345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 19.Hochbaum D., Hong K., Barila G., Ribeiro-Neto F., Altschuler D.L. Epac, in synergy with cAMP-dependent protein kinase (PKA), is required for cAMP-mediated mitogenesis. J Biol Chem. 2008;283:4464–4468. doi: 10.1074/jbc.C700171200. [DOI] [PubMed] [Google Scholar]
- 20.Haag S., Warnken M., Juergens U., Racke K. Role of Epac1 in mediating anti-proliferative effects of prostanoid EP2 receptors and cAMP in human lung fibroblasts. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:617–630. doi: 10.1007/s00210-008-0334-3. [DOI] [PubMed] [Google Scholar]
- 21.Wong L.F., Harding T., Uney J., Murphy D. cAMP-dependent protein kinase A mediation of vasopressin gene expression in the hypothalamus of the osmotically challenged rat. Mol Cell Neurosci. 2003;24:82–90. doi: 10.1016/s1044-7431(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 22.Ohba Y., Mochizuki N., Matsuo K., Yamashita S., Nakata M., Hashimoto Y. Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol Cell Biol. 2000;20:6074–6083. doi: 10.1128/mcb.20.16.6074-6083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen A.E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K.K. cAMP analog mapping of Epac1 and cAMP kinase — discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 24.Enserink J.M., Christensen A.E., de Rooij J., van Triest M., Schwede F., Genieser H.G. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 25.Enyeart J.A., Enyeart J.J. Metabolites of an Epac-selective cAMP analog induce cortisol synthesis by adrenocortical cells through a cAMP-independent pathway. PLoS ONE. 2009;4:e6088. doi: 10.1371/journal.pone.0006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enyeart J.A., Liu H., Enyeart J.J. cAMP analogues and their metabolites enhance TREK-1 mRNA and K + current expression in adrenocortical cells. Mol Pharmacol. 2010;77:469–482. doi: 10.1124/mol.109.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poppe H., Rybalkin S.D., Rehmann H., Hinds T.R., Tang X.B., Christensen A.E. Cyclic nucleotide analogues as probes of signaling pathways. Nat Meth. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 28.Hogarth D.K., Sandbo N., Taurin S., Kolenko V., Miano J.M., Dulin N.O. Dual role of PKA in phenotypic modulation of vascular smooth muscle cells by extracellular ATP. Am J Physiol Cell Physiol. 2004;287:C449–C456. doi: 10.1152/ajpcell.00547.2003. [DOI] [PubMed] [Google Scholar]
- 29.Rubinfeld B., Munemitsu S., Clark R., Conroy L., Watt K., Crosier W.J. Molecular-cloning of a GTPase activating protein-specific for the Krev-1 protein p21Rap1. Cell. 1991;65:1033–1042. doi: 10.1016/0092-8674(91)90555-d. [DOI] [PubMed] [Google Scholar]
- 30.Tantini B., Manes A., Fiumana E., Pignatti C., Guarnieri C., Zannoli R. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol. 2005;100:131–138. doi: 10.1007/s00395-004-0504-5. [DOI] [PubMed] [Google Scholar]
- 31.Cook S.J., Rubinfeld B., Albert I., McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook S.J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 33.Hochbaum D., Tanos T., Ribeiro-Neto F., Altschuler D.L., Coso O. Activation of JNK by EPAC is independent of its activity as a Rap guanine nucleotide exchanger. J Biol Chem. 2003;278:33738–33746. doi: 10.1074/jbc.M305208200. [DOI] [PubMed] [Google Scholar]
- 34.Assoian R.K., Zhu X.Y. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. doi: 10.1016/s0955-0674(97)80157-3. [DOI] [PubMed] [Google Scholar]
- 35.Bond M., Sala-Newby G., Newby A. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. J Biol Chem. 2004;279:37304–37310. doi: 10.1074/jbc.M404307200. [DOI] [PubMed] [Google Scholar]
- 36.Cass L., Summers S., Prendergast G., Backer J., Birnaum M., MeinKoth J. Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol. 1999;19:5882–5891. doi: 10.1128/mcb.19.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obara Y., Labudda K., Dillon T.J., Stork P.J.S. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci. 2004;117:6085–6094. doi: 10.1242/jcs.01527. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt J., Stork P. PKA phosphorylation of Src mediated cAMP's inhibition of cell growth via Rap1. Mol Cell. 2002;9:85–94. doi: 10.1016/s1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- 39.Shi G., Rehmann H., Andres D. A novel cyclic AMP-dependent Epac–Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol. 2006;26:9136–9147. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howe A. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta: Mol Cell Res. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Laulajainen M., Muranen T., Carpen O., Gronholm M. Protein kinase A-mediated phosphorylation of the NF2 tumor suppressor protein merlin at serine 10 affects the actin cytoskeleton. Oncogene. 2008 May;27:3233–3243. doi: 10.1038/sj.onc.1210988. [DOI] [PubMed] [Google Scholar]
- 42.Pelletier S., Julien C., Popoff M., Lamarche-Vane N., Meloche S. Cyclic AMP induces morphological changes of vascular smooth muscle cells by inhibiting a Rac-dependent signaling pathway. J Cell Physiol. 2005;204:412–422. doi: 10.1002/jcp.20308. [DOI] [PubMed] [Google Scholar]
- 43.Bond M., Wu Y.J., Sala-Newby G.B., Newby A.C. Rho GTPase, Rac1, regulates Skp2 levels, vascular smooth muscle cell proliferation, and intima formation in vitro and in vivo. Cardiovasc Res. 2008;80:290–298. doi: 10.1093/cvr/cvn188. [DOI] [PubMed] [Google Scholar]
- 44.Cospedal R., Lobo M., Zachary I. Differential regulation of extracellular signal-regulated protein kinases (ERKs) 1 and 2 by cAMP and dissociation of ERK inhibition from anti-mitogenic effects in rabbit vascular smooth muscle cells. Biochem J. 1999;342:407–414. [PMC free article] [PubMed] [Google Scholar]
- 45.Giasson E., Servant M.J., Meloche S. Cyclic AMP-mediated inhibition of angiotensin II-induced protein synthesis is associated with suppression of tyrosine phosphorylation signaling in vascular smooth muscle cells. J Biol Chem. 1997;272:26879–26886. doi: 10.1074/jbc.272.43.26879. [DOI] [PubMed] [Google Scholar]
- 46.Graves L.M., Bornfeldt K.E., Argast G.M., Krebs E.G., Kong X.M., Lin T.A. Camp-sensitive and rapamycin-sensitive regulation of the association of eukaryotic initiation-factor 4e and the translational regulator Phas-I in aortic smooth-muscle cells. Proc Natl Acad Sci USA. 1995;92:7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]